Abstract
Barttin is an accessory subunit of a subgroup of ClC-type chloride channels expressed in renal and inner ear epithelia. In this study, we examined the effects of barttin on two ClC-K channel isoforms, rat ClC-K1 and human ClC-Kb, using heterologous expression, patch clamping, confocal imaging, and flow cytometry. In the absence of barttin, only a small percentage of rClC-K1 and hClC-Kb channels are inserted into the plasma membrane. Coexpression of barttin enhances surface membrane insertion and furthermore modifies permeation and gating of ClC-K channels. hClC-Kb channels are nonfunctional without barttin and require the coexpressed accessory subunit to become anion conducting. In contrast, rClC-K1 channels are active without barttin, but at the cost of reduced unitary conductance as well as altered voltage dependence of activation. We mapped the separate functions of barttin to structural domains by a deletion analysis. Whereas the transmembrane core is necessary and sufficient to promote ClC-K channel exit from the endoplasmic reticulum, a short cytoplasmic segment following the second transmembrane helix modifies the unitary conductance. The entire cytoplasmic carboxyl terminus affects the open probability of ClC-K channels. The multiple functions of barttin might be necessary for a tight adjustment of epithelial Cl− conductances to ensure a precise regulation of body salt content and endocochlear potential.
Keywords: chloride channels, gating, kidney, ClC family, accessory subunit
Barttin was identified as a protein encoded by the disease gene of a variant of Bartter’s syndrome, BSND, that combines a salt-losing nephropathy with sensorineural deafness and renal failure (1, 2), and later shown to be an accessory subunit of a subclass of ClC channels, namely the ClC-K channels (3, 4). ClC-K channels are necessary for chloride absorption in various sections of the nephron (5, 6) as well as for potassium secretion in the stria vascularis and the vestibular labyrinth. For the majority of these channels, heterologous expression results in measurable anion currents only when expressed together with barttin (3, 4, 7, 8). So far, this effect of barttin on ClC channels has been interpreted in terms of trafficking alone, i.e., that ClC-K channels are retained in intracellular membrane systems and reach the cell surface after association with barttin (3, 4, 9).
We examined the functional and molecular basis of the regulation of two different ClC-K channels, rat ClC-K1 (10) and human ClC-Kb (11), by barttin using a combination of heterologous expression, site-directed mutagenesis, confocal imaging, flow cytometry, and cellular electrophysiology. Our study demonstrates that this accessory subunit modifies not only the surface membrane density, but also permeation and gating of ClC-K channels. By controlling these diverse parameters, barttin effectively and tightly controls the macroscopic chloride conductance in renal and inner ear epithelia.
Results
Barttin Promotes Surface Membrane Insertion of rClC-K1 and hClC-Kb.
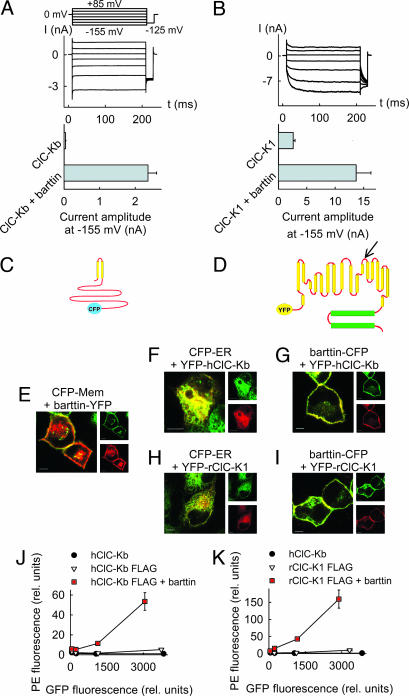
We expressed rClC-K1 and hClC-Kb channels alone or together with human barttin in mammalian cells and measured anion currents with the patch clamp technique. Consistent with previous reports, we observed anion currents in the absence of barttin only in cells expressing rClC-K1 (10), but not in those expressing hClC-Kb (11). Coexpression of barttin with rClC-K1 and hClC-Kb, respectively, results in large whole-cell anion current amplitudes in tsA201 cells (Fig. 1A and B). To study the effect of barttin on the subcellular ClC-K channel distribution, we generated fluorescent protein-tagged constructs (Fig. 1 C and D) and examined transfected cells by confocal imaging and flow cytometry. Barttin inserts itself into the plasma membrane (Fig. 1E), whereas ClC-K1 and ClC-Kb channels are predominantly present in the endoplasmic reticulum (Fig. 1 F and H). Cotransfection of barttin results in a redistribution of the chloride channels to the surface membrane (Fig. 1 G and I). Barttin exerts similar effects on ClC-K channel trafficking in MDCKII cells, a cell line forming confluent monolayers with characteristics of in vivo renal epithelia (12) (Fig. 1), and in tsA201 cells (Fig. 6, which is published as supporting information on the PNAS web site), the cell line we routinely use for electrophysiological experiments.
