Abstract
The nuclear hormone receptors farnesoid X receptor (FXR) and pregnane X receptor have been implicated in regulating bile acid, lipid, carbohydrate, and xenobiotic metabolism. Bile duct ligation was used to increase endogenous bile acids and evaluate the roles of these receptors in modulating cholestatic liver injury. FXR knockout (KO) mice were found to be protected from obstructive cholestasis. Concurrent deletion of FXR also could ameliorate an increase in liver injury that is seen usually in pregnane X receptor KO mice with cholestasis. Mechanisms proposed for this protection include the lowering of bile acid concentrations and altered expression of the hepatic transporters Mdr1, Mdr2, BSEP, and Mrp4. FXR KO mice also exhibit a biphasic lipid profile after bile duct ligation, with an increase in high-density lipoprotein cholesterol and triglycerides by day 6. The expression of apolipoprotein AV was reduced in these mice, implicating FXR in triglyceride regulation. We show that FXR modulates cholestasis by controlling bile acids within the hepatocyte and is involved in bile acid synthesis, bile excretion via BSEP, and serum export via Mrp4. This study strongly suggests a potential clinical role for FXR antagonists in the treatment of obstructive cholestatic liver disorders.
Keywords: bile acids, Mrp4, pregnane X receptor, apolipoprotein AV, triglycerides
Cholestatic liver disorders include a spectrum of hepatobiliary diseases of diverse etiologies that are characterized by impaired hepatocellular secretion of bile, resulting in accumulation of bile acids, bilirubin, and cholesterol. Pharmacological therapy for cholestasis is limited, and ursodeoxycholic acid (UDCA) is the only disease-modifying therapy with evidence of efficacy, so new therapeutic approaches are essential.
Farnesoid X receptor (FXR) (NR1H4) and pregnane X receptor (PXR) (NR1I2) are nuclear hormone receptors that function as ligand-activated transcription factors. FXR is known to regulate genes involved in lipid and lipoprotein metabolism, including apolipoprotein (apo) AI, apoCII, very low-density lipoprotein (VLDL) receptor, PPARα, and the phospholipid transfer protein (1, 2), but the role of FXR in lipid homeostasis remains to be clarified. Other FXR targets include genes involved in bile acid synthesis such as cytochrome P450 7A1 (CYP7A1) and CYP8B1, and bile acid transporters including bile salt export pump (BSEP; ABCB11), sodium-taurocholate cotransporting polypeptide (NTCP; SLC10A1), and intestinal bile acid-binding protein (1, 3). PXR also regulates genes involved in bile acid synthesis, metabolism, and transport, including CYP7A1, CYP3A, sulfotransferase, and Na+-dependent organic anion transporter 2 (Oatp2; Slc21a6) (4, 5).
Bile acids are the end products of hepatic cholesterol metabolism, which also act as signaling molecules that regulate their own synthesis and metabolism and other metabolic pathways, via nuclear receptors (1). Bile acids are ligands for both PXR and FXR, with affinities differing for individual bile acids (6, 7). It has been suggested that bile acids can lower triglyceride levels via a pathway in which FXR induces the small heterodimer partner (SHP; NR1I0) that, in turn, represses sterol regulatory element-binding protein-1c (SREBP-1c) (8).
Hepatic bile acid accumulation and hyperlipidemia are features of cholestasis. Bile acid-induced liver injury may progress to cirrhosis and be complicated by endotoxemia and sepsis, because of a reduction in intestinal bile acids that have an important defensive role, fragmenting bacterial lipopolysaccharide (LPS) and enteroendotoxin to reduce intestinal absorption of these molecules (9, 10). During the body’s acute-phase response to injury or infection, extensive changes in plasma lipoproteins occur, including an increase in production and a decrease in catabolism of circulating lipoproteins (11). Lipoproteins also play an important role in LPS detoxification by binding to its bioactive lipid A portion and neutralizing its toxic effects. Specifically, triglyceride-rich lipoproteins are important components of innate host-immune response to infection, and in rodent models, VLDL and chylomicrons can effectively protect against LPS-induced death (11, 12).
Bile duct ligation (BDL) is an established animal model of cholestasis, with complete biliary obstruction and accumulation of multiple primary bile acids in liver and serum (13). Changes in serum lipids and increases in circulating endotoxin also parallel those that occur in humans with cholestasis (9). BDL therefore represents a powerful in vivo model to study the roles of the nuclear receptors FXR and PXR in regulation of lipid and bile acid metabolism in cholestasis. By using BDL, we show that FXR null genotypes are relatively protected after BDL and that deletion of FXR is able to reverse the marked increase in liver injury that occurs in PXRKO mice with cholestasis. This protection appears to occur as a result of lower bile acid concentrations and altered expression of hepatic transporters, including BSEP, multidrug resistance protein (Mdr) 1, Mdr2, and multidrug resistance-associated protein (Mrp) 4. FXRKO mice also have elevated high-density lipoprotein (HDL) cholesterol and marked hypertriglyceridemia after BDL with reduced expression of apoAV. We propose a potential therapeutic role for FXR antagonists in the treatment of obstructive cholestatic liver disease.
