Abstract
To investigate functional mechanisms underlying cortical motor plasticity in the intact and injured brain, we used “behaviorally relevant,” long-duration intracortical microstimulation. We now report the existence of complex, multijoint movements revealed with a 500-msec duration intracortical stimulation in rat motor cortex. A consistent topographic distribution of these complex motor patterns is present across the motor cortex in naïve rats. We further document the plasticity of these complex movement patterns after focal cortical injury, with a significant expansion of specific complex movement representations in response to rehabilitative training after injury. Notably, the degree of functional recovery attained after cortical injury and rehabilitation correlates significantly with a specific feature of map reorganization, the ability to reexpress movement patterns disrupted by the initial injury. This evidence suggests the existence of complex movement representations in the rat motor cortex that exhibit plasticity after injury and rehabilitation, serving as a relevant predictor of functional recovery.
Keywords: complex movements, intracortical microstimulation, motor cortex, rehabilitation
The ability of sensory and motor cortices to dynamically reorganize is an important component of normal learning and recovery after neural injury (1–7). Cortical reorganization, or map plasticity, is believed to reflect the integration of molecular, cellular, synaptic, and anatomic plasticity over large populations of neurons (7–11). Map plasticity in the motor cortex is observed traditionally as a reorganization of cortically encoded muscle groups (such as those controlling the wrist, elbow, and shoulder) identified by intracortical microstimulation (ICMS) (3, 5, 12). For example, skilled motor training of the distal forelimb in rats increases the proportion of ICMS-evoked distal forelimb movements in the motor cortex (3, 5, 12).
Plasticity of ICMS-derived cortical maps also occurs in association with functional recovery after brain injury. In both rats (4, 8, 13–16) and primates (2, 17), discrete lesions placed within the forelimb area of motor cortex result in functional deficits in skilled-reaching performance. Subsequent rehabilitative training promotes functional recovery and results in the reorganization of forelimb motor representations (2, 4, 18–21). Disrupting cortical reorganization significantly reduces recovery (4), and ablating or inactivating newly responsive forelimb sites reinstates the functional deficit after recovery has occurred (4, 15, 17, 22), suggesting that the reorganization was responsible for the functional recovery. Despite the substantial evidence demonstrating reorganization of ICMS-derived cortical maps after focal brain injury, it is still not clear how this reorganization contributes to behavioral recovery. In part, this lack of understanding can be ascribed to a limitation inherent to the technique: ICMS elicits contractions of individual muscles but does not reveal integrative aspects of neural encoding such as coordinated actions across muscles, movement velocity, or movement force (23–27).
Recently, Graziano and colleagues (28–34) have reported that intracortical stimulation in primates over behaviorally relevant time spans of 500 msec elicits complex, multijoint movements coordinated through space and time. Stimulation of specific cortical sites causes coordinated limb movements toward identical locations and postures, regardless of the initial limb position. Moreover, a topographical organization of complex movements across both motor and premotor cortex was identified. Although controversial (35, 36), it has been proposed that long-duration stimulation reveals complex aspects of motor encoding previously unattainable through short duration ICMS (28, 29, 35). In this study, we used long-duration intracortical microstimulation to identify potential neural mechanisms underlying motor learning and functional recovery after cortical injury. We now report the existence of topographical maps generating complex, multijoint movements within the motor cortex of rats and a significant correlation between the reorganization of disrupted complex representations and behavioral recovery after brain injury.
Results
A total of 58 adult male F344 rats (225–250 g of starting weight) were used in this study in three separate experiments: (i) To characterize the types and topography of movements evoked by a long-duration stimulation paradigm, 11 naive animals were mapped by using a long-duration stimulation paradigm (29). (ii) To assess the effects of motor learning on the distribution of these complex movement representations, 11 animals were trained to perform a skilled forelimb reaching task and were mapped by using ICMS techniques; 10 additional animals served as untrained, naïve controls. (iii) To examine whether plasticity of complex movement representations relates to functional recovery after brain injury, 26 rats received focal cortical lesions and rehabilitative training as described in ref. 4. Briefly, rats were trained to acquire a skilled forelimb reaching task and then received bilateral focal electrolytic lesions targeting sites controlling distal (wrist) forelimb movements (4). One group of animals (n = 15) then received 5 weeks of rehabilitative training, whereas a second group (n = 11) was not rehabilitated. Both groups then were mapped by using the long-duration stimulation paradigm to assess potential plasticity of complex movement representations and their correlation with functional recovery.
Qualitative Features of Forelimb Movements Elicited by Long-Duration Stimulation.