Fig. 1.
Barttin promotes surface membrane insertion of ClC-K channels. (A and B) Representative currents from cells coexpressing hClC-Kb (A) or rClC-K1 (B) with barttin and mean isochronal current amplitudes determined 2 ms after a voltage step to −155 mV (n > 8) from cells expressing channels with and without barttin. (C and D) Topology model of barttin (C) and ClC-K channels (D). The position of the FLAG epitope used for FACS analysis is marked by an arrow. (E) Confocal images of live MDCK cells coexpressing a barttin-YFP fusion protein and a fluorescent marker for the membrane surface (CFP-Mem; Clontech). CFP is shown in green, and YFP is shown in red. This color code results in an orange coloring of regions where both proteins overlap. (F and H) Confocal images of live MDCK cells coexpressing a fluorescent marker for the endoplasmic reticulum (CFP-ER; Clontech) together with YFP-hClC-Kb (F) and YFP-rClC-K1 (H). (G and I) Confocal images of live MDCK cells coexpressing a barttin-CFP fusion protein and YFP-hClC-Kb (G) or YFP-rClC-K1 (I). (Scale bars: 5 μm.) (J and K) Surface expression of hClC-Kb (J) and rClC-K1 (K) with (■) and without (▿) barttin in transiently transfected tsA201 cells determined by flow cytometry. Shown are plots of fluorescence levels of an anti-Flag antibody versus the GFP fluorescence. Data were binned in four groups where means and SEMs were obtained. GFP-hClC-Kb without FLAG (●) was used as a control. For both channels, antibody fluorescence is significantly different from control level (P < 0.05), and barttin significantly increases the fluorescence levels (P < 0.05).
We next quantified the effect of barttin on the subcellular distribution of ClC-K channels by means of flow cytometry. We expressed mutant ClC-K channels with an amino-terminal GFP protein and a FLAG epitope between transmembrane domains L and M (Fig. 1D) in tsA201 cells, applied anti-FLAG antibodies to nonpermeabilized cells, and individually measured GFP and antibody fluorescence levels. Because the engineered FLAG epitope is located extracellularly (13, 14), a plot of the antibody fluorescence levels versus the GFP fluorescence (Fig. 1 J and K) provides information about the fraction of channels inserted into the plasma membrane. Antibody fluorescence levels were negligible in cells expressing channel constructs lacking the FLAG-epitope (Fig. 1 J and K), demonstrating the specificity of antibody binding. In the absence of barttin, antibody fluorescences of cells with FLAG-tagged ClC-K constructs are significantly larger than background levels (P < 0.05; Fig. 1 J and K). Therefore, hClC-Kb and rClC-K1 insert into the plasma membrane even without barttin. Barttin increases the ratio of antibody fluorescence to GFP fluorescence and thus the fraction of channels in the surface membrane severalfold (Fig. 1 J and K). Both in the presence and in the absence of barttin, a larger percentage of rClC-K1 channels could traffic to the surface membrane than hClC-Kb.
ClC-Kb Channels Are Nonfunctional in the Absence of Barttin.
Because hClC-Kb channels can insert into the cell surface even in the absence of barttin (Fig. 1J), the complete lack of anion currents (Fig. 1A) suggests either that hClC-Kb channels are nonfunctional under this condition or that the membrane densities of hClC-Kb are simply too low for whole-cell recordings. To distinguish between these possibilities, we studied heterodimeric channels consisting of hClC-Kb and another ClC isoform that inserts into the plasma membrane independently of barttin, hClC-1 (15). We reasoned that hClC-Kb might reach the surface membrane at higher density as a component of a heterodimeric channel together with such related protein. By means of flow cytometry, we first compared surface membrane expression in cells expressing hClC-Kb alone and in cells coexpressing hClC-Kb and hClC-1. Cotransfection of hClC-1 increases surface insertion of hClC-Kb (P < 0.05; Fig. 2A), demonstrating that the two different ClC isoforms can assemble to heterodimeric channels and that these heterodimeric channels effectively travel to the plasma membrane. If one assumes that hClC-Kb does not display anion currents in the absence of barttin because of the low membrane insertion, the cotransfection with hClC-1 should result in the appearance of an hClC-Kb current component. In contrast to this prediction, coexpression of hClC-1 and hClC-Kb without barttin resulted in anion currents that were undistinguishable from pure hClC-1 currents (data not shown).