Results
Mortality and Liver Injury Are Reduced in FXRKO Mice After BDL.
To study the impact of nuclear receptor deletion on cholestasis outcomes, we assessed mortality, morbidity, and liver injury 6 days after BDL in FXRKO, PXRKO, and PXR and FXR knockout (P/FXRKO) mice. FXRKO mice appeared healthier with significantly heavier mean weights on day 6 (24.8 ± 1.8 g) after BDL than WT and PXRKO mice (20.6 g and 19.3 g, respectively). There was minimal mortality in FXRKO and P/FXRKO BDL mice (0% and 7.7%, respectively) compared with WT and PXRKO mice (15% and 23%, respectively).
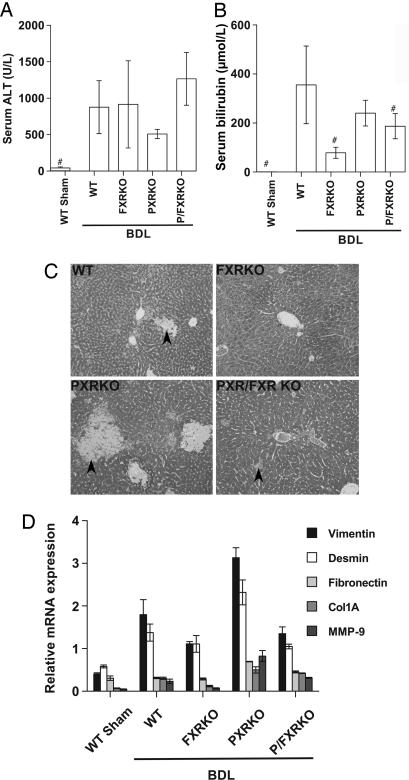
BDL results in liver injury secondary to intrahepatic bile acid overload, which can be assessed by using serum markers of injury and cholestasis [alanine aminotransaminase (ALT) and bilirubin] and liver histology. Serum ALT and bilirubin were elevated markedly in WT animals after BDL (Fig. 1A and B). ALT also was elevated in all KO animals, but serum bilirubin was not elevated significantly in the FXRKO genotype and was significantly lower in both FXR-null genotypes compared with WT BDL (Fig. 1B), suggesting a reduction in cholestatic injury. When liver histology was examined, bile infarcts were observed predominantly in acinar zones 1 and 2 in WT BDL mice (Fig. 1C), with an increase in the area of bile infarcts in PXRKO mice, as described in refs. 14 and 15. PXRKO mice also had markedly increased expression of genes involved in fibrosis and tissue remodeling (Fig. 1D), consistent with increased susceptibility of this genotype to bile acid-induced injury. In contrast, the FXRKO and P/FXRKO mice had a marked reduction in bile infarcts compared with other genotypes after BDL (Fig. 1C), although some disseminated liver cell necrosis and hepatocyte steatosis were observed. Additionally, the expression of liver injury genes, including matrix metalloproteinase 9 (MMP-9), vimentin, and desmin were reduced in the P/FXRKO compared with the single PXRKO genotype (Fig. 1D). These data, when combined with the mortality and serum bilirubin results, demonstrate that FXR deletion is protective in obstructive cholestasis and is able to abrogate the increased propensity to liver injury usually observed in PXRKO mice.
Fig. 1.
Deletion of PXR or FXR results in differential patterns of liver injury after BDL. Serum and liver samples were collected 6 days after BDL or sham operation in WT, FXRKO, PXRKO, and P/FXRKO mice. (A) Serum ALT was significantly elevated in all BDL animals compared with shams. (B) Total serum bilirubin was significantly elevated in WT, PXRKO, and P/FXRKO BDL mice relative to sham and was significantly lower in FXR-null mice than WT after BDL. #, P < 0.01 relative to WT BDL. (C) Livers were fixed for histological examination and stained with Gomori’s Trichrome to evaluate liver injury and necrosis. WT livers show modest areas of bile infarct/necrosis (arrowheads); PXRKO mice have increased areas of bile infarct/necrosis compared to WT; FXRKO and P/FXRKO mice have a reduction in bile infarcts, but disseminated liver cell necrosis and mild to moderate hepatocyte steatosis is still observed. (D) Effects of BDL on hepatic expression of genes involved in fibrosis and tissue remodeling. Relative mRNA expression of vimentin, desmin, fibronectin, collagen 1A (Col1A), and matrix metalloproteinase 9 (MMP-9) were examined by real-time RT-PCR and normalized for U36B4 expression.
Bile Acids Are Lower in FXRKO Mice After BDL.