To determine whether complex movement representations exist in rat motor cortex, long-duration (500-msec) ICMS was applied, as described in ref. 29. At each site, the stimulating current was gradually increased from 1 μA until movement was detected, and the current then was raised to magnify that movement and facilitate characterization. Notably, raising the current did not alter the quality or sequencing of the evoked complex movement. As with short-duration stimulation (4, 5), a site was deemed nonresponsive if movement could not be elicited with a maximum stimulus of 200 μA.
Long-duration stimulation elicited a variety of movement patterns, ranging from “simple movements” (muscle contractions across a single joint) to very complex movement patterns across multiple joints. Three patterns of complex movements were commonly observed, described here as reaching, grasping, and retraction (Figs. 1–3). Along with these complex movements, long-duration stimulation also produced sequential combinations of these reaching, grasping, and retraction movements (Figs. 2 and 3). For example, a single 500-msec stimulation could elicit a sequentially coordinated reach, grasp, and retract movement (Fig. 2; see also Movie 1, which is published as supporting information on the PNAS web site). Other complex movement sequences included combinations of reaching followed by grasping, or grasping followed by retractions. Long-duration stimulation rarely elicited dysynergic or apparently random patterns of motor activation (i.e., stimulation never resulted in a grasp coinciding with, or preceding, a reach). Rather, elicited movements nearly always occurred in the sequence of reach-grasp-retract, resembling patterns spontaneously generated by animals during purposeful behaviors. Complex movement sequences (reach-grasp, grasp-retract, and reach-grasp-retract) required lower stimulation intensities than individual complex movements, (117 ± 10 μA vs. 141 ± 10 μA, respectively; P < 0.01), suggesting that sequential movements are not caused simply by cortical “overstimulation.”
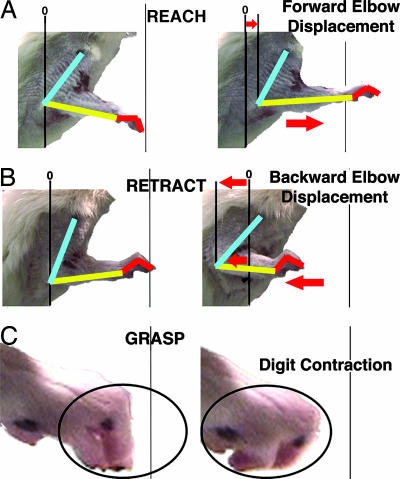
Fig. 1.
Complex movements elicited by long-duration microstimulation. (A–C) Three types of complex movements evoked by long-duration stimulation within motor cortex. Complex movements elicited by long-duration microstimulation occur across multiple joints. (A) Reaching movement characterized by rostral displacement of the elbow and shoulder, without change in wrist configuration. (B) Retraction characterized by caudal displacement of the elbow and forepaw. (C) Grasping movement characterized by contraction of all digit joints simultaneously.
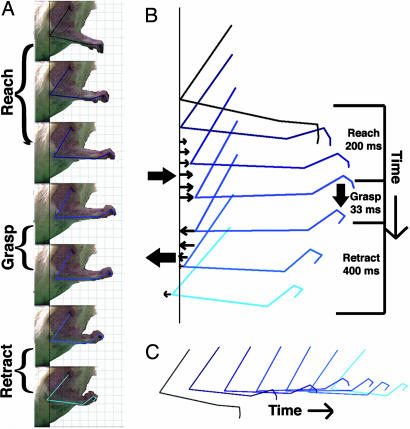
Fig. 2.
Coordinated sequence of complex movements elicited by prolonged simulation in rostral forelimb area of motor cortex. (A) Coordinated sequence of reach, grasp, and retract movements elicited by a single 500-msec stimulus within the rostral portion of the motor cortex. (B and C) Illustrated, using a digitized kinematic analysis, are the temporal sequence of this complex movement. Repeated stimulation at the same site elicited the same sequence of complex movements.
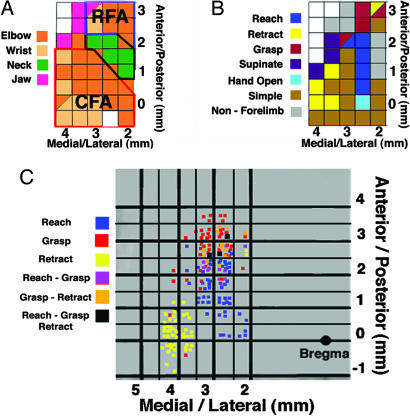
Fig. 3.