Fig. 2.
hClC-Kb protopores are functional only in the presence of barttin. (A) Surface expression of hClC-Kb in transiently transfected tsA201 cells determined by flow cytometry, when expressed alone (▿), together with hClC-1 (■), or with barttin (◇). GFP-hC1C-Kb without FLAG (●) was used as a control. (B and C) Representative current recordings from a cell expressing the hClC-1-hClC-Kb concatamer alone (B) and from another cell expressing it together with barttin (C).
In coexpression experiments, a quantitative analysis of the effect of barttin is difficult, because the contribution of homodimeric channels to the observed current amplitudes is not known. To solely express heterodimeric channels (16), we engineered a concatameric construct that links one hClC-1 and one hClC-Kb coding region into a single ORF. In the absence of barttin, expression of this construct alone results in anion currents whose gating properties are indistinguishable from hClC-1 homodimeric currents (Fig. 2B). Although no effect of barttin on homodimeric hClC-1 channels was observed (data not shown), macroscopic currents of cells coexpressing the heteroconcatamer with barttin resemble a superposition of hClC-1 and hClC-Kb/barttin (Fig. 2C). hClC-1 deactivates at negative potentials and exhibits an inwardly rectifying isochronal conductance (16). In contrast, hClC-Kb/barttin channels do not gate and only slightly rectify (Fig. 1A). The effect of barttin on the heterodimer can therefore be quantified by measuring late current amplitudes at positive and at negative potentials. Barttin increases both values [at −155 mV: Ilate/Iinst = 0.54 ± 0.02 with barttin compared with 0.15 ± 0.01 without barttin, P < 0.01; at +105 mV: Ilate(+105 mV)/Iinst(−155 mV) = 0.23 ± 0.01 with barttin compared with 0.10 ± 0.01 without barttin, P < 0.01]. Because the expression of the hetero-concatamer is expected to result in a preferential formation of heterodimeric channels and therefore in equal numbers of hClC-1 and hClC-Kb protopores, we conclude that hClC-Kb protopores are nonfunctional in the absence of barttin.
An alternative explanation represents the principal formation of hClC-1/hClC-1 or hClC-Kb/hClC-Kb homodimeric channels by association of two concatamers. In this scenario, barttin would increase the hClC-Kb current by a mere stimulation of channel insertion into the surface membrane. This possibility is refuted by various experimental results. Flow cytometry demonstrates that hClC-1/hClC-Kb heterodimers can form upon expression of monomeric constructs (Fig. 2A). Thus, covalently linked hClC-1 and hClC-Kb subunits should preferentially form heterodimeric channels. Expression of a comparable concatameric construct was shown to result in a homogenous population of heterodimeric channels (16). Moreover, a mutant barttin, T61X (see below), causes a redistribution of hClC-Kb to the surface membrane but does not increase macroscopic current amplitudes in cells expressing hClC-Kb (see Fig. 4). All results are only satisfactorily explained by assuming that hClC-Kb is not functional without barttin and that barttin switches hClC-Kb to an active form.
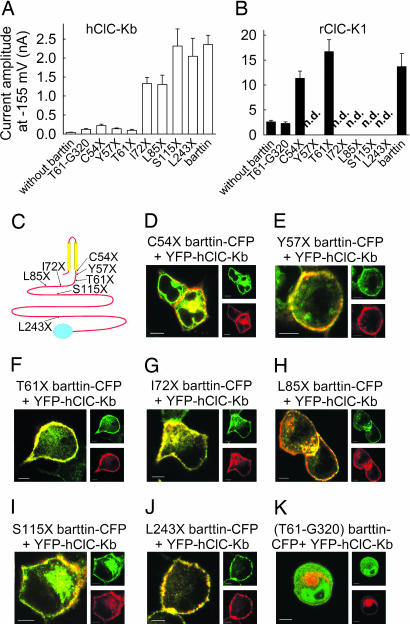
Fig. 4.
A deletion analysis defines functional domains within barttin. (A and B) Mean isochronal current amplitudes determined 2 ms after a voltage step to −155 mV (n > 8) on cells coexpressing ClC-Kb (A) and ClC-K1 (B) and WT barttin or various truncated versions, respectively. (C) Localization of the tested carboxyl-terminal truncations in a transmembrane topology model of barttin. (D–K) Confocal images of live MDCK cells coexpressing hClC-Kb with various barttin mutants. (Scale bars: 5 μm.) CFP is shown in green, and YFP is shown in red.
