Abstract
Cannabinoids can evoke antihyperalgesia and antinociception at a peripheral site of action. However, the signaling pathways mediating these effects are not clearly understood. We tested the hypothesis that certain cannabinoids directly inhibit peripheral capsaicin-sensitive nociceptive neurons by dephosphorylating and desensitizing transient receptor potential vanilloid 1 (TRPV1) via a calcium calcineurin-dependent mechanism. Application of the cannabinoid WIN 55,212-2 (WIN) to cultured trigeminal (TG) neurons or isolated skin biopsies rapidly and significantly inhibited capsaicin-activated inward currents and neuropeptide exocytosis by a mechanism requiring the presence of extracellular calcium. The inhibitory effect did not involve activation of G protein-coupled cannabinoid receptors, because neither pertussis toxin nor GDPβS treatments altered the WIN effect. However, application of WIN-activated calcineurin, as measured by nuclear translocation of the nuclear factor of activated T cells (NFAT)c4 transcription factor, dephosphorylated TRPV1. The WIN-induced desensitization of TRPV1 was mediated by calcineurin, because the application of structurally distinct calcineurin antagonists (calcineurin autoinhibitory peptide and cyclosporine/cyclophilin complex) abolished WIN-induced inhibition of capsaicin-evoked inward currents and neuropeptide exocytosis. This mechanism also contributed to peripheral antinociceptive/antihyperalgesic effects of WIN because pretreatment with the calcineurin antagonist calcineurin autoinhibitory peptide (CAIP) significantly reduced peripherally mediated WIN effects in two behavioral models. Collectively, these data demonstrate that cannabinoids such as WIN directly inhibit TRPV1 functional activities via a calcineurin pathway that represents a mechanism of cannabinoid actions at peripheral sites.
Keywords: desensitization, pain, capsaicin, TRPA1, dephosphorylation
Although cannabinoids have been used to treat pain for millennia, the mechanisms of their actions are still not well understood. Cannabinoids activate numerous signaling pathways because they bind to both G protein-coupled receptors (GPCRs), such as CB1 and CB2, as well as ionotropic receptors, such as transient receptor potential vanilloid 1 (TRPV1), TRPV4, and TRPA1 (1–5). Spinal and supraspinal administration of cannabinoids leads to antihyperalgesia or antinociception in several acute and chronic pain models (6–8). Cannabinoids also evoke peripherally mediated antihyperalgesia or antinociception in several pain models (9–12). Although the CNS effects of cannabinoids on nociception appear to be mediated primarily by the CB1 cannabinoid receptor (13), the peripheral mechanisms of cannabinoid effects on nociceptive neurons appear to be indirect or possibly involve non-CB1 or -CB2 receptors because of the low level of expression of CB1 or CB2 receptors in nociceptive neurons (14–16).
The thermal and capsaicin-sensitive TRPV1 channel plays a key role in thermal inflammatory hyperalgesia and neuropathic pain (17–19). TRPV1 channel activity is modulated by its phosphorylation status (17) and the phosphorylation of TRPV1 by kinases such as PKA, PKC, and calcium/calmodulin dependent kinase II α (CaMKIIα) sensitizes its functions, whereas dephosphorylation by the protein phosphatase 2B (also known as calcineurin) desensitizes its activity (17, 20–27). Thus, highly efficacious TRPV1 agonists such as capsaicin can desensitize the channel in a calcium-dependent fashion via the calcineurin-pathway leading to dephosphorylation of the channel (25, 28–31). The cannabinoid agonist WIN 55,212-2 (WIN) evokes calcium-dependent neuropeptide release from cultured sensory neurons (32). However, it is not known whether cannabinoids such as WIN can dephosphorylate and desensitize TRPV1 and whether this mechanism contributes to the peripheral antihyperalgesic effects of cannabinoids.
Results
Cannabinoid Agonist WIN Inhibits Capsaicin-Evoked Responses in Cultured Nociceptors and Superfused Nociceptor Terminals.
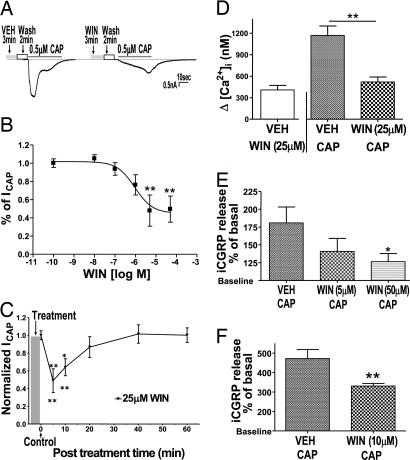
The mixed cannabinoid agonist WIN (CB1/CB2) was selected as a prototypical cannabinoid because it evokes a profound peripherally mediated thermal antihyperalgesic effect in TRPV1-dependent behavioral models (9). In whole-cell voltage clamp experiments (Fig. 1A), the pretreatment with WIN inhibited capsaicin-induced inward current (ICAP) in ≈80% of the cells that responded to capsaicin (n = 132/168). Most of these cells were small-to-medium diameter (15–40 μm). The WIN-induced inhibition of Icap was concentration dependent with a maximal inhibition of ≈48% (Fig. 1B). This inhibition occurred at concentrations greater than the known nanomolar affinity of WIN at GPCR cannabinoid receptors (33). The WIN-evoked inhibitory effects displayed a rapid onset, a duration that persisted at least 10 min and recovered by 40 min (Fig. 1C). WIN altered the kinetics of ICAP, producing ≈5-fold increase in the duration of the 5–95% rise time of ICAP (5.1 ± 0.7 s, n = 40 vs. 22.4 ± 1.7 s, n = 55; P < 0.0001). The application of WIN (25 μM) by itself induced a transient calcium influx in a subset of capsaicin-sensitive neurons. After a return to basal levels, the neurons were exposed to capsaicin (0.5 μM). Compared with the vehicle-treated cells, WIN significantly inhibited capsaicin-induced calcium influx (≈57%; Fig. 1D). Importantly, this effect occurred only in cells that responded to WIN pretreatment. To exclude the possibility that the WIN effects were an artifact of culturing conditions, we used two in vitro superfusion assays to evaluate WIN effects in acutely isolated tissue. In acutely dissociated and superfused trigeminal (TG) neurons, WIN pretreatment inhibited capsaicin-evoked immunoreactive calcitonin gene-related peptide (iCGRP) release (Fig. 1E). In the WIN pretreatment fraction, we observed a statistically insignificant (≈15%) increase in iCGRP release above the basal release (data not shown), possibly because of the small, transient increase in calcium evoked by WIN in these neurons. Because most behavioral assays evaluating peripheral cannabinoids have involved hindpaw testing, we next evaluated the effects of WIN on iCGRP release from acutely isolated and superfused hindpaw skin biopsies. Similar to the TG superfusion experiments, WIN pretreatment alone resulted in a small (≈17%), but statistically insignificant, increase in iCGRP release above the basal release (data not shown). Importantly, pretreatment with WIN significantly inhibited capsaicin-evoked iCGRP release from the peripheral nerve terminals (Fig. 1F).