Comparison of cortical motor maps derived by using short-duration (30 msec) and long-duration (500 msec) intracortical microstimulation. (A) Representative motor map of forelimb motor cortex derived by using a short-duration (30-msec) microstimulation paradigm. Two regions of classic forelimb cortex, caudal forelimb area and rostral forelimb area, are separated by intervening neck-responsive sites. Distal forelimb movements (wrist) are elicited generally in lateral caudal forelimb areas. (B) Representative motor map derived by using the long-duration (500 msec) ICMS paradigm, demonstrating complex movement representations. Complex movements elicited by the prolonged stimulation paradigm include reaching, grasping, and retractions. Stimulation in rostral forelimb areas elicited complex sequences of movements such as grasp-retract or reach-grasp (shown as split squares in the figures). (C) Cumulative distribution of complex movement patterns in 11 naïve animals. The distribution of complex movements demonstrates a clear topography across motor cortex: Retractions are elicited by stimulation within lateral caudal forelimb area, reaches by stimulation spanning medial aspects of caudal and rostral forelimb areas, and grasps by stimulation within rostral motor cortex. Complex movement combinations are elicited by stimulation within rostral forelimb area (black squares).
To compare the topography of movements elicited with long-duration (500-msec) and short-duration (30-msec) microstimulation, motor maps were next derived by using a standard short-duration stimulation paradigm. Short-duration motor maps were similar to those described in refs. 37 and 38 and included the presence of two distinct forelimb areas (caudal and rostral forelimb area), separated by a region associated with vibrissa and neck movements (Fig. 3A). Forelimb movements evoked by using short-duration ICMS typically consisted of brief twitches of the elbow or shoulder (proximal limb movements), wrist (distal limb movements), or simultaneous twitches of both muscle groups. The mean stimulation threshold measured in previous experiments for evoking forelimb (elbow) movements with short-duration ICMS was 57.7 ± 3.1 μA (4, 5).
Notably, a distinct topography of complex movements existed (Fig. 3 B and C). Long-duration stimulation in the rostral-most portion of the forelimb area (classically referred to as the rostral forelimb area) most often elicited grasping movements; stimulation within the lateral aspect of the classically defined caudal forelimb area elicited retraction of both the wrist and forepaw; stimulation in a region intermediate between the rostral forelimb area and caudal forelimb area typically elicited forward reaching movements of the forelimb and paw. Complex movement sequences described above were exclusively elicited by stimulation within the rostral portions of the forelimb area (Fig. 3 B and C).
As noted above, long-duration stimulation also evoked movements that were simpler in nature, defined as movements across only one joint. Some of these movements, such as wrist and elbow contractions, were similar in form to “twitches” observed after short-duration stimulation. Other single-joint movements, such as supination of the arm or extension of the elbow or hand, were never observed during short-term stimulation. These simple, single-joint movements were distributed across both caudal and rostral forelimb area (Fig. 3B), and their topography was not examined in detail in the present study.
Thus, complex movements are produced by long-duration stimulation consisting of either individual complex movements (reach, grasp, or retract) or complex movement sequences (sequential combinations of reach, grasp, and retract). The topographic distribution of individual complex movements (reach, grasp, and retract) and complex movement sequences (reach-grasp, grasp-retract, and reach-grasp-retract) is consistent across animals (Fig. 3C), suggesting a common neural organization underlying these movement patterns.
Complex Movement Representations Do Not Expand After Normal Motor Learning.
Having identified a general topography of complex multijoint movements evoked by long-duration stimulation within the motor cortex of naïve rats, we next investigated whether the distribution of these complex movements changes after the acquisition of a skilled motor behavior (5). The forelimb reaching task requires animals to use their forepaw to retrieve small food pellets from a platform next to the testing chamber (5). Behavioral and EMG studies have suggested that success in reaching, grasping, and retrieving food pellets requires the animal to coordinate and modify complex motor synergies (39–41). Thus, it is possible that skilled motor learning would be associated with an increase in cortical resources devoted to the generation of complex movements, reflected by an increase in the total cortical area where complex movements could be elicited by long-duration stimulation.
Acquisition of the motor skill was measured as percent success in pellet retrieval. Animals acquired a level of skilled reaching performance comparable with previous reports (mean 70 ± 5% retrieval accuracy) (4, 5). Skilled motor learning did not alter the distribution or qualitative nature of complex movements and sequences (reach, retract, grasp, or any combination thereof). The mean area of cortex coding for all complex movements did not differ between naïve and trained animals (2.9 ± 0.3 mm2 vs. 2.7 ± 0.3 mm2, respectively; P = 0.6). Further, the mean area of cortex coding for simple movements did not change between naïve and trained animals after skilled motor learning (2.9 ± 0.2 mm2 vs. 2.6 ± 0.3 mm2, respectively; P = 0.4). Average stimulation amplitudes used to evoke complex movements also did not differ between naïve and trained rats (138 ± 5 μA vs. 128 ± 6 μA, respectively, P = 0.2). See Table 1, which is published as supporting information on the PNAS web site, for a full description of movement topographies in these animals.