Barttin Modifies Gating and Ion Conduction of rClC-K1.
The regulation of rClC-K1 by barttin is far more complex than that of hClC-Kb. Fig. 3 shows current recordings from cells expressing WT rClC-K1 alone (Fig. 3A) and with barttin (Fig. 3B). Cells were held at 0 mV, and voltage steps between −155 mV and +85 mV were applied. WT rClC-K1 channels are open at the holding potential, and current amplitudes change instantaneously upon voltage steps in both directions. For membrane hyperpolarization, this instantaneous rise is followed by a time-dependent current increase. The time-dependent changes of rClC-K1 currents are comparable for rClC-K1 channels with and without barttin (Fig. 3 A and B). However, the voltage dependence of the relative open probability is shifted to more positive potentials by barttin (Fig. 3C). Therefore, barttin increases the channel open probability in the physiological voltage range.
Fig. 3.
Barttin modifies the gating of WT and mutant rClC-K1. (A, B, D, and E) Representative current recordings from cells expressing WT rClC-K1 (A and B) or V166E rClC-K1 (D and E) channels, with or without barttin, respectively. (C and F) Voltage dependence of relative open probabilities of WT (C) and absolute open probabilities of V166E (F) rClC-K1 channels, expressed either alone (●) or together with barttin (▿).
We then used nonstationary noise analysis to study the effects of barttin on unitary channel properties. This analysis was not feasible for WT rClC-K1 channels because of the limited time-dependent changes of the macroscopic current amplitude upon voltage steps (Fig. 3 A and B). We therefore used a mutant rClC-K1 bearing a single point mutation, V166E. Because single channel experiments on ClC-0 channels demonstrated that substitutions of this acidic side chain did not affect the single channel amplitude (17), we expect unitary current amplitudes of V166E rClC-K1 to be identical to those of WT channel. V166E alters gating of rClC-K1 (Fig. 3 D–F). Without barttin, V166E rClC-K1 channels are activated by membrane hyperpolarization (Fig. 3 D and F), whereas cotransfection of barttin inverts the voltage dependence of activation (Fig. 3E), resulting in a depolarization-induced activation (Fig. 3F). Nonstationary noise analyses (Supporting Text and Fig. 7, which are published as supporting information on the PNAS web site) demonstrated that the unitary conductance of V166E rClC-K1 was ≈3-fold increased by barttin (without barttin, 6.5 ± 0.3 pS; with barttin, 19.4 ± 0.9 pS; n > 6). These results were corroborated by direct measurements of unitary current amplitudes (Supporting Text and Fig. 8, which is published as supporting information on the PNAS web site). Single channel recordings demonstrated the occurrence of channels with a unitary conductance of 20.5 pS in cells cotransfected with V166E rClC-K1 and barttin and of 8.6 pS in cells expressing V166E rClC-K1 alone.
Barttin thus changes gating and ion permeation of rClC-K1 channels. Assuming that ClC-K channels exhibit a three-dimensional structure comparable with the recently solved structure of an Escherichia coli ClC transporter (14), barttin most likely does not contribute to the formation of the ion conduction pathway, but rather allosterically modifies the ion conduction pathway of V166E rClC-K1.
Separate Domains of Barttin Exhibit Distinct Functions.
The predicted transmembrane topology of barttin contains two transmembrane helices encompassing the amino acids between 9 and 54 and a cytoplasmic carboxyl terminus of 266 aa. We generated various truncation mutants and studied the effect of these truncated barttins on the subcellular distribution and on the functional properties of ClC-K channels (Fig. 4). An isolated carboxyl-terminal fragment Cterm(T61-G320) and three barttin truncation mutants (C54X, Y57X, and T61X) do not result in macroscopic chloride currents in cells when expressed with hClC-Kb (Fig. 4A). In contrast, all other tested mutants with longer carboxyl termini (I72X, L85X, S115X, and L243X) result in current amplitudes comparable with WT barttin. For rClC-K1, even C54X and T61X cause significant increases of macroscopic current amplitudes (Fig. 4B). Confocal microscopy revealed that all truncated barttin mutants longer than C54X cause hClC-Kb channels to be present predominantly in the surface membrane (Fig. 4 D–J). Our results demonstrate that trafficking and function of ClC-K channels are conferred by separate regions of barttin. Whereas the transmembrane core of barttin is sufficient for ClC-K channel exit from the endoplasmic reticulum and incorporation into the surface membrane, an additional stretch of 15–17 aa (I72X barttin) is necessary to turn hClC-Kb into a conductive anion channel.