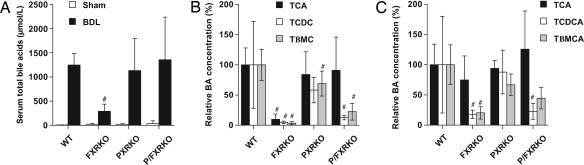
Individual bile acids differ in their relative cytotoxicity, and changes to the composition of the bile acid pool can modulate hepatic injury; therefore, we quantified the individual bile acid concentrations in each genotype after BDL. Serum total bile acid concentrations were significantly elevated in all BDL mice compared with shams (Fig. 2A), with increases in all major primary bile acids and their respective metabolites in WT mice as reported in ref. 13. In PXRKO mice, a relative reduction in concentration of the hydroxylated bile acid, tauro-β-muricholic acid (Tβ-MCA), was observed (Fig. 2B), suggesting that these mice have impaired 6β-hydroxylation of bile acids (a phase I detoxification step). FXRKO mice had markedly reduced bile acid concentrations, with at least 4-fold reductions in individual and total serum bile acids relative to WT BDL (Fig. 2 A and B). In the double knockouts, there was a reduction in serum tauro-chenodeoxycholic acid (TCDCA) and Tβ-MCA but preservation of tauro-cholic acid (TCA) (Fig. 2B), and this pattern was similarly observed in the livers of FXR-null genotypes (Fig. 2C). Therefore, although the hepatic ratio of cholic acid (CA) to chenodeoxycholic acid (CDCA) increases in FXRKO mice after BDL (14), the absolute concentration of CA does not actually increase with loss of FXR-mediated repression of Cyp8b1 (Fig. 5, which is published as supporting information on the PNAS web site); instead concentrations of CDCA, a toxic bile acid, and its derivative Tβ-MCA decrease, altering the bile acid ratio. This overall reduction in bile acid concentrations may contribute to the observed reduction in liver injury. Urinary total bile acid concentrations also were quantified and found not to significantly differ between WT and FXRKO BDL mice (306 ± 172 μmol/liter, 385 ± 189 μmol/liter, respectively). This result is surprising in the context of markedly reduced serum concentrations in FXRKO mice and implies that these mice excrete bile acids from serum to urine with greater efficiency than WT mice.
Fig. 2.
Relative bile acid concentrations of CDCA and its metabolites decrease in FXR-null BDL mice. Bile acid concentrations were measured 6 days after BDL or sham operation. (A) Total serum bile acids increased after BDL in all genotypes but were reduced in FXRKO relative to WT BDL mice. (B) Individual serum bile acids were quantitated by liquid chromatography/MS and expressed as a percentage of WT BDL concentration. Serum tauro-cholic acid (TCA), Tβ-MCA, and tauro-chenodeoxycholic acid (TCDCA) were significantly reduced in FXRKO mice; TCDCA and Tβ-MCA were reduced in P/FXRKO mice, but TCA concentrations were preserved in this genotype. (C) Individual hepatic bile acids were quantitated by liquid chromatography/MS and expressed as a percentage of WT BDL concentration. All FXR-null genotypes had a significant relative reduction in TCDCA and Tβ-MCA relative to WT BDL, whereas concentrations of TCA were preserved. #, P < 0.01 relative to WT BDL.
However, in contrast to the relative decrease in serum and liver bile acid concentrations, the expression levels of most of the bile acid synthesis genes were increased in the null genotypes (Fig. 5). Repression of Cyp8b1 and Cyp7b1 was abrogated in all knockouts, with a greater effect seen from PXR deletion on Cyp7b1 and FXR deletion on Cyp8b1. A marked increase in the relative expression of Cyp7a1 in the P/FXRKO mice confirms the significance of the combined role of both nuclear receptors in the negative regulation of this rate-limiting step in bile acid synthesis. In contrast, induction of cholesterol 25-hydroxylase was attenuated in all FXR-null BDL mice, suggesting that FXR positively regulates this gene, which has a poorly defined role in oxysterol synthesis (1). These results demonstrate considerable overlap between FXR and PXR in regulating genes involved in bile acid synthesis and suggest that bile acid synthesis is increased in FXRKO mice.
FXR Positively Regulates Multiple Hepatic Canalicular Transporters.
Adaptive changes in the expression of hepatic transporters are observed in cholestasis (14, 16), and loss of transporter function may contribute to liver injury, so we quantified the expression of these genes after BDL (Table 1). The results indicate that PXR regulates the basolateral uptake transporters, Oatp2, and Oatp-c (Slc21a6) as described in refs. 4 and 15. The multidrug resistance-associated proteins 1 and 4 (Mrp1/ABCC1 and Mrp4/ABCC4) are postulated to act as alternative basolateral bile acid efflux transporters (17) and were induced in WT BDL mice. Although induction of Mrp3 was modest, FXRKO mice had a 22-fold induction of Mrp4, suggesting that these mice have a significantly increased capacity to export bile acids out of the hepatocyte back into the circulation, thereby contributing to a reduction in hepatotoxicity.