Plasticity of Complex Movement Representations Correlates with Functional Recovery After Focal Cortical Injury and Rehabilitation.
Although plasticity of complex movement representations was not identified in association with normal motor learning, we postulated that reorganization of complex movement representations could occur as a neural mechanism underlying functional recovery after injury. Evidence suggests that cortical plasticity occurring in response to injury and rehabilitation may differ from that arising during normal learning (13, 18, 42–45). Further, more extensive cortical reorganization is often required to support functional recovery after injury relative to that required for normal skilled motor learning (4, 5).
Animals were subjected to a focal injury and rehabilitation paradigm as previously described (4). After 3 weeks of training to acquire a skilled reaching behavior, rats received bilateral electrolytic lesions of the lateral aspect of the caudal forelimb area, a region associated primarily with retraction movements (Fig. 4). To control for possible variability in lesion size, animals were reassessed for 3 days (beginning 2 weeks after the initial injury) on the reaching task to establish the magnitude of the functional deficit defined as follows: % deficit = (prelesion accuracy − postlesion accuracy) × 100%/prelesion accuracy.
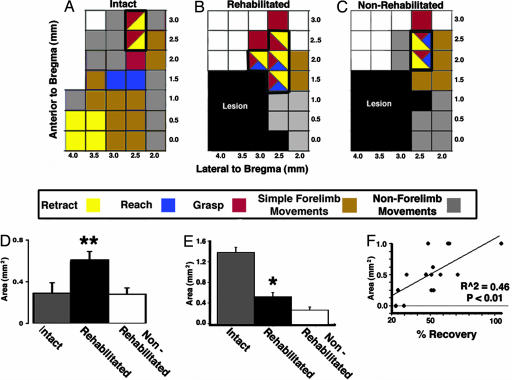
Fig. 4.
Focal brain injury and rehabilitative training are associated with significant plasticity of complex movement representations. (A) Characteristic topography of complex movements in intact animals: Retractions are located laterally, reaches are medial, and grasps and complex movement sequences are rostral. (B and C) After a lesion targeting the lateral aspect of the caudal forelimb area, forelimb movements can no longer be elicited in and around the lesion site. (B) Moreover, rehabilitated animals exhibit a significant expansion of complex movements (outlined) within undamaged rostral forelimb area relative both to naïve control rats (A) and to nonrehabilitated, lesioned animals (C). (D) Quantification of plasticity within the RFA demonstrates that rehabilitative training after a lesion results in significant expansion of complex movement sequences (reach-grasp, grasp-retract, and reach-grasp-retract) above both naïve controls and nonrehabilitated controls (ANOVA, P < 0.01; Fisher’s post hoc between rehab vs. nonrehab, P < 0.01; Fisher’s post hoc between rehab vs. prelesion control, P < 0.01). (E) The area encoding retraction movements is significantly reduced by 67% after the lesion in nonrehabilitated animals (ANOVA, P < 0.001; Fisher’s post hoc, P < 0.0001). Notably, rehabilitative training significantly increased the area of cortex encoding retraction movements (P < 0.05 compared with nonrehabilitated animals), partially restoring the specific loss of retraction movements imposed by the lesion. Paralleling the extent of behavioral recovery, the area encoding retraction movements in rehabilitated animals recovers to 64% of intact controls. (F) The area of cortex encoding stimulus-evoked retraction movements significantly correlates with the degree of functional recovery in rehabilitated animals (R2 = 0.46, P < 0.05). No significant correlation was found between the cortical area encoding reaching or grasping movements and functional recovery (data not shown).
Animals were then divided into two groups (rehabilitated and nonrehabilitated), matched for extent of functional deficit (average deficit = 82.3 ± 3.4% vs. 80.5 ± 4.1%, respectively; P = 0.84). Animals were either subjected to rehabilitation training for an additional five weeks (n = 15) or were treated as nonrehabilitated controls for the same duration (n = 11); without rehabilitation, rats do not recover forelimb function after this lesion (4). Functional recovery in the rehabilitated group was then calculated as the percent improvement of each animal’s initial deficit over the course of rehabilitation as follows (4): % recovery = (postrehabilitation accuracy − initial postlesion accuracy) × 100%/ lesion-induced deficit.