The isolated carboxyl-terminal fragment Cterm(T61-G320)-CFP did not change ClC-K currents (Fig. 4B). It is soluble and small enough to diffuse through nuclear pores into the cell nucleus, resulting in a fluorescent staining of the whole cell (Fig. 4K). There is no redistribution of CFP fluorescence in experiments involving coexpression with hClC-Kb. Our data suggest that the carboxyl-terminal fusion protein of barttin does not stably bind to the ClC channel protein.
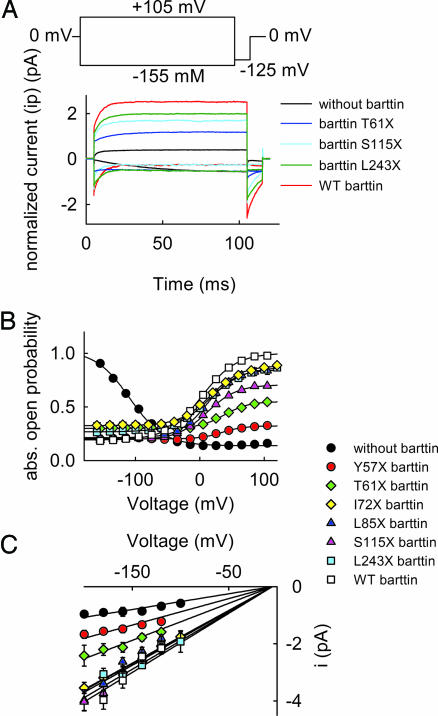
To define barttin domains involved in modifying unitary current amplitudes and in gating of V166E rClC-K1 channels, we next studied the effect of truncated barttins on these channels. Fig. 5A shows normalized representative current recordings from cells expressing V166E rClC-K1 alone, with three truncated barttin constructs (T61X, S115X, and L243X) and with WT barttin. Whereas channels without barttin are activated by hyperpolarization, all other tested barttin constructs induce channel activation by membrane depolarization. Fig. 5 B and C shows the effects of various barttin truncations on the voltage dependences of the absolute open probabilities and unitary current amplitudes determined by nonstationary noise analysis. V166E rClC-K1 channels with truncated barttins exhibit a smaller absolute open probability than those with WT barttin at positive potentials. However, at negative potentials, all tested barttins reduce the absolute open probability compared with barttin-less V166E rClC-K1. The alteration of unitary current amplitude by mutant barttin requires domains differing from those involved in the setting of the absolute open probability. All barttins longer than I72X result in the same unitary current amplitude as WT barttin, whereas Y57X and T61X barttin increase the unitary current amplitude, but not to the WT level (Fig. 5C and Table 1). The transmembrane core together with a short stretch of 15–17 aa is sufficient to increase the unitary conductance of V166E rClC-K1 channels to the level of their WT barttin-associated counterparts.
Fig. 5.
Role of the carboxyl terminus of barttin in modifying the unitary conductance and the absolute open probability of V166E rClC-K1. (A) Representative normalized current amplitudes from cells coexpressing V166E rClC-K1 and truncated barttins. To normalize our data, currents were divided by the number of channels per cell determined by noise analysis. (B and C) Voltage dependences of the absolute open probability (B) and the unitary current amplitudes (C) of V166E rClC-K1/mutant barttin channels.
Table 1.