Table 1.
Hepatic expression of transporter genes after BDL
| Transporter | WT | FXRKO | PXRKO | P/FXRKO |
|---|---|---|---|---|
| ABCG5 | +1.0 ± 0.4 | +1.0 ± 0.2 | −1.5 ± 0.9 | +1.1 ± 0.4 |
| ABCG8 | −1.9 ± 0.8 | +1.8 ± 0.4 | −2.2 ± 0.5 | +1.5 ± 0.4 |
| Oatp-c | −3.1 ± 0.0 | −1.7 ± 0.1 | n.c. | n.c. |
| Oatp2 | +1.9 ± 0.1 | +2.2 ± 0.2 | −2.3 ± 0.5 | +1.3 ± 0.2 |
| Bsep | n.c. | −3.5 ± 0.2 | −1.7 ± 0.5 | −3.2 ± 0.4 |
| Mrp1 | +3.9 ± 2.1 | +2.2 ± 0.9 | +10.5 ± 2.7 | +5.4 ± 0.6 |
| Mrp3 | +1.5 ± 0.3 | +2.1 ± 0.3 | +1.3 ± 0.4 | +1.1 ± 0.5 |
| Mrp4 | +3.4 ± 1.3 | +22.1 ± 8.4 | +2.5 ± 0.9 | +4.9 ± 1.7 |
| Mdr1 | +7.3 ± 0.9 | +1.4 ± 0.1 | +10.1 ± 1.1 | n.c. |
| Mdr2 | +1.5 ± 0.2 | n.c. | +2.1 ± 0.0 | n.c. |
Relative hepatic expression of mRNA for ABC membrane and basolateral (sinusoidal) transporter genes. Livers were removed from WT and KO mice 6 days after BDL or sham operation. Relative mRNA expression of genes in liver were examined by real-time RT-PCR and normalized for 36B4. Data are expressed as the fold change relative to sham-operated animals. n.c., no change.
Multidrug resistance proteins 1 and 2 (Mdr1/ABCB1; Mdr2/ABCB4) were significantly induced in WT and PXRKO mice but not in FXR-null mice after BDL (Table 1). Mdr2 codes for the phospholipid export pump at the canalicular membrane and hMDR3 (ABCB4) is known to be regulated by FXR (18). Mdr1 can be induced by xenobiotics via PXR (19), but our results show that in cholestasis, FXR regulates induction of both Mdr1 and 2 as well as BSEP and, therefore, is a major regulator of these important canalicular transporters. Loss of FXR therefore would be expected to reduce bile formation and prevent hydrostatic rupture of the canals of Herring after BDL (14). Biliary cholesterol secretion is mediated by the ABC transporters ABCG5 and ABCG8 and is a major pathway for cholesterol elimination (20). We found that ABCG8 expression was repressed in WT mice after BDL but induced in FXRKO mice, suggesting that FXRKO mice may have greater capacity to excrete cholesterol into bile.
FXR Influences Serum Lipids in Cholestasis.
To further evaluate the role of FXR in lipid metabolism, we measured serum lipid concentrations after BDL. Total cholesterol concentrations were similar in all sham mice (Fig. 3A) and increased after BDL to a mean of 11.2 ± 1.9 mmol/liter in WT mice (day 6). In FXRKO mice, however, a transient increase at day 3 was followed by a decline to less than one-half of WT levels by day 6 (Fig. 3B). This decrease may be a consequence of increased utilization of cholesterol as a substrate for bile acid synthesis in these mice. PXRKO mice also displayed an attenuated increase in total cholesterol (mean increase 2.8 mmol/liter) by day 6 after BDL. HDL cholesterol was significantly higher at baseline in FXR-null mice (Fig. 3 C and D) and also displayed a biphasic profile after BDL (Fig. 3D). Fasting triglycerides were not significantly different in sham animals (Fig. 3E). After BDL, a progressive 11-fold increase in serum triglycerides was observed in FXR-null genotypes (Fig. 3F), indicating that FXR has a significant role in the negative regulation of serum triglycerides in cholestasis.
Fig. 3.
FXRKO mice have a biphasic lipid profile after BDL with marked hypertriglyceridemia. Blood samples were collected from mice before BDL or sham operation on day 0 and day 6. (A) Total cholesterol concentrations were similar in all sham mice. (B) After BDL, total cholesterol increased significantly in WT mice (P < 0.01). In FXRKO mice, a transient increase at day 3 was followed by a decline to less than one-half of WT levels by day 6. PXRKO mice also had an attenuated increase in total cholesterol. (C) Serum HDL cholesterol was significantly higher at baseline in FXRKO mice. (D) After BDL, FXRKO mice had a biphasic HDL cholesterol profile, with a transient decrease in HDL on day 3 and subsequent increase by day 6. (E) Fasting serum triglycerides in sham animals showed no genotype-specific differences by using the Cholestech LDX Analyzer. (F) After BDL, a marked increase in serum triglycerides was observed in FXR-null mice. (G) FXR regulates expression of apoAV. Relative mRNA expression of apolipoprotein genes in liver were examined by real-time RT-PCR. apoAV is induced in WT BDL mice, and its expression depends on FXR.