Animals that underwent rehabilitation training exhibited a 51.5 ± 5.3% recovery of function on the forelimb reach task by the fifth week of postlesion rehabilitation, comparable with previous studies (4). At the conclusion of rehabilitation training, long-duration ICMS was used to derive maps of complex movement representations. In all cases, the cortex contralateral to the forepaw used for grasping pellets was analyzed. In the few animals (n = 4) that used both forepaws, each cortex was mapped, and the size of the complex movement representations was averaged across hemispheres (after determining that map topographies did not differ significantly between hemispheres and between unilaterally vs. bilaterally reaching animals; P > 0.4 for all comparisons).
In nonrehabilitated animals, focal lesions placed in the lateral part of caudal forelimb area (Fig. 4), centered in the region associated with retraction movements, resulted in a complete loss of stimulation-evoked retraction movements in and around the ablated region (Fig. 4 B and C) and an overall 66% loss in the total cortical area evoking retraction movements (ANOVA, P < 0.01; Fisher’s post hoc test, P < 0.0001 compared with intact animals; Fig. 4). The rostral forelimb area in these nonrehabilitated subjects showed no reorganization of the complex map, indicated by a lack of change in the area of cortex evoking complex movement sequences compared with intact controls (Fig. 4D).
In marked contrast, lesioned and rehabilitated rats exhibited significant plasticity of complex movement representations relative both to intact and lesioned, nonrehabilitated animals (Fig. 4; see also Table 2, which is published as supporting information on the PNAS web site). The number of cortical sites from which complex movement sequences could be elicited increased in rehabilitated animals (Fig. 4D). Rehabilitation also resulted in a 90% increase in the area of cortex specifically evoking retraction movements compared with nonrehabilitated controls (P < 0.05, Fisher’s post hoc test; Fig. 4E). Correlational analysis between the size of individual movement representations (reach, grasp, or retract) and the extent of functional recovery in each rehabilitated subject indicated that functional recovery correlated significantly with the size of cortex specifically encoding retract movements (Fig. 4F; R2 = 0.49; Z = 2.93; P < 0.005). Other complex movements (reach or grasp) did not demonstrate a statistically significant correlation with behavioral recovery (R2 = 0.21, P = 0.09 for grasp; R2 = 0.1, P = 0.26 for reach). Thus, functional recovery correlated best with plasticity specifically associated with restoration of retract movements within the cortex, the type of complex movement originally eliminated by the caudal motor cortex lesion.
Stimulation intensity thresholds to evoke complex movements did not differ between rehabilitated and nonrehabilitated groups (171 ± 10 μA rehabilitated vs. 164 ± 4 μA, respectively; P = 0.6).
Discussion
The present study documents the existence of complex movement representations within the motor cortex of rats, extending previous reports of stimulation-evoked complex movements in primates (29, 34) to the less complex rodent system. Moreover, the present study demonstrates that complex representations reorganize in response to injury and rehabilitation. Indeed, functional recovery after brain injury directly correlates with the ability of an animal to encode complex movement patterns (in this case, retractions) specifically abolished by the injury. These data thus provide a demonstration of a measure of motor map plasticity that specifically correlates with recovery of skilled motor behaviors after brain injury.
It is important to consider whether the complex movement patterns elicited by long-duration stimulation reflect the activation of cortical circuitry associated with behaviorally relevant movements. Graziano et al. (35) have suggested that stimulating motor cortex for behaviorally relevant durations activates interrelated motor networks, potentially eliciting a more realistic depiction of complex motor actions than achieved with short-duration stimulation. They infer that movements elicited by long-duration stimulation are ethologically valid and electrophysiologically and behaviorally meaningful (30–33, 46). Others, however, have argued that the use of long-duration stimulus trains may lead simply to a nonspecific current spread beyond the original stimulation site, generating seemingly complex movements by randomly activating a large number of spinal motor units (36). Results from the present study tend to support the former interpretation for several reasons. One would predict that a random spread of current would result in the indiscriminate activation of large numbers of neurons associated with various discrete but nonpurposeful movement patterns. Our findings demonstrate that long-duration stimulation paradigms result in reproducible, sequential activation of groups of muscles to achieve what, at times, are remarkably complex movements in rats, including sequential reach-grasp-retract movements (see Movie 1 and Fig. 2). Further, the topographic distribution of these movements is consistent across animals. Importantly, complex movement sequences always progress in an apparently purposeful order, consistent with behaviors the animal actually uses (i.e., reach always precedes grasp, and grasp always precedes retraction of the forelimb). Behaviorally “nonpurposeful” sequences (for example, a grasp followed by a reach) are never seen. If multiple individual movements contributing to a complex sequence were generated truly by a random spread of current, one would expect that the order of movements also would be random based upon the pseudorandom selection of stimulation sites within motor cortex. Further, one would expect variability in the order of these sequences between different animals, but these features were not observed. The finding of a direct correlation between the plasticity of complex movement representations and the extent of behavioral recovery after lesions further supports the physiological relevance of long-duration cortical stimulation. Because cortical excitability, measured by stimulation threshold intensity, was equivalent in rehabilitated and nonrehabilitated animals, the plasticity cannot be explained by differences in random current spread between the two groups. All of these arguments strongly support the notion that long-duration stimulation reveals a physiologically relevant measure of motor function.