Permeation and gating properties of V166E rClC-K1 channels coexpressed with WT and mutant barttin or without barttin, respectively
| Pore-forming and accessory subunit | Unitary conductance, pS | V0.5, mV | Apparent gating charge, eo | Popenmax | Popenmin |
|---|---|---|---|---|---|
| V166E rClC-K1 | 6.5 ± 0.3, n = 11 | −110.2 ± 2.8, n = 8 | −1.0 ± 0.0, n = 8 | 1.0 | 0.14 |
| + Y57X barttin | 9.1 ± 0.3, n = 6 | 31.2 ± 1.4, n = 16 | 1.1 ± 0.1, n = 16 | 0.32 | 0.19 |
| + T61X barttin | 13.1 ± 0.5, n = 7 | 38.0 ± 2.3, n = 12 | 1.3 ± 0.1, n = 12 | 0.55 | 0.27 |
| + I72X barttin | 18.1 ± 0.4, n = 12 | 17.7 ± 1.9, n = 19 | 1.1 ± 0.0, n = 19 | 0.88 | 0.21 |
| + L85X barttin | 16.6 ± 0.6, n = 9 | 15.3 ± 2.3, n = 14 | 1.1 ± 0.0, n = 14 | 0.71 | 0.19 |
| + S115X barttin | 18.7 ± 0.6, n = 7 | 11.4 ± 1.3, n = 17 | 1.2 ± 0.0, n = 17 | 0.84 | 0.33 |
| + L243X barttin | 18.5 ± 0.7, n = 10 | 14.7 ± 2.8, n = 6 | 1.0 ± 0.1, n = 6 | 0.87 | 0.30 |
| + WT barttin | 19.4 ± 0.9, n = 6 | 11.6 ± 3.5, n = 15 | 0.9 ± 0.0, n = 15 | 1.00 | 0.20 |
Discussion
Barttin is an accessory subunit of a subgroup of renal and inner ear ClC-type chloride channels. In this study, we have demonstrated barttin’s crucial role for the intracellular trafficking as well as for the functional properties of these channels. Barttin is an integral membrane protein consisting of a transmembrane core and a cytoplasmic carboxyl terminus (Fig. 1C). The transmembrane core is necessary and sufficient to redistribute ClC-K/barttin channels out of the endoplasmic reticulum and into the surface membrane (Fig. 4). The transmembrane region and 15–17 adjacent amino acids of the carboxyl terminus switch hClC-Kb channels from closed to open state (Fig. 4A) and increase the unitary current amplitudes of rClC-K1 ≈3-fold (Fig. 5C). The remainder of the carboxyl-terminal tail is necessary for setting the absolute channel open probability (Fig. 5B). These results define barttin as a protein with separate functional domains: the transmembrane core associates with the pore-forming subunit of the channel and acts as a chaperone for intracellular trafficking. The cytoplasmic carboxyl terminus modifies the functional properties of the ClC-K/barttin channel when associated with ClC-K channels via the transmembrane core. The modular architecture of barttin might allow the design of small molecules that selectively modify the various functions of the ClC-K/barttin interactions in the future. Because ClC-K channels are the only human ClC isoforms that are modulated by barttin, such components could be used as selective ClC-K channel modifiers to stimulate water and salt excretion in the treatment of hypertension or renal failure.
Thus far, rClC-K1 is the only mammalian ClC-K isoform that is functional without barttin in a heterologous system. Neither hClC-Ka, the functional homologue to rClC-K1 in humans (8), nor rClC-K2 and hClC-Kb can be functionally expressed in the absence of this accessory subunit (3, 4, 11). The regulation of hClC-Ka by barttin is therefore more similar to hClC-Kb than to rClC-K1, indicating a species specificity in the regulation of ClC-K channels by barttin. This is not a surprising result because hClC-Ka and hClC-Kb exhibit a higher sequence identity to each other than to rClC-K1 or to rClC-K2 (11).
ClC-K channels are the only mammalian ClC channels lacking a glutamate residue in the signature sequence GKE/VGPXXH (18) close to the selectivity filter of this channel family (14). The functional modification of rClC-K1 by barttin assigns a likely physiological significance to this uniqueness. Whereas barttin causes a shift of the relative open probability of WT rClC-K1 to more positive potentials and thus increases the open probability at physiological voltages (Fig. 3), it slightly decreases this value for V166E rClC-K1 (Popen at −75 mV: 0.27 ± 0.02 (n = 6) without barttin and 0.24 ± 0.02 (n = 6) with barttin). ClC-K channels might have lost the conserved negative side chain as an evolutionary adjustment to enable an increase of the channels’ open probability by barttin.
Barttin affects the subcellular trafficking as well as the ion conduction and gating of renal and inner ear chloride channels. The number of functions of barttin is unprecedented among accessory subunits, demonstrating that ClC-type chloride channels are unique not only in molecular architecture and gating mechanisms, but also in their regulation by non pore-forming subunits.
Methods
Mutagenesis and Channel Expression.
tsA201 and MDCKII cells were transfected with 0.1–10 μg of plasmid DNA encoding WT and mutant rClC-K1, hClC-Kb, and human barttin by using a calcium phosphate precipitation method (16). Cells were typically examined 2 days after transient transfection. For electrophysiological experiments, cells were cotransfected with a plasmid encoding the CD8 antigen and incubated 5 min before use with polystyrene microbeads precoated with anti-CD8 antibodies (Dynabeads M-450 CD 8; Dynal, Great Neck, NY) (19). Only cells decorated with microbeads were used for electrophysiological recordings. Methods for generation of the transfected plasmids are provided in Supporting Text.