FXR Regulates apoAV.
To elucidate mechanisms for these FXR-dependent alterations in lipid homeostasis, we quantified the expression of a range of genes involved in lipid regulation. After BDL, SREBP-1c was down-regulated in both WT mice as expected (21) and, surprisingly, also in FXRKO mice, demonstrating that repression of SREBP-1c in cholestasis does not depend on FXR (Fig. 6, which is published as supporting information on the PNAS web site). apoAI is a major protein component of HDL, and PXR and FXR both have been implicated in its regulation (2). We observed a minor decrease in apoAI expression in WT BDL mice and 2-fold induction in all knockout mice (Fig. 3G), so differential expression of this gene is unlikely to explain the FXRKO-specific changes in HDL. Similarly, apoB and apoE were induced in all BDL KO mice. apoAV, another HDL-associated protein, has been shown to respond to bile acids and FXR (22). KO mice lacking apoAV have increased serum triglyceride levels, and altered apoCIII levels have been implicated in this effect (23). We found no change in the expression of apoCIII, but apoAV was induced after BDL in WT mice, with a 6-fold decrease in expression in FXRKO mice, indicating that FXR regulates apoAV (Fig. 3G).
Discussion
This work uses a BDL model of cholestasis in WT and KO animals to elucidate the pivotal role of FXR in the regulation of multiple hepatic metabolic pathways. We have shown that deletion of FXR confers a survival and morbidity advantage after BDL and is able to reverse the marked increase in liver injury that occurs in PXRKO mice with severe cholestasis. Several factors are likely to contribute to this protection, including reduced concentrations of bile acids and altered expression of canalicular and basolateral transporters, particularly reduced expression of BSEP and marked induction of Mrp4.
We have also shown that FXRKO mice have a biphasic lipid profile after BDL, with an increase in HDL cholesterol, marked hypertriglyceridemia by day 6, and a markedly reduced expression of apoAV. Bile acids have been shown to reduce triglyceride levels, possibly via down-regulation of SREBP-1c, which controls fatty acid synthesis. Studies have suggested that this regulation occurs via PGC-1α, FXR, and SHP (8, 21); FXRKO mice show modest increases in triglycerides on control diets that decrease after CA feeding (3). However, in our cholestasis model, we found a progressive increase in serum triglycerides in FXRKO mice after BDL, and down-regulation of SREBP-1c occurred independently of either FXR or PGC-1α (data not shown). Our findings suggest that reduced expression of apoAV is implicated in the hypertriglyceridemia seen in these FXRKO mice. apoAV may act to reduce plasma triglycerides by inhibiting VLDL-triglyceride production and stimulating lipoprotein lipase-mediated VLDL-triglyceride hydrolysis (24). In cell culture, CDCA can induce the human apoAV gene promoter via FXR (22), and we demonstrate the in vivo induction of apoAV in WT mice with elevated concentrations of CDCA after BDL. Mice with elevated apoAV have a reduction in serum triglycerides and HDL cholesterol and, conversely, mice lacking apoAV have increased serum triglyceride levels (23, 24). Therefore, the marked increases in triglycerides and HDL cholesterol that we found in FXRKO mice after BDL are consistent with reduced apoAV expression and the inability to stimulate VLDL-triglyceride hydrolysis in response to cholestasis.
An intriguing observation is the biphasic nature of the changes in serum HDL and total cholesterol in these mice. HDL concentrations are known to decrease in response to acute inflammation or sepsis, because they have after BDL in the present work, and it has been suggested that HDL acts as the dominant LPS acceptor until HDL levels fall below a critical level. However, in hypertriglyceridemic serum, VLDL and LDL collectively become the dominant LPS acceptor lipoproteins (12). Our data suggest that the continued increase in TG concentrations, conferred by apoAV deficiency in the FXRKO genotype, results in a partial recovery of HDL concentrations several days after BDL. We hypothesize that these changes may be related to changes in dominance of LPS acceptor lipoproteins. These changes to endogenous circulating lipoproteins as the result of apoAV deficiency in the FXRKO BDL mice may be a significant contributor to the improvement in morbidity and mortality that we observe in this genotype.