Plasticity of complex movement representations did not occur as a function of normal skilled motor learning in this study. However, it has been reported that plasticity of “muscle synergies” occurs after motor learning (41), and it remains possible that patterns of precise muscle activation, measured by electromyographic recording, would reveal plasticity associated with normal learning by using long-duration stimulation paradigms, a possibility that can be addressed in future studies.
Notably, significant plasticity of complex motor representations was readily apparent after rehabilitative training after cortical injury. Rehabilitation training produced a significant increase in the amount of cortex-evoking complex movement sequences in comparison with both nonrehabilitated lesioned animals and intact controls. This expansion resulted in a restoration of stimulus-evoked retraction movements in the cortex of rehabilitated animals. Further, the extent of functional recovery after brain injury significantly correlated with the degree of plasticity associated specifically with retraction movements. It is important to note that, although previous studies using short-duration (30-msec) ICMS have reported cortical reorganization after a lesion (2, 21), no significant correlation has been reported between the magnitude of cortical remodeling and the extent of functional recovery (4). Thus, the plasticity of complex motor sequences identified in this study appears to represent a measure of motor encoding that actually reflects behavioral performance. Future studies of detailed kinematics of forelimb movement (39) could be useful in understanding the contribution of complex motor representations to normal function and plasticity after cortical injury. These findings shed light on both mechanisms and potential limitations of cortical plasticity related to functional recovery after nervous system injury, with implications for the design of strategies to promote recovery in humans. Complex motor actions may require specific training to optimally recover after cortical lesions: Complex postinjury training could lead to better recovery than repetition of simple motor acts in rehabilitation programs. This possibility is a testable hypothesis in the clinical realm.
Materials and Methods
Behavioral Training and Rehabilitative Testing.
Motor training was carried out by using single-pellet retrieval boxes as described in refs. 5 and 47). This task requires animals to use the forepaw to reach through a small slit in a Plexiglas chamber and grasp and retrieve a small food pellet positioned on a platform near the chamber. During the acquisition phase of testing, rats performed 60 reaches per day, 5 days per week, for 3 weeks. Rehabilitative training consisted of 40–50 trials per day, 5 days per week, for 5 weeks.
Motor Cortex Lesions.
The focal motor cortex lesion used in this study is a modification of lesion paradigms used by others (48–50). Small electrolytic lesions were made bilaterally at two sites (Site 1: anterior/posterior = 0, medial/lateral ± 3.5 mm; Site 2: anterior/posterior = +1.5 mm, medial/lateral ± 3.5 mm relative to bregma), specifically targeting the distal forelimb representation in caudal forelimb motor cortex (4). Bilateral lesions were performed to eliminate the possibility that rats would switch paw preference to the unaffected hemisphere. At each site, a 100-μm, teflon-coated, stainless-steel electrode was initially lowered to a depth of 1.7 mm, and 1 mA dc (Grass Model DCLM5A) was passed for 20 sec. The electrode was raised 1 mm, and current was applied for another 20 sec.
Functional ICMS Mapping.
For all mapping procedures, animals were anesthetized with ketamine hydrochloride (70 mg/kg i.p.) and xylazine (5 mg/kg i.p.) and received supplementary doses of the ketamine/xylazine mixture as needed. Pulled glass microelectrodes (input impedance ≈0.5 MΩ at 300 Hz) filled with 3 M NaCl and containing a 125-μm chlorided silver wire were used. Microelectrode penetrations were made at 500-μm intervals at a depth of ≈1,800 μm (corresponding to cortical layers V–VI).
To obtain standard somatotopic maps by using short-duration stimulation, a 30-msec train of 200-μsec duration monophasic cathodal pulses was delivered at 333 Hz from an electrically isolated, constant current stimulator (Isoflex; AMPI, Inc., Jerusalem, Israel) under the control of a programmable pulse generator (AMPI, Inc.). Two pulse trains were delivered 1.2 sec apart, with additional pulse trains delivered as needed to assess body movements evoked by the stimulation. Evoked movements were examined with the animal maintained in a prone position and limbs free. At each penetration site, the stimulating current was increased gradually until a movement could be detected (threshold current). The average stimulation threshold measured in previous experiments for evoking forelimb (elbow) movements with short-duration stimulation was 57.7 ± 3.1 μA (4, 5). If no movement could be detected up to 200 μA, the site was defined as “nonresponsive.”