Fig. 1.
The cannabinoid agonist WIN 55,212-2 (WIN) reversibly inhibits capsaicin responses from nociceptors. (A) Effect of vehicle (VEH) or WIN pretreatment on capsaicin-induced inward current in cultured rat TG neurons. Cultured TG neurons were grown in presence of nerve growth factor (NGF) for 24–48 h. The intact neurons were exposed to VEH/WIN (25 μM) for 3 min, washed for 2 min, and then ICAP [0.5 μM capsaicin (CAP) for 40 s] were recorded in whole-cell configuration. Representative traces denote capsaicin current (ICAP) after VEH or WIN treatment. (B) Effect of pretreatment with different concentrations of WIN on Icap. Data are normalized to ICAP after VEH pretreatment (n = 8–13 cells per condition; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). (C) The time course of WIN (25 μM for 3 min, then wash) inhibition of ICAP is shown (n = 8–12 cells per condition; ∗, P < 0.05; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). (D) Effect of VEH or WIN (25 μM) pretreatment on capsaicin-induced calcium influx in cultured TG neurons. The Fura-loaded cells were exposed to either vehicle or WIN (25 μM) for 3 min. After the WIN-induced calcium influx returned to the basal level, the cells were exposed to capsaicin (0.5 μM for 40 s). The elevation (Δ) in [Ca2+]i in VEH-WIN group denotes the calcium influx evoked by WIN alone over baseline values, and the elevation (Δ) in [Ca2+]i in the VEH-CAP and WIN-CAP groups denotes the CAP-evoked calcium influx over baseline levels after VEH or WIN pretreatment (n = 16–25; ∗∗, P < 0.05, ANOVA with Bonferroni post hoc test). (E) Effect of VEH or WIN pretreatment on capsaicin-induced CGRP release from acutely dissociated rat TG neurons. Freshly isolated and dissociated TG were pretreated with either vehicle or WIN for 7 min, washed, and then pulsed with capsaicin alone (100 μM) for 2 min. Capsaicin-evoked iCGRP release was measured by radioimmunoassay (RIA) and is represented as percent of basal release (n = 8; ∗, P < 0.05, ANOVA with Bonferroni post hoc test). (F) Effect of VEH or WIN pretreatment on capsaicin-induced CGRP release from acutely isolated hindpaw skin. Freshly isolated hindpaw skin was pretreated with VEH or WIN (10 μM), washed (10 min), and then pulsed with capsaicin alone (100 μM) for 2 min. Capsaicin-evoked iCGRP release was measured by RIA and is represented as percent of basal release (n = 6; ∗∗, P < 0.01, Student’s t test).
WIN Inhibition of Capsaicin Responses Is Independent of G Protein Activation.
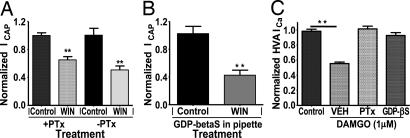
The preponderance of evidence (18, 19, 34) indicates either a very low (<5%) or no detectable expression of CB1/CB2 receptors on TRPV1-positive nociceptors. These observations suggest the possibility of non-CB1/-CB2 mechanisms for WIN inhibitory actions in sensory neurons, and it is possible that either a non-CB1/-CB2 cannabinoid GPCR or a cannabinoid ionotropic receptor might mediate an effect. To exclude the role of a GPCR, we first evaluated whether the inhibitory G protein (Gi/o)-protein signaling pathway was involved in WIN inhibition of ICAP. Pretreatment with pertussis toxin (PTx) did not affect WIN inhibition of ICAP (Fig. 2A), even though control experiments verified that PTx treatment abolished the actions of the μ-opioid agonist [d-Ala2,N-MePhe4,Gly5-ol]enkephalin (DAMGO) on ICa (Fig. 2C). To exclude other GPCRs including inhibitory Gz and G16 pathways, we applied GDP-βS, a nonhydrolysable GDP analogue that traps all G proteins in an inactive conformation (35), and we found no change in WIN-induced inhibition of ICAP (Fig. 2B), although control experiments verified that it abolished the DAMGO-inhibition of ICa (Fig. 2C and Fig. 6, which is published as supporting information on the PNAS web site). Collectively, these data demonstrate that WIN inhibition of capsaicin responses is unlikely to be mediated by cannabinoid GPCRs.