Electrophysiology.
Standard whole-cell or excised outside-out patch clamp recordings were performed by using an EPC9 or 10 (HEKA Electronics, Lambrecht, Germany) amplifier (20, 21). Pipettes were pulled from borosilicate glass and had resistances between 1.0 MΩ and 2.2 MΩ for whole-cell recordings and >10 MΩ for single-channel recordings. In whole-cell experiments, 60–80% of the series resistance was compensated by an analog procedure, so that the calculated voltage error due to access resistance was always <2 mV. The standard extracellular solution contained 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes; the standard intracellular solution contained 120 mM NaCl, 2 mM MgCl2, 5 mM EGTA, and 10 mM Hepes. All solutions were adjusted to pH 7.4 with NaOH. Cells/patches were clamped to 0 mV between test sweeps. For single-channel recordings, currents were filtered with a 0.5 or 1 kHz low-pass Bessel filter and sampled at 10 kHz. Capacitive and leak currents were compensated by subtracting scaled control traces recorded at the calculated anion reversal potential (−5 mV).
Data were analyzed by a combination of PulseTools (HEKA Electronics, Lambrecht, Germany) and SigmaPlot (Jandel Scientific, San Rafael, CA) programs. Isochronal current amplitudes were measured 2 ms after the voltage step. To determine the unitary current amplitudes as well as absolute open probabilities of rClC-K1 channels, nonstationary noise analysis was performed as described (20). Amplitude histograms were generated from 10-s voltage steps to various potentials. The single-channel amplitude and the absolute open probability were determined by fitting Gaussian distributions to these histograms. In patches with more than one channel, the open probability of a single channel was calculated assuming a binomial distribution of channel openings. Unitary conductances were calculated by fitting linear relationships to the voltage dependences of the unitary current amplitudes (Figs. 5 and 8). To obtain the voltage dependence of activation, isochronal current amplitudes were determined at a voltage step to −125 mV after 200-ms prepulses to various voltages, normalized by their maximum value and plotted versus the preceding potential. The absolute voltage dependence was obtained by adjusting the relative activation curve to the open probability at +75 mV calculated by nonstationary noise analyses. Activation curves were fit with a sum of a voltage-independent minimum open probability (Pmin) and a voltage-dependent term: I(V) = Amp/(1 + ezeo(V − V0.5)/kT)) + Pmin, with zeo being the apparent gating charge and Amp + Pmin being the maximum open probability. For the determination of mean peak current amplitudes (Fig. 4), whole cell and excised outside-out patch currents were averaged. To account for the distinct membrane area in patches compared with a whole cell, the patch current amplitudes were multiplied by the ratios of resting conductance before excision by the corresponding value after excision (21).
Confocal Microscopy and Flow Cytometry.
Live cell confocal imaging was carried out with transiently transfected MDCKII or tsA201 cells on a glass coverslip 48–72 h after transfection as described (21). For flow cytometry, 5 × 105 to 1 × 106 transfected tsA201 cells were resuspended in cold PBS supplemented with 5% FCS and 0.1% sodium azide (PBS/azide) and incubated with 2 μg/ml mouse anti-flag antibody (Sigma) for 30 min at 4°C. Cells were washed with cold PBS/azide and subsequently incubated in darkness with a 1:50 dilution of an R-phycoerythrin-conjugated α-mouse antibody for 30 min at 4°C. Cells were washed again and resuspended in PBS/azide. Then 104 cells per sample were analyzed by flow cytometry using a FACScalibur (Becton Dickinson) equipped with a 488-nm argon laser. Data were analyzed by a combination of CellQuest Pro (BD Biosciences), WinMDI version 2.8, and SigmaPlot (Jandel Scientific). GFP fluorescence of individual cells was sorted into four bins, and average antibody fluorescence in each of these bins was plotted against average GFP fluorescence. For statistical evaluation, ratios of average GFP and antibody fluorescences of the region with the largest GFP fluorescence were compared by using Student’s t test.