PXRKO mice consistently exhibit increased liver injury in different models of cholestasis (6, 15). This injury has been attributed to the loss of various detoxification mechanisms, because concentrations of bile acids are not increased in these mice (13). This study shows that concurrent deletion of another nuclear receptor is able to reduce this liver injury and explore mechanisms for this protection. Bile acid concentrations, particularly CDCA and Tβ-MCA, are markedly reduced in serum and liver of FXR-null genotypes compared with WT mice after BDL, despite reduced expression of the canalicular transporters that usually excrete bile acids into the bile. Additionally, we found a relative increase in the expression of genes involved in bile acid synthesis. Therefore, these mice must have an enhanced capacity to excrete bile acids, which correlates with our observed induction of Mrp4, a hepatocyte-to-serum bile acid transporter. From the serum, bile acids can be efficiently excreted through the kidney into the urine. Despite a 4-fold reduction in serum bile acids, the urinary contents do not fall, presumably because of enhanced secretion. In support of this hypothesis, FXRKO mice recently have been shown to have increased 24-hour urinary bile acid excretion at earlier time points after BDL, resulting in lower serum BA levels (25). Thus, at 6 days after BDL, urinary levels of BAs are ≈4-fold elevated relative to the lowered serum concentrations.
UDCA is currently the only effective therapy in cholestasis and, interestingly, has been shown to inhibit CDCA activation of FXR (26, 27). UDCA treatment in humans results in induction of MRP4 (28), and our study suggests that the probable mechanism for this induction is via antagonism of FXR. Mrp4 transports a range of bile acids across the basolateral membrane of the hepatocyte, with the exception of unconjugated UDCA (17), so this mechanism could potentially contribute to accumulation of UDCA, which is a relatively hydrophilic bile acid of low toxicity. The volume and composition of bile is expected to be altered in FXRKO mice, because we have shown reduced expression of multiple canalicular transporters that generate bile, including Mdr1, Mdr2, and Bsep. The decrease in bile acid formation provides a mechanism for the observed decrease in biliary hydrostatic pressure in FXRKO mice after BDL and has been implicated in a reduction in bile infarcts, tight junction disruption, and bile regurgitation into plasma in these mice (14). This work therefore provides compelling evidence that a high-affinity FXR antagonist is likely to be beneficial in obstructive cholestasis (Fig. 4), particularly in combination with UDCA, which may work partially through this mechanism. This suggestion is in apparent contrast to work showing benefits from FXR agonists in cholestasis (29, 30). Although one study reported a protective effect of GW 4064 in rats after BDL, their data showed no effect on mortality, bilirubin, or bile acids, important indicators of severity of cholestasis (30). In models of bile acid overload or drug-induced cholestasis, activation of BSEP and excretion of bile acids into bile still would be beneficial if there was sufficient bile flow, a different situation from biliary obstruction where the converse applies. We propose that pharmacological treatment of intrahepatic cholestasis should differ from that of obstructive cholestasis.
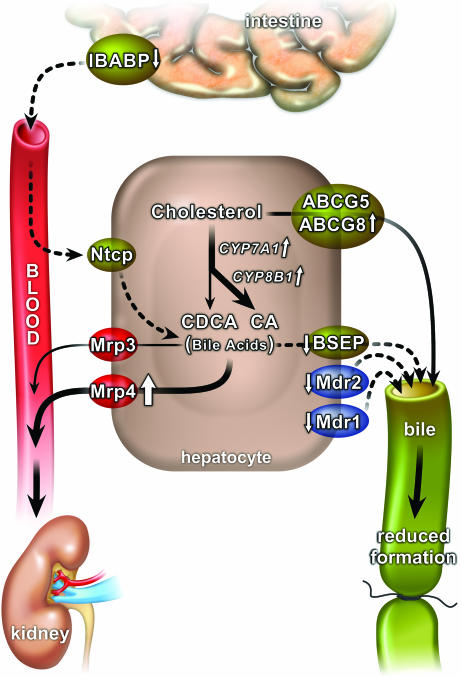
Fig. 4.
Model showing the benefit of FXR deletion or antagonism on FXR target genes in obstructive cholestasis. Although overall bile acid synthesis is increased, there is reduced synthesis of CDCA and reduced absorption of bile acids from the intestine. Canalicular transporters involved in bile formation are down-regulated, reducing pressure in the obstructed bile ducts, and the basolateral transporter Mrp4 is up-regulated, facilitating export of bile acids into blood where they are available for renal excretion. Dashed arrows represent down-regulation; continuous or block arrows depict up-regulation.
The MDR transporters are induced in human cholestasis (16) and are also important mediators of drug resistance. Our results suggest that physiological concentrations of bile acids induce Mdr1 and 2 via FXR, independently of PXR, so regulation of these important transporters in disease states can differ significantly from regulation by xenobiotics. These findings may have important implications for drug therapy in patients with cholestatic liver disease.
In summary, we have shown that the deletion of FXR can ameliorate the increased liver injury that is usually seen in BDL mice by lowering bile acid concentrations and altering expression of the transporters Mdr1, Mdr2, BSEP, and Mrp4. FXR therefore is a key regulator of intrahepatic bile acids, including synthesis within the hepatocyte and transport into bile and blood. Additionally, FXR positively regulates key metabolic genes, including apoAV, which influences serum triglycerides and HDL cholesterol. These findings suggest a role for targeted therapy for different cholestatic syndromes, and specifically, a clinical role for FXR antagonists in the treatment of obstructive cholestatic liver disease.