Fig. 2.
WIN inhibition of capsaicin-evoked responses is independent of G protein activation. (A) Effect of pretreatment of TG neurons with pertussis toxin (PTx). Cultured TG neurons were grown in the presence or absence of PTx (500 ng/ml, 24–36 h). Vehicle (VEH) or WIN was applied with ICAP as described in the legend for Fig. 1. (n = 7–10 cells; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). (B) Effect of trapping of G protein activities by dialyzing neurons (5–8 min) with GDP-βS (2.5 mM) on WIN inhibition of ICAP (n = 8–9 cells; ∗∗, P < 0.01, Student’s t test). (C) Positive control experiments, demonstrating the inhibitory effect of pertussis toxin or GDP-βS on [d-Ala2,N-MePhe4,Gly5-ol]enkephalin (DAMGO) (1 μM) inhibition of high voltage-activated calcium currents (HVA ICa, n = 8–11 cells; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test).
WIN Inhibition of Capsaicin Responses Is Dependent on the Presence of Extracellular Calcium and the Activation of Calcineurin.
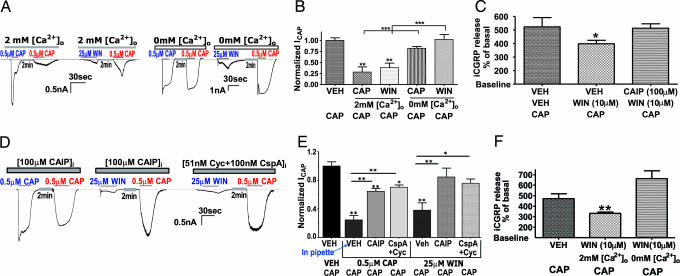
Previous studies have demonstrated that ICAP can be inhibited via a Ca2+-dependent pharmacological desensitization (including tachyphylaxis) (28, 29, 31, 36). Because WIN application elevates intracellular calcium ([Ca2+]i) (Fig. 1D and ref. 32), we evaluated whether external calcium was necessary for WIN-induced inhibition of ICAP. Similar to capsaicin-induced tachyphylaxis, WIN inhibition of ICAP was dependent on extracellular calcium (Fig. 3A and B). In contrast to this dependence on extracellular calcium, the presence or absence of calcium in the internal pipette solution had no effect (Fig. 7, which is published as supporting information on the PNAS web site) (24, 25). In addition, WIN inhibition of capsaicin-evoked iCGRP release from superfused peripheral skin terminals was dependent on the presence of extracellular calcium during WIN pretreatment (Fig. 3C). The exclusive reliance on extracellular calcium is consistent with the hypothesis that the site of calcium actions could be in a vicinity of the channel pore through which calcium enters (24). Because calcium influx activates protein phosphatase 2B (PP2B or calcineurin) that is critically involved in regulating TRPV1-capsaicin responses (25, 30, 37), we evaluated whether calcineurin mediates WIN-induced inhibition of capsaicin responses. The application of either calcineurin autoinhibitory peptide (CAIP) or the cyclosporine-cyclophilin complex (CspA+Cyc), two structurally distinct calcineurin inhibitors (30), completely reversed WIN inhibition of ICAP (Fig. 3 D and E), and positive control experiments verified their effectiveness for blocking capsaicin-induced tachyphylaxis. The involvement of the calcineurin pathway was confirmed in skin superfusion assay by using a cell permeable CAIP T (38) (Fig. 3F). The CAIP pretreatment alone did not change the basal release of iCGRP release.
Fig. 3.
WIN inhibition of capsaicin-evoked responses is dependent on activation of the calcium-calcineurin pathway. (A) Evaluation of calcium dependency of WIN and CAP for inhibition of ICAP. Electrophysiology experiments were conducted as described in the legend to Fig. 1, except that WIN and CAP treatment was administered in the presence of either 2 mM calcium (normal) or 0 mM calcium in the external solution. Representative traces showing capsaicin/WIN desensitization of ICAP in the presence or absence of external calcium. (B) Graphical representation of traces in A demonstrating the calcium dependency of WIN and CAP for inhibition of ICAP (n = 14 cells; ∗∗, P < 0.01; ∗∗∗, P < 0.001, ANOVA with Bonferroni post hoc test). (C) Effect of WIN pretreatment in calcium-free buffer on WIN inhibition of capsaicin evoked iCGRP release from isolated hindpaw skin (n = 12; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). The superfusion experiments were conducted as described in the legend to Fig. 1, except that WIN pretreatment was administered in the presence of either 2 mM calcium (normal) or 0 mM calcium in the external solution; all groups then were returned to 2 mM calcium concentrations for the 10 min wash and during the application of capsaicin. (D) Evaluation of the calcineurin dependency of WIN and CAP for inhibition of ICAP. Electrophysiology experiments were conducted as described in the legend to Fig. 1, except that WIN or CAP treatment was administered in presence of CAIP (100 μg) or the complex of 51 nM cyclosporine/100 nM cyclophilin. Representative traces are shown. (E) Graphical representation of traces in D demonstrating the effect of inhibition of calcineurin on WIN and CAP inhibition of ICAP (n = 6–15 cells; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, ANOVA with Bonferroni post hoc test). (F) Effect of WIN pretreatment in the presence of CAIP (100 μM) on WIN inhibition of capsaicin-evoked iCGRP release from isolated hindpaw skin (n = 6; ∗, P < 0.01, ANOVA with Bonferroni post hoc test).