Supplementary Material
Acknowledgments
We thank Drs. A. L. George (Vanderbilt University, Nashville, TN) and S. Uchida (Tokyo Medical and Dental University) for providing the expression constructs for hClC-Kb, rClC-K1, and barttin. We thank Drs. Tania Alekova, Patricia Hidalgo, Heider Linder, Günther Schmalzing, and Saba Sile for helpful discussions; Dr. S. Radtke for help with the flow cytometry; Drs. A. Lückhoff and P. Heinrich for support; Barbara Poser for excellent technical assistance; and Dr. Stefan Wüllner (RWTH, Aachen, Germany) for providing the MDCK cells. These studies were supported by Deutsche Forschungsgemeinschaft Grants FOR450, TP10 (to C.F.), and SFB 542, Zentralprojekt Z1 (to G.M.-N.) and by the Studienstiftung des deutschen Volkes (U.S.).
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hebert S. C. Curr. Opin. Nephrol. Hypertens. 2003;12:527–532. doi: 10.1097/00041552-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Birkenhager R., Otto E., Schurmann M. J., Vollmer M., Ruf E. M., Maier-Lutz I., Beekmann F., Fekete A., Omran H., Feldmann D., et al. Nat. Genet. 2001;29:310–314. doi: 10.1038/ng752. [DOI] [PubMed] [Google Scholar]
- 3.Estevez R., Boettger T., Stein V., Birkenhager R., Otto E., Hildebrandt F., Jentsch T. J. Nature. 2001;414:558–561. doi: 10.1038/35107099. [DOI] [PubMed] [Google Scholar]
- 4.Waldegger S., Jeck N., Barth P., Peters M., Vitzthum H., Wolf K., Kurtz A., Konrad M., Seyberth H. W. Pflügers Arch. 2002;444:411–418. doi: 10.1007/s00424-002-0819-8. [DOI] [PubMed] [Google Scholar]
- 5.Uchida S., Sasaki S., Nitta K., Uchida K., Horita S., Nihei H., Marumo F. J. Clin. Invest. 1995;95:104–113. doi: 10.1172/JCI117626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi K., Uchida S., Okamura H. O., Marumo F., Sasaki S. J. Am. Soc. Nephrol. 2002;13:1992–1998. doi: 10.1097/01.asn.0000023434.47132.3d. [DOI] [PubMed] [Google Scholar]
- 7.Jentsch T. J., Stein V., Weinreich F., Zdebik A. A. Physiol. Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 8.Uchida S., Sasaki S. Annu. Rev. Physiol. 2005;67:759–778. doi: 10.1146/annurev.physiol.67.032003.153547. [DOI] [PubMed] [Google Scholar]
- 9.Hayama A., Rai T., Sasaki S., Uchida S. Histochem. Cell Biol. 2003;119:485–493. doi: 10.1007/s00418-003-0535-2. [DOI] [PubMed] [Google Scholar]
- 10.Uchida S., Sasaki S., Furukawa T., Hiraoka M., Imai T., Hirata Y., Marumo F. J. Biol. Chem. 1993;268:3821–3824. [PubMed] [Google Scholar]
- 11.Kieferle S., Fong P., Bens M., Vandewalle A., Jentsch T. J. Proc. Natl. Acad. Sci. USA. 1994;91:6943–6947. doi: 10.1073/pnas.91.15.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson J. C., Scalera V., Simmons N. L. Biochim. Biophys. Acta. 1981;673:26–36. [PubMed] [Google Scholar]
- 13.Kuchenbecker M., Schu B., Kurz L., Rüdel R. Pflügers Arch. 2001;443:280–288. doi: 10.1007/s004240100688. [DOI] [PubMed] [Google Scholar]
- 14.Dutzler R., Campbell E. B., Cadene M., Chait B. T., MacKinnon R. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 15.Steinmeyer K., Ortland C., Jentsch T. J. Nature. 1991;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- 16.Fahlke C., Knittle T., Gurnett C. A., Campbell K. P., George A. L., Jr. J. Gen. Physiol. 1997;109:93–104. doi: 10.1085/jgp.109.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutzler R., Campbell E. B., MacKinnon R. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]
- 18.Fahlke C., Yu H. T., Beck C. L., Rhodes T. H., George A. L., Jr. Nature. 1997;390:529–532. doi: 10.1038/37391. [DOI] [PubMed] [Google Scholar]
- 19.Jurman M. E., Boland L. M., Liu Y., Yellen G. BioTechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- 20.Hebeisen S., Heidtmann H., Cosmelli D., Gonzalez C., Poser B., Latorre R., Alvarez O., Fahlke C. Biophys. J. 2003;84:2306–2318. doi: 10.1016/S0006-3495(03)75036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebeisen S., Biela A., Giese B., Müller-Newen G., Hidalgo P., Fahlke C. J. Biol. Chem. 2004;279:13140–13147. doi: 10.1074/jbc.M312649200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.