Materials and Methods
Animals.
PXRKO and FXRKO mice (obtained from Frank J. Gonzalez, National Cancer Institute, National Institutes of Health, Bethesda) were crossed to generate double-null mice (P/FXRKO) (31). All mice were 8- to 10-week-old males of C57BL background and were allowed food and water ad libitum, except for 6 h of fasting before blood sampling. Two separate identical experiments were performed with KO mice (n = 5–6 mice per group per genotype in both experimental groups). The use of mice in this study complied with all relevant federal guidelines and institutional policies.
Bile Duct Ligation.
Mice were anesthetized and aseptically subjected to BDL or sham operation as described in ref. 13, then killed by exsanguination under anesthesia 6 days after surgery. Blood samples (35 μl) were collected into heparinized capillary tubes from the tail vein of mice before the initial surgical procedure on day 0 and repeated on days 3 and 6 before killing. Total cholesterol, HDL, and triglycerides were measured by using the Cholestech LDX (Hayward, CA). Additional serum was collected on day 6 to measure ALT and bilirubin by using automated procedures (Clinical Chemistry, Institute of Clinical Pathology and Medical Research) as a marker of liver injury and cholestasis, respectively. Serum, urine, and liver samples were frozen at −70°C for bile acid analysis, and liver was snap frozen in liquid nitrogen for subsequent RNA extraction.
Liver Histology.
Consecutive sections of liver (4 μm thick) from paraffin-embedded liver were cut for hematoxylin and eosin staining and Gomori’s Trichrome staining for evaluation of liver injury and necrosis.
Quantitation of Bile Acids.
Individual bile acids in liver, serum, urine, and bile were quantified by high-performance liquid chromatography/MS, as described in ref. 15. Total serum bile acid concentrations were determined by using a 3α-hydroxysteroid dehydrogenase assay (Randox Laboratories, Antrim, U.K.).
Gene Expression.
Total RNA was extracted from frozen tissue samples or cells by using TRIzol (Invitrogen). RNA was treated with DNase I (Ambion, Austin, TX) at 37°C for 30 min, followed by inactivation at 75°C for 5 min. cDNA was synthesized from total RNA by using SuperScript First-Strand Synthesis System (Invitrogen) and random hexamer primers. Real-time PCR measurement of individual cDNAs was performed in triplicate by using either TaqMan probe or SYBR green dye technology on an ABI Prism 7700 or 7900 (Applied Biosystems, Foster City, CA) and normalized to m36B4 expression. Primers and probes used are available upon request.
Statistics.
Treatment groups were compared by using factorial ANOVA, and post hoc analysis was performed by using the Bonferroni/Dunn test. Results are presented as mean ± SD.
Supplementary Material
Acknowledgments
R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology. This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Nuclear Receptor Signaling Atlas orphan receptor Grant U19DK62434, the Enviromental Protection Agency Superfund Program Grant (P42 ES10337), and a grant from the National Health and Medical Research Council of Australia (NHMRC). Additional support was given by the Howard Hughes Medical Institute. C.S. received financial support from a Westmead Millennium Institute Initiating Grant; Clinical Hepatology Trust Fund Westmead Hospital; New Zealand Society of Gastroenterology/Ferring Research Fellowship; and a NHMRC Postgraduate Medical Research Scholarship.
Abbreviations
- ALT
alanine aminotransaminase
- apo
apolipoprotein
- BDL
bile duct ligation
- CDCA
chenodeoxycholic acid
- FXR
farnesoid X receptor
- HDL
high-density lipoprotein
- KO
knockout
- Mdr
multidrug resistance protein
- Mrp
multidrug resistance-associated protein
- PXR
pregnane X receptor
- P/FXRKO
PXR and FXR knockout
- Tβ-MCA
tauro-β-muricholic acid
- UDCA
ursodeoxycholic acid
- VLDL
very low-density lipoprotein.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Russell D. W. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 2.Sirvent A., Claudel T., Martin G., Brozek J., Kosykh V., Darteil R., Hum D. W., Fruchart J. C., Staels B. FEBS Lett. 2004;566:173–177. doi: 10.1016/j.febslet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 4.Staudinger J. L., Madan A., Carol K. M., Parkinson A. Drug Metab. Dispos. 2003;31:523–527. doi: 10.1124/dmd.31.5.523. [DOI] [PubMed] [Google Scholar]