WIN Activates Calcineurin in TG Neurons and Dephosphorylates TRPV1.
We next evaluated the coexpression of calcineurin subunits with TRPV1 in cultured TG neurons (Fig. 4A) and whether WIN activates calcineurin in these neurons (Fig. 4 B and C). NFATc4 was found to be ubiquitously expressed in trigeminal sensory neurons. TRPV1 immunoreactivity was found in 60.7 ± 2.4% (n = 12) of NFATc4-positive TG neurons. Application of both capsaicin and ionomycin evoked nuclear translocation of NFATc4 in TG neurons suggestive of calcineurin activation (Fig. 4 B and C) (39). Pretreatment with the calcineurin inhibitor, CAIP, significantly inhibited the capsaicin effect (Fig. 4C). Similarly, WIN evoked nuclear translocation of NFATc4 that was significantly inhibited by CAIP pretreatment (Fig. 4C). The percent of cells showing calcineurin activity (as measured by nuclear NFATc4) post-WIN treatment was slightly lower than after capsaicin treatment (39.5% vs. 53.0%). These numbers were consistent with the electrophysiology and calcium imaging experiments where only 75–80% of capsaicin responsive cells were also WIN responsive. The TRPV1 phosphorylation at threonine residues plays a critical role in channel sensitization and desensitization (25, 26). The application of WIN decreased basal phosphorylation of TRPV1 at threonine residues, and this effect was blocked by a pretreatment with the calcineurin inhibitor CAIP (Fig. 4 D and E). Additional experiments confirmed WIN-induced dephosphorylation of TRPV1 by measuring a reduction in 32P-TRPV1 (Fig. 8, which is published as supporting information on the PNAS web site).
Fig. 4.
WIN activates calcineurin in TG neurons and dephosphorylates TRPV1. (A) Calcineurin subunits are coexpressed with TRPV1. The colocalization of calcineurin A subunit (Upper) and B subunit (Lower) with TRPV1 is demonstrated in the respective panels. (B) The effect of various treatments on the nuclear translocation of NFATc4 (calcineurin activation). Cultured TG neurons were exposed to vehicle, ionomycin (1 μM), capsaicin (1 μM), WIN (25 μM), or WIN+CAIP (25 μM/50 μM), and immunohistochemistry was performed by using an antibody against NFATc4. (C) Graphical representation of the percent of NFATc4 positive neurons showing nuclear translocation of NFAFc4 after treatment with VEH/VEH, VEH/WIN (25 μM), CAIP (50 μM)/WIN, VEH/CAP (1 μM), or CAIP (50 μM)/CAP (n = 4 independent cultures assessed by blinded observer; n = 152–180 cells per condition; ∗∗, P < 0.01, ANOVA with Bonferroni post hoc test). (D) Effect of WIN and CAIP/WIN treatment on TRPV1 phosphorylation. Cultured TG neurons (6 ganglia per 10-cm plate) were treated with VEH, WIN (25 μM), or CAIP (50 μM)+WIN. A representative Western blot demonstrating phosphothreonine content (Upper) in TRPV1 immunoprecipitated TG lysates (Lower). (E) Quantification of multiple experiments performed as described in D. The band density was normalized to total TRPV1 protein (n = 3 independent cultures; ∗, P < 0.05, ANOVA with Bonferroni post hoc test).
Role of Calcineurin in Peripheral WIN-Evoked Inhibition of Nocifensive Behavior and Hyperalgesia.
We next evaluated the in vivo relevance of the calcineurin pathway in mediating peripheral WIN-induced antihyperalgesia and antinociception in trigeminal and dorsal root ganglia pain models. Local injection of WIN into the vibrissal pad inhibited the second phase of formalin nocifensive behavior in a CAIP reversible manner (Fig. 5A). Control experiments indicated that the WIN effect was peripherally mediated (Fig. 5A). The intraplantar (ipl) injection of capsaicin resulted in thermal hyperalgesia (10.4 ± 0.5 s vs. 4.4 ± 0.6 s, P < 0.001; Fig. 5B). Pretreatment with a peripherally selective dose of WIN (9) completely (P < 0.001) reversed capsaicin-induced thermal hyperalgesia, and this WIN effect was blocked by CAIP (Fig. 5B). Thus in vivo experiments confirm the involvement of the calcineurin pathway in mediating peripheral WIN-induced inhibition in models of nociception and hyperalgesia.
Fig. 5.
WIN inhibition of orofacial nocifensive behavior and hindpaw hyperalgesia is dependent on activation of calcineurin. (A) After habituation to the testing chamber, lightly restrained rats were injected in the right vibrissal pad with VEH, WIN (10 μg), CAIP (400 μg), or CAIP/WIN along with 5% formalin. In a separate group of animals, the same dose (10 μg) of WIN was injected systemically (SYS, s.c.) before formalin injection. The results are represented as the mean number of seconds the animal displayed nocifensive behavior as measured by blinded observers (n = 6 animals per group; ∗∗∗, P < 0.001, two-way ANOVA with Bonferroni post hoc test). (B) Lightly restrained rats were injected with VEH, WIN (10 μg), CAIP (400 μg), or WIN+CAIP in the right hindpaw. The same paws were injected with capsaicin (10 μg) 15 min later. The thermal withdrawal latencies were measured 5 and 10 min after capsaicin injection by blinded observers. Data are depicted as paw withdrawal latency in seconds. To evaluate the development of hyperalgesia, post-capsaicin withdrawal latencies were compared with the basal withdrawal latencies (n = 6–10 animals per group, two-way ANOVA with Bonferroni post hoc test; ###, P < 0.001; ##, P < 0.01). To evaluate the WIN-evoked antihyperalgesia and its modulation by CAIP, the statistical comparison was made with respect to the VEH-CAP group (n = 6–10 animals per group; ∗∗∗, P < 0.001, two-way ANOVA with Bonferroni post hoc test).