To identify complex motor movements by using long-duration stimulation, a 300- to 500-msec train of 200-μsec duration bipolar pulses was delivered at 200–333 Hz. Bipolar pulses are used to minimize damage that may occur during long-duration stimulation (29). No differences were detected when changing either the stimulation time (300 vs. 500 msec) or the frequency of stimulation (200 vs. 333 Hz), similar to findings reported by Graziano with stimulation durations between 500 and 1,000 msec. Evoked movements were examined with the animal supported in a fixed position in an elevated stereotaxic frame. At each site, the stimulating current was increased gradually until a movement could be detected. Once a movement was detected, the current was raised to optimize that movement and ease its characterization.
Characterization of Evoked Movements.
Movements were visually monitored and identified during mapping sessions or videotaped at 30 frames/sec. Videotaped movements were analyzed frame-by-frame by using Quicktime and iMovie software (Apple Computers). Complex movement sequences too difficult to visually characterize were analyzed by digitizing joint positions frame-by-frame in NIH Image software. To standardize movements from different animals and at different levels of camera magnification, movements were calibrated to each subject’s arm length. “Reaches” were defined as movement of the elbow in the horizontal direction over a distance exceeding 10% of the subject’s forearm length. “Retracts” were defined as movements in the opposite direction over a distance exceeding 10% of the subject’s forearm length. “Grasps” were defined as a change in angle of the digit joints by >30°. Other movements, including contraction, supination, pronation, and extension of both the arm and wrist, were grouped together as “noncomplex” movements.
Statistics.
Multiple group comparisons were made by using ANOVA, with a significance threshold of P < 0.05. Post hoc comparisons were made by using Fisher’s least-square difference. Two-group comparisons were made by using unpaired, two-tailed t tests. Regression analysis was used to test correlations by using Statview II (SAS Institute, Cary, NC) software.
Supplementary Material
Acknowledgments
We thank Peter Masny for assisting with the kinematic analysis and with preparation of the figures. This work was supported by grants from the National Institutes of Health and the Department of Veterans Affairs.
Abbreviation
- ICMS
intracortical microstimulation.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Xerri C., Merzenich M. M., Peterson B. E., Jenkins W. J. Neurophysiol. 1998;79:2119–2148. doi: 10.1152/jn.1998.79.4.2119. [DOI] [PubMed] [Google Scholar]
- 2.Nudo R. J., Milliken G. W., Jenkins W. M., Merzenich M. M. J. Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleim J. A., Barbay S., Cooper N. R., Hogg T. M., Reidel C. N., Remple M. S., Nudo R. J. Neurobiol. Learn. Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 4.Conner J. M., Chiba A. A., Tuszynski M. H. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Conner J. M., Culberson A., Packowski C., Chiba A. A., Tuszynski M. H. Neuron. 2003;38:819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- 6.Recanzone G. H., Merzenich M. M., Jenkins W. M., Grajski K. A., Dinse H. R. J. Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 7.Kilgard M. P., Merzenich M. M. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 8.Sanes J. N., Donoghue J. P. Annu. Rev. Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 9.Pons T. P., Garraghty P. E., Ommaya A. K., Kaas J. H., Taub E., Mishkin M. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 10.Florence S. L., Taub H. B., Kaas J. H. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 11.Kaas J. H. Prog. Brain Res. 2002;138:167–176. doi: 10.1016/S0079-6123(02)38077-4. [DOI] [PubMed] [Google Scholar]
- 12.Kleim J. A., Hogg T. M., VandenBerg P. M., Cooper N. R., Bruneau R., Remple M. J. Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nudo R. J. J. Rehabil. Med. 2003;(Suppl. 41):7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- 14.Jones T. A., Chu C. J., Grande L. A., Gregory A. D. J. Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro-Alamancos M. A., Borrel J. Neuroscience. 1995;68:793–805. doi: 10.1016/0306-4522(95)00178-l. [DOI] [PubMed] [Google Scholar]