- 5.Sonoda J., Xie W., Rosenfeld J. M., Barwick J. L., Guzelian P. S., Evans R. M. Proc. Natl. Acad. Sci. USA. 2002;99:13801–13806. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie W., Radominska-Pandya A., Shi Y., Simon C. M., Nelson M. C., Ong E. S., Waxman D. J., Evans R. M. Proc. Natl. Acad. Sci. USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe M., Houten S. M., Wang L., Moschetta A., Mangelsdorf D. J., Heyman R. A., Moore D. D., Auwerx J. J. Clin. Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements W. D., Erwin P., McCaigue M. D., Halliday I., Barclay G. R., Rowlands B. J. Gut. 1998;42:293–299. doi: 10.1136/gut.42.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertok L. Ann. N.Y. Acad. Sci. 1998;851:408–410. doi: 10.1111/j.1749-6632.1998.tb09016.x. [DOI] [PubMed] [Google Scholar]
- 11.Harris H. W., Gosnell J. E., Kumwenda Z. L. J. Endotoxin Res. 2000;6:421–430. [PubMed] [Google Scholar]
- 12.Kitchens R. L., Thompson P. A., Munford R. S., O’Keefe G. E. J. Lipid Res. 2003;44:2339–2348. doi: 10.1194/jlr.M300228-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Stedman C., Robertson G., Coulter S., Liddle C. J. Biol. Chem. 2004;279:11336–11343. doi: 10.1074/jbc.M310258200. [DOI] [PubMed] [Google Scholar]
- 14.Wagner M., Fickert P., Zollner G., Fuchsbichler A., Silbert D., Tsybrovskyy O., Zatloukal K., Guo G. L., Schuetz J. D., Gonzalez F. J., et al. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 15.Stedman C. A., Liddle C., Coulter S. A., Sonoda J., Alvarez J. G., Moore D. D., Evans R. M., Downes M. Proc. Natl. Acad. Sci. USA. 2005;102:2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zollner G., Fickert P., Silbert D., Fuchsbichler A., Marschall H. U., Zatloukal K., Denk H., Trauner M. J. Hepatol. 2003;38:717–727. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 17.Rius M., Hummel-Eisenbeiss J., Hofmann A. F., Keppler D. Am. J. Physiol. 2006;290:G640–G649. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- 18.Huang L., Zhao A., Lew J. L., Zhang T., Hrywna Y., Thompson J. R., de Pedro N., Royo I., Blevins R. A., Pelaez F., et al. J. Biol. Chem. 2003;278:51085–51090. doi: 10.1074/jbc.M308321200. [DOI] [PubMed] [Google Scholar]
- 19.Synold T. W., Dussault I., Forman B. M. Nat. Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 20.Yu L., Gupta S., Xu F., Liverman A. D., Moschetta A., Mangelsdorf D. J., Repa J. J., Hobbs H. H., Cohen J. C. J. Biol. Chem. 2005;280:8742–8747. doi: 10.1074/jbc.M411080200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Castellani L. W., Sinal C. J., Gonzalez F. J., Edwards P. A. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieur X., Coste H., Rodriguez J. C. J. Biol. Chem. 2003;278:25468–25480. doi: 10.1074/jbc.M301302200. [DOI] [PubMed] [Google Scholar]
- 23.Pennacchio L. A., Olivier M., Hubacek J. A., Cohen J. C., Cox D. R., Fruchart J. C., Krauss R. M., Rubin E. M. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 24.Schaap F. G., Rensen P. C., Voshol P. J., Vrins C., van der Vliet H. N., Chamuleau R. A., Havekes L. M., Groen A. K., van Dijk K. W. J. Biol. Chem. 2004;279:27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 25.Marschall H. U., Wagner M., Bodin K., Zollner G., Fickert P., Gumhold J., Silbert D., Fuchsbichler A., Sjovall J., Trauner M. J. Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Howard W. R., Pospisil J. A., Njolito E., Noonan D. J. Toxicol. Appl. Pharmacol. 2000;163:195–202. doi: 10.1006/taap.1999.8869. [DOI] [PubMed] [Google Scholar]
- 27.Downes M., Verdecia M. A., Roecker A. J., Hughes R., Hogenesch J. B., Kast-Woelbern H. R., Bowman M. E., Ferrer J. L., Anisfeld A. M., Edwards P. A., et al. Mol. Cell. 2003;11:1079–1092. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marschall H. U., Wagner M., Zollner G., Fickert P., Diczfalusy U., Gumhold J., Silbert D., Fuchsbichler A., Benthin L., Grundstrom R., et al. Gastroenterology. 2005;129:476–485. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Fiorucci S., Clerici C., Antonelli E., Orlandi S., Goodwin B., Sadeghpour B. M., Sabatino G., Russo G., Castellani D., Willson T. M., et al. J. Pharmacol. Exp. Ther. 2005;313:604–612. doi: 10.1124/jpet.104.079665. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Binz J., Numerick M. J., Dennis S., Luo G., Desai B., MacKenzie K. I., Mansfield T. A., Kliewer S. A., Goodwin B., et al. J. Clin. Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie W., Barwick J. L., Downes M., Blumberg B., Simon C. M., Nelson M. C., Neuschwander-Tetri B. A., Brunt E. M., Guzelian P. S., Evans R. M. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.