Discussion
In this study, we demonstrate that the cannabinoid agonist WIN directly and reversibly inhibits capsaicin responses in nociceptors via a signaling pathway that involves calcium influx and activation of calcineurin. The activation of calcineurin by WIN dephosphorylates TRPV1 and thus desensitizes/inhibits TRPV1 activities. In vivo studies using two distinct models of nociception involving both the trigeminal and dorsal root ganglia systems demonstrated the calcineurin dependency of peripheral WIN-induced thermal antihyperalgesia.
WIN was selected because this cannabinoid is peripherally active in pain models, including inflammation (9, 39–41). WIN activates CB1 and CB2, permitting evaluation of both of these receptors in mediating peripheral WIN mechanisms. In addition, these results might be generalized to a variety of structurally distinct cannabinoids that share CB1-/CB2-binding domains. Indeed, a CB1 agonist, such as ACEA, and a CB2 agonist, such as AM1241, desensitize TRPV1 in sensory neurons via a similar pathway (A.N.A. and K.M.H., unpublished data). Moreover, we previously have demonstrated that WIN evokes a calcium influx in TG nociceptors in a TRPV1 independent manner (32). One possible candidate for this WIN-evoked response appears to be TRPA1 (A.N.A. and K.M.H., unpublished data).†† Thus, understanding the signaling mediating the desensitization of TRPV1 by cannabinoids, possibly by a heterologous desensitization mechanism not requiring direct activation of TRPV1, could have profound mechanistic and therapeutic value.
TRPV1 plays an important role in chemical nociception and thermal hyperalgesia (18, 19) and appears to contribute to mechanical hyperalgesia (42, 43). Thus cannabinoid desensitization of TRPV1 may explain the efficacy of peripheral cannabinoids in various pain models. The behavioral effects demonstrate that calcineurin mediates the antihyperalgesic effects of WIN but do not by themselves implicate a direct neuronal hypothesis. Instead, this hypothesis was tested by using multiple independent in vitro experiments.
In the electrophysiology experiments, WIN treatment reduced ICAP and produced ≈5-fold increase in the 5–95% rise time of ICAP. A similar effect is observed with capsaicin tachyphylaxis (37), and this altered rate of activation might explain the nocifensive and nonnocifensive nature of certain compounds due to altering the kinetics of ligand-gated channels, thereby influencing action potential frequency and duration. Thus, compounds that produce a delayed rate of activation lead to inactivation of certain voltage-gated channels and therefore evoke little to no nocifensive behavior (44). This effect might have implications for the in vivo WIN effects because a delay in ICAP kinetics might result in a decrease in nocifensive behavior/hyperalgesia.
Multiple studies performed by using point mutation strategy have demonstrated that TRPV1 activity is modulated by its phosphorylation status at various serine and threonine residues (21, 22, 25, 26). WIN decreased phosphorylation of TRPV1 at threonine residues in a calcineurin-dependent fashion, and these results were confirmed with the 32P experiment. It should be recognized that calcineurin is a serine-threonine phosphatase, and thus it is possible that WIN-evoked dephosphorylation of TRPV1 occurs at serine residues as well. Further studies will be required to pinpoint the specific residues involved in calcineurin-dependent WIN-induced dephosphorylation of TRPV1.
In our in vivo experiments, CAIP alone did not significantly alter nocifensive responses, possibly because of an inability to further potentiate maximally active doses of both capsaicin (10 μg) and formalin (5%).
An important question raised by these results is the relative contribution of GPCR (i.e., metabotropic) vs. channel (i.e., ionotropic) mechanisms in mediating peripheral cannabinoid antihyperalgesia/antinociception. Depending on the agonist used, various studies using mainly behavioral pharmacology methods have reported the involvement of CB1 and/or CB2 in peripheral cannabinoid antihyperalgesia/antinociception (9, 10, 16, 39–41, 45). Moreover, some studies have demonstrated a CB1-/CB2-independent component of peripheral cannabinoid inhibition of nociceptors (46, 47). In our experimental conditions, at a single cell level, cannabinoid-inhibition of ICAP was independent of activation of GPCRs. Importantly, cannabinoid inhibition of ICAP and CGRP release was dependent on the presence of extracellular calcium, a finding consistent with a possible involvement of a channel in mediation of this effect. Moreover, the inhibition of calcineurin completely abolished WIN inhibition of chemical nociception and capsaicin-induced CGRP release, denoting the critical role of this pathway in cannabinoid effects. Although there are no reported pathways that link activation of either CB1 or CB2 to calcineurin, that possibility cannot be ruled out in the in vivo and skin superfusion assays. Thus, a link between calcineurin and CB1/CB2 may exist in peripheral nonneuronal cells such as skin cells, immune cells, and so on. Based on these issues, this data demonstrates a peripheral cannabinoid ionotropic mechanism for antihyperalgesia, but the presence of both ionotropic and metabotropic cannabinoid mechanisms in other peripheral assays cannot be excluded.