- 16.Castro-Alamancos M. A., Donoghue J. P., Connors B. W. J. Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouiller E. M., Yu X. H., Moret V., Tempini A., Wiesendanger M., Liang F. Eur. J. Neurosci. 1998;10:729–740. doi: 10.1046/j.1460-9568.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- 18.Nudo R. J., Plautz E. J., Frost S. B. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- 19.Friel K. M., Heddings A. A., Nudo R. J. Neurorehabil. Neural Repair. 2000;14:187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- 20.Plautz E. J., Barbay S., Frost S. B., Friel K. M., Dancause N., Zoubina E. V., Stowe A. M., Quaney B. M., Nudo R. J. Neurol. Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 21.Frost S. B., Barbay S., Friel K. M., Plautz E. J., Nudo R. J. J. Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 22.Castro-Alamancos M. A., Garcia-Segura L. M., Borrell J. Eur. J. Neurosci. 1992;4:853–863. doi: 10.1111/j.1460-9568.1992.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 23.Georgopoulos A. P., Schwartz A. B., Kettner R. E. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 24.Georgopoulos A. P., Ashe J., Smyrnis N., Taira M. Science. 1992;256:1692–1695. doi: 10.1126/science.256.5064.1692. [DOI] [PubMed] [Google Scholar]
- 25.Scott S. H., Kalaska J. F. J. Neurophysiol. 1995;73:2563–2567. doi: 10.1152/jn.1995.73.6.2563. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz A. B., Moran D. W. J. Neurophysiol. 1999;82:2705–2718. doi: 10.1152/jn.1999.82.5.2705. [DOI] [PubMed] [Google Scholar]
- 27.Kakei S., Hoffman D. S., Strick P. L. Science. 1999;285:2136–2139. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- 28.Graziano M. S., Taylor C. S., Moore T., Cooke D. F. Neuron. 2002;36:349–362. doi: 10.1016/s0896-6273(02)01003-6. [DOI] [PubMed] [Google Scholar]
- 29.Graziano M. S., Taylor C. S., Moore T. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 30.Graziano M. S., Cooke D. F., Taylor C. S., Moore T. Exp. Brain Res. 2004;155:30–36. doi: 10.1007/s00221-003-1701-4. [DOI] [PubMed] [Google Scholar]
- 31.Graziano M. S., Patel K. T., Taylor C. S. J. Neurophysiol. 2004;92:395–407. doi: 10.1152/jn.01241.2003. [DOI] [PubMed] [Google Scholar]
- 32.Cooke D. F., Taylor C. S. R., Moore T., Graziano M. S. A. Proc. Natl. Acad. Sci. USA. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke D. F., Graziano M. S. J. Neurophysiol. 2004;91:1648–1660. doi: 10.1152/jn.00955.2003. [DOI] [PubMed] [Google Scholar]
- 34.Stepniewska I., Fang P.-C., Kaas J. H. Proc. Natl. Acad. Sci. USA. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graziano M. S., Taylor C. S., Moore T. Nat. Neurosci. 2002;5:921. doi: 10.1038/nn1002-921. [DOI] [PubMed] [Google Scholar]
- 36.Strick P. L. Nat. Neurosci. 2002;5:714–715. doi: 10.1038/nn0802-714. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Kurata K. Brain Res. 1998;781:135–147. doi: 10.1016/s0006-8993(97)01223-7. [DOI] [PubMed] [Google Scholar]
- 38.Liang F., Rouiller E. M., Wiesendanger M. Somatosens. Mot. Res. 1993;10:51–61. doi: 10.3109/08990229309028823. [DOI] [PubMed] [Google Scholar]
- 39.VandenBerg P. M., Hogg T. M., Kleim J. A., Whishaw I. Q. Brain Res. Bull. 2002;59:197–203. doi: 10.1016/s0361-9230(02)00865-1. [DOI] [PubMed] [Google Scholar]
- 40.Whishaw I. Q., Gorny B., Foroud A., Kleim J. A. Behav. Brain Res. 2003;145:221–232. doi: 10.1016/s0166-4328(03)00143-8. [DOI] [PubMed] [Google Scholar]
- 41.Kargo W. J., Nitz D. A. J. Neurosci. 2003;23:11255–11269. doi: 10.1523/JNEUROSCI.23-35-11255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmichael S. T. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- 43.Florence S. L., Kaas J. H. J. Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleim J. A., Jones T. A., Schallert T. Neurochem. Res. 2003;28:1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 45.Jones T. A., Kleim J. A., Greenough W. T. Brain Res. 1996;733:142–148. doi: 10.1016/0006-8993(96)00792-5. [DOI] [PubMed] [Google Scholar]
- 46.Cooke D. F., Graziano M. S. Neuron. 2004;43:585–593. doi: 10.1016/j.neuron.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Whishaw I. Q. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 48.Kozlowski D. A., James D. C., Schallert T. J. Neurosci. 1996;16:4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humm J. L., Kozlowski D. A., James D. C., Gotts J. E., Schallert T. Brain Res. 1998;783:286–292. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- 50.Jones T. A., Schallert T. J. Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.