The activation of nociceptive sensory neurons could lead to nociception. However, some compounds are capable of altering nociceptor activity without producing nociceptive behavior. Thus, some cannabinoids such as anandamide, Δ9THC, and, in this case, WIN induce sufficient calcium influx in nociceptors to activate the calcineurin pathway and desensitize the TRPV1 responses, but in vivo assays do not detect a WIN-evoked nociception/hyperalgesia before desensitizing nociceptors. This observation also holds true with TRPV1 agonists such as anandamide (AEA) that fail to evoke nociception by using in vivo assays (12, 48). The mechanism for this phenomenon is unknown, although it is possible that the comparatively small and slow WIN- or AEA-gated currents might not be sufficient to generate action potentials leading to nociception. This property of WIN to evoke desensitization without evoking nociception could be of considerable therapeutic importance in clinical settings where local application of WIN (or similar compounds) could produce analgesia without exacerbating pain.
Materials and Methods
Animals and Compounds.
Adult male Sprague–Dawley (Charles River Laboratories) rats weighing 250–300 g were used in this Institutional Animal Care and Use Committee (IACUC)-approved study. WIN (Tocris Cookson, Ellisville, MO) and capsaicin and cyclosporine (both from Fluka/Sigma–Aldrich) were made in DMSO, and then, on the day of use, were dissolved in respective buffers (Hanks’ solution/standard external solution). For behavioral experiments, WIN and capsaicin were dissolved in a mixture of 5% Tween-80 and 5% DMSO in 0.9% saline. CAIP (Calbiochem), [d-Ala2,N-MePhe4,Gly5-ol]enkephalin (DAMGO), GDP-βs, and cyclophilin stocks were made in water and diluted in the respective buffer.
TG Cultures.
TG were cultured similar to ref. 49, except the trypsin step was omitted and the collagenase treatment included dispase (1 mg/ml; Roche) For the experiments evaluating changes in the TRPV1 phosphorylation, six TGs collected from three rats were plated onto one 10-cm plate.
Electrophysiology.
Whole-cell voltage-clamp recordings of ICAP from cannabinoid- versus vehicle-treated neurons were compared in separate neurons to avoid interference from progressive reduction of ICAP amplitudes (i.e., tachyphylaxis) caused by successive application of capsaicin (28). The experimental protocol included either a vehicle or WIN (3 min) pretreatment and wash (2 min), followed by a capsaicin exposure (0.5 μM for 40 s). The recordings were made at 22–24°C from the somata of neurons (15–45 picofarads) by using an Axopatch 200B amplifier and pclamp 9.0 software (Axon Instruments, Union City, CA). Recordings were performed at holding potential (Vh) of −60 mV. Data were filtered at 0.5–5 kHz and sampled at 2–10 kHz depending on current kinetics. ICa was elicited every 15 s with 50 or 100 ms pulses to 0 mV from a Vh of −60 mV at pH 7.4. Solutions are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Calcium Imaging.
To measure intracellular [Ca2+] levels, the dye Fura-2 AM (2 μM; Molecular Probes) was loaded into cells in the presence of 0.05% Pluronic (Calbiochem) for 60 min at 37°C. Fluorescent imaging was performed by using a 20×/0.75 numerical aperture Fluor objective (Nikon); the light source was a xenon lamp equipped with filter wheel (Sutter Instruments, Novato, CA) controlled by Lambda 10–3 (Sutter Instruments) and metafluor software (Universal Imaging, Downingtown, PA). Cultured TG neurons preloaded with Fura-2 were exposed to either vehicle or WIN (25 μM) for 3 min. WIN evoked a transient calcium influx in a subset of cells. After the calcium levels returned to basal levels in these cells, the neurons were exposed to capsaicin (0.5 μM for 40 s). The ratiometric data were converted to [Ca2+]i (as a micromolar concentration) by using the equation [Ca2+]i = K* (R − Rmin)/(Rmax − R), where R is the 340/380 nm fluorescence ratio. Rmin, Rmax, and K* (0.29, 2.75, and 1.44 μM, respectively) were measured according to a method described in ref. 50.
In Vitro Skin and TG Superfusion.
To measure CGRP release from skin (51), freshly isolated rat hindpaw skin biopsies were incubated (Hanks’ solution) for 20 min for basal iCGRP release and then pretreated with either vehicle or WIN (10 μM) for 20 min, washed for 10 min (to remove WIN), and stimulated with 100 μM capsaicin for 2 min. The evoked release was collected over three 20-min fractions, and iCGRP release was measured by radioimmunoassay (RIA) as described (49). The experiments using CAIP (100 μM) had a 20-min pre- and cotreatment of the peptide with WIN followed by a wash (10 min). In experiments evaluating the calcium dependence of WIN effect, WIN pretreatment was performed in calcium-free Hanks’ solution buffer containing 10 mM EGTA; however, 2 mM calcium was restored during the 10-min wash, and the capsaicin exposure was done in Hanks’ solution containing 2 mM calcium.
To measure CGRP release from acutely isolated TG (49), dissociated TGs (four per chamber) were washed with oxygenated Hanks’ solution buffer for 30 min to attain a basal iCGRP release and then exposed to either vehicle or WIN (5–50 μM) for 7 min, washed (7 min), and then pulsed with capsaicin (100 μM for 2 min).
Behavioral Assays.
Formalin-evoked nocifensive behavior tests in the orofacial pain model were carried out as described (52). The right vibrissal pad was injected with 50 μl of 5% formalin (Fisher Scientific)-containing vehicle, 10 μg of WIN, and/or membrane permeable form of CAIP (400 μg). Scratching, grooming, or rubbing (nocifensive behavior) events at the injection side was collected by 3-min bins by blinded observers.
Capsaicin-evoked thermal paw withdrawal latencies (PWL) were measured as described (53). After collection of basal PWL, rats were injected intraplantarly (50 μl) with Veh (9), WIN (10 μg), CAIP (400 μg), or WIN/CAIP, and then were injected with capsaicin (10 μg in 10 μl, intraplantarly) 15 min later. PWL was measured 5 and 10 min later by blinded observers.
Immunohistochemistry.
Immunohistochemical studies evaluated calcineurin expression in TG neurons by using anti-A and anti-B antisera to the subunits of calcineurin (Chemicon) with colocalization to anti-TRPV1 antisera (15) (Neuromics; Bloomington, MN). As a measure of calcineurin activation, the nuclear translocation of NFATc4 was determined in cultured TG neurons exposed to various treatments for 20 min. Neurons were fixed, and immunohistochemistry was performed by using a previously characterized antibody specific to NFATc4.
Dephosphorylation Assay.
To determine the effects of WIN on TRPV1 dephosphorylation, 10-cm TG cultures were incubated with 1 mCi of [32P]orthophosphate (PerkinElmer) for 4 h at 37°C in phosphate-free DMEM (GIBCO), pretreated with either vehicle or 50 μM CAIP for 30 min at 37°C, and then with either vehicle or WIN (25 μM) for 15 min, and harvested (54). The cleared lysates were immunoprecipitated with 1 μg of anti-TRPV1 antisera (Ab-2; Calbiochem), resolved on 15% SDS/PAGE, and transferred to poly(vinylidene difluoride) (Millipore). Western blots were either exposed to film at −80°C overnight for autoradiography or blocked in 5% BSA in Tris-buffered saline plus 0.05% Tween 20 and visualized by using antibodies to TRPV1 (anti-TRPV1, Ab-1; Calbiochem) or phosphothreonine (Calbiochem), followed by appropriate secondary antisera linked to horseradish peroxidase and enhanced chemiluminescence (General Electric Healthcare). All autoradiographic and phosphospecific bands were quantified using National Institutes of Health Image 162 and normalized to values obtained from total immunoprecipitated TRPV1.
Data Analysis.
Data are presented as mean ± SEM. The electrophysiology data were normalized to the ICAP under respective conditions, and comparisons were made to the respective ICAP. Data were analyzed by using graphpad prism software version 4 (GraphPad, San Diego). Multifactor experimental data were analyzed by using two-way ANOVA; single-factor, multiple treatment data were analyzed by using one-way ANOVA; and individual groups were compared by using a Bonferroni post hoc test, whereas experiments examining differences between two groups were analyzed by using Student’s t test.
Supplementary Material
Acknowledgments
We thank Gabriela Helesic and Jaime Cerecero for their technical support and Dr. Jennifer Gooch for discussions of the calcineurin experiments. This work was supported by National Institute on Drug Abuse Grant R01-DA19585 (to K.M.H.) and National Institute of Dental and Craniofacial Research Grant F32-DE016500 (to N.A.J.).
Abbreviations
- CAIP
calcineurin autoinhibitory peptide
- GPCR
G protein-coupled receptors
- ICAP
capsaicin-induced inward current
- iCGRP
immunoreactive calcitonin gene-related peptide
- NFAT
nuclear factor of activated T cells
- TG
trigeminal
- TRPV1
transient receptor potential vanilloid 1
- WIN
cannabinoid WIN 55,212-2.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
††Ruparel, N. B., Akopian, A. N., Patwardhan, A. M., Jeske, N. A. & Hargreaves, K. M. (2005) Soc. Neurosci. Abstr., 622.3 (abstr.).
References
- 1.Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 2.Munro S., Thomas K. L., Abu-Shaar M. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 3.Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sorgard M., Di Marzo V., Julius D., Hogestatt E. D. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 5.Jordt S. E., Bautista D. M., Chuang H. H., McKemy D. D., Zygmunt P. M., Hogestatt E. D., Meng I. D., Julius D. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 6.Croxford J. L. CNS Drugs. 2003;17:179–202. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Goutopoulos A., Makriyannis A. Pharmacol. Ther. 2002;95:103–117. doi: 10.1016/s0163-7258(02)00250-4. [DOI] [PubMed] [Google Scholar]
- 8.Rice A. S., Farquhar-Smith W. P., Nagy I. Prostaglandins Leukotrienes Essent. Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- 9.Johanek L. M., Heitmiller D. R., Turner M., Nader N., Hodges J., Simone D. A. Pain. 2001;93:303–315. doi: 10.1016/S0304-3959(01)00336-0. [DOI] [PubMed] [Google Scholar]
- 10.Malan T. P., Jr, Ibrahim M. M., Deng H., Liu Q., Mata H. P., Vanderah T., Porreca F., Makriyannis A. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 11.Calignano A., La Rana G., Giuffrida A., Piomelli D. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 12.Richardson J. D., Kilo S., Hargreaves K. M. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- 13.Ledent C., Valverde O., Cossu G., Petitet F., Aubert J. F., Beslot F., Bohme G. A., Imperato A., Pedrazzini T., Roques B. P., et al. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 14.Bridges D., Rice A. S., Egertova M., Elphick M. R., Winter J., Michael G. J. Neuroscience. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 15.Price T. J., Helesic G., Parghi D., Hargreaves K. M., Flores C. M. Neuroscience. 2003;120:155–162. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim M. M., Porreca F., Lai J., Albrecht P. J., Rice F. L., Khodorova A., Davar G., Makriyannis A., Vanderah T. W., Mata H. P., Malan T. P., Jr Proc. Natl. Acad. Sci. USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortright D. N., Szallasi A. Eur. J. Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 18.Davis J. B., Gray J., Gunthorpe M. J., Hatcher J. P., Davey P. T., Overend P., Harries M. H., Latcham J., Clapham C., Atkinson K., et al. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 19.Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., Koltzenburg M., Basbaum A. I., Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 20.Premkumar L. S., Ahern G. P. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 21.Bhave G., Hu H.-J., Glauner K. S., Zhu W., Wang H., Brasier D. J., Oxford G. S., Gereau R. W., IV Proc. Natl. Acad. Sci. USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhave G., Zhu W., Wang H., Brasier D. J., Oxford G. S., Gereau R. W., IV Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 23.Mandadi S., Numazaki M., Tominaga M., Bhat M. B., Armati P. J., Roufogalis B. D. Cell Calcium. 2004;35:471–478. doi: 10.1016/j.ceca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Numazaki M., Tominaga T., Takeuchi K., Murayama N., Toyooka H., Tominaga M. Proc. Natl. Acad. Sci. USA. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohapatra D. P., Nau C. J. Biol. Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 26.Mohapatra D. P., Nau C. J. Biol. Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 27.Lopshire J. C., Nicol G. D. J. Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koplas P. A., Rosenberg R. L., Oxford G. S. J. Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L., Simon S. A. J. Neurophysiol. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- 30.Docherty R. J., Yeats J. C., Bevan S., Boddeke H. W. Pflügers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 31.Cholewinski A., Burgess G. M., Bevan S. Neuroscience. 1993;55:1015–1023. doi: 10.1016/0306-4522(93)90315-7. [DOI] [PubMed] [Google Scholar]
- 32.Price T. J., Patwardhan A. M., Akopian A. N., Hargreaves K. M., Flores C. M. Br. J. Pharmacol. 2004;142:257–266. doi: 10.1038/sj.bjp.0705778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felder C. C., Joyce K. E., Briley E. M., Mansouri J., Mackie K., Blond O., Lai Y., Ma A. L., Mitchell R. L. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 34.Hohmann A. G., Herkenham M. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 35.Guo J., Schofield G. G. J. Physiol. (London) 2002;545:767–781. doi: 10.1113/jphysiol.2002.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L., Simon S. A. Brain Res. 1998;809:246–252. doi: 10.1016/s0006-8993(98)00853-1. [DOI] [PubMed] [Google Scholar]
- 37.Piper A. S., Yeats J. C., Bevan S., Docherty R. J. J. Physiol. (London) 1999;518:721–733. doi: 10.1111/j.1469-7793.1999.0721p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terada H., Matsushita M., Lu Y. F., Shirai T., Li S. T., Tomizawa K., Moriwaki A., Nishio S., Date I., Ohmoto T., Matsui H. J. Neurochem. 2003;87:1145–1151. doi: 10.1046/j.1471-4159.2003.02098.x. [DOI] [PubMed] [Google Scholar]
- 39.Ulugol A., Karadag H. C., Ipci Y., Tamer M., Dokmeci I. Neurosci. Lett. 2004;371:167–170. doi: 10.1016/j.neulet.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 40.Yesilyurt O., Dogrul A., Gul H., Seyrek M., Kusmez O., Ozkan Y., Yildiz O. Pain. 2003;105:303–308. doi: 10.1016/s0304-3959(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 41.Dogrul A., Gul H., Akar A., Yildiz O., Bilgin F., Guzeldemir E. Pain. 2003;105:11–16. doi: 10.1016/s0304-3959(03)00068-x. [DOI] [PubMed] [Google Scholar]
- 42.Birder L. A., Nakamura Y., Kiss S., Nealen M. L., Barrick S., Kanai A. J., Wang E., Ruiz G., De Groat W. C., Apodaca G., et al. Nat. Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 43.Walker K. M., Urban L., Medhurst S. J., Patel S., Panesar M., Fox A. J., McIntyre P. J. Pharmacol. Exp. Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- 44.Liu L., Lo Y., Chen I., Simon S. A. J. Neurosci. 1997;17:4101–4111. doi: 10.1523/JNEUROSCI.17-11-04101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malan T. P., Jr, Ibrahim M. M., Vanderah T. W., Makriyannis A., Porreca F. Chem. Phys. Lipids. 2002;121:191–200. doi: 10.1016/s0009-3084(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 46.Sagar D. R., Smith P. A., Millns P. J., Smart D., Kendall D. A., Chapman V. Eur. J. Neurosci. 2004;20:175–184. doi: 10.1111/j.1460-9568.2004.03481.x. [DOI] [PubMed] [Google Scholar]
- 47.Costa B., Giagnoni G., Franke C., Trovato A. E., Colleoni M. Br. J. Pharmacol. 2004;143:247–250. doi: 10.1038/sj.bjp.0705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price T. J., Patwardhan A. M., Akopian A. N., Hargreaves K. M., Flores C. M. Br. J. Pharmacol. 2004;141:1118–1130. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patwardhan A. M., Berg K. A., Akopain A. N., Jeske N. A., Gamper N., Clarke W. P., Hargreaves K. M. J. Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gamper N., Shapiro M. S. J. Gen. Physiol. 2003;122:17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kilo S., Harding-Rose C., Hargreaves K. M., Flores C. M. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 52.Gilbert S. D., Clark T. M., Flores C. M. Pain. 2001;89:159–165. doi: 10.1016/s0304-3959(00)00358-4. [DOI] [PubMed] [Google Scholar]
- 53.Hargreaves K. M., Dubner R., Brown F., Flores C., Joris J. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 54.Jeske N. A., Berg K. A., Cousins J. C., Ferro E. S., Clarke W. P., Glucksman M. J., Roberts J. L. J. Neurochem. 2006;97:13–21. doi: 10.1111/j.1471-4159.2006.03706.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.