Abstract
We previously reported that the methicillin resistance gene mecA is carried by a novel type of mobile genetic element, SCCmec (staphylococcal cassette chromosome mec), in the chromosome of methicillin-resistant Staphylococcus aureus (MRSA). These elements are precisely excised from the chromosome and integrated into a specific site on the recipient chromosome by a pair of recombinase proteins encoded by the cassette chromosome recombinase genes ccrA and ccrB. In the present work, we detected homologues of the ccr genes in Staphylococcus hominis type strain GIFU12263 (equivalent to ATCC 27844), which is susceptible to methicillin. Sequence determination revealed that the ccr homologues in S. hominis were type 1 ccr genes (ccrA1 and ccrB1) that were localized on a genetic element structurally very similar to SCCmec except for the absence of the methicillin-resistance gene, mecA. This genetic element had mosaic-like patterns of homology with extant SCCmec elements, and we designated it SCC12263 and considered it a type I staphylococcal cassette chromosome (SCC). The ccrB1 gene identified in the S. hominis strain is the first type 1 ccrB gene discovered to retain its function through the excision process as judged by two criteria: (i) SCC12263 was spontaneously excised during cultivation of the strain and (ii) introduction of the S. hominis ccrB1 into an MRSA strain carrying a type I SCCmec whose ccrB1 gene is inactive generated SCCmec excisants at a high frequency. The existence of an SCC without a mec determinant is indicative of a staphylococcal site-specific mobile genetic element that serves as a vehicle of transfer for various genetic markers between staphylococcal species.
The emergence and worldwide spread of methicillin-resistant Staphylococcus aureus (MRSA) since the early 1960s has long posed serious problems for antimicrobial chemotherapy. The genetic basis of methicillin resistance in MRSA is the acquisition of the mecA gene (30, 39) that encodes the low-affinity penicillin-binding protein 2′ (PBP 2′ [see references 9, 37, and 46]). The mecA gene is carried by an exogenous DNA element inserted in the Staphylococcus aureus chromosome. Ito et al. sequenced the entire element (17, 18), and Katayama et al. showed that it is a novel class of mobile genetic element, designated SCCmec (staphylococcal cassette chromosome mec) (20). The SCCmec element is integrated in the S. aureus chromosome at the attBSCC site located in orfX, an open reading frame (ORF) of unknown function (17, 18, 20). The SCCmec element has both inverted repeats (IR) and direct repeats (DR) at its termini and carries a set of site-specific recombinase genes, cassette chromosome recombinase genes ccrA and ccrB, which are responsible for the integration and excision of SCCmec, and the mecA gene complex that encodes inducible methicillin resistance (17, 18, 20). So far, four distinct types of SCCmec have been identified in MRSA strains isolated from around the world (12, 13, 18). The type I SCCmec group includes the older MRSA strains prevalent in the 1960s and is exemplified by NCTC 10442, the first MRSA strain isolated in 1960 in England. The type II SCCmec group first identified by studies of a Japanese pre-MRSA strain, N315, isolated in 1982 (14, 17), is ubiquitous among recent MRSA isolates from Japan, Korea, and the United States (12). The type III SCCmec group is found in strains prevalent in the countries that have close geographical or historical ties to England, such as European countries, Saudi Arabia, India, Singapore, Hong Kong, Australia, and New Zealand (18). Recently, Ma et al. have identified a fourth type of SCCmec from community-acquired MRSA strains (29, 36).
The type of an SCCmec element is defined by the allotype of the ccr genes and the structural class of the mecA gene complex it possesses. Three allotypes of ccr genes (type 1, type 2, and type 3) and four classes of mecA gene complex (class A, class B, class C, and class D) have been identified (21). The class A mecA gene complex maintains the prototypic structure, mecI- mecR1-mecA-IS431mec, where mecI and mecR1 encode a repressor and a signal transducer of beta-lactam-induced mecA gene transcription, respectively (11, 16, 27, 31). In the other classes, the mecI and mecR1 genes are either partially or completely deleted in presumable association with an integrated copy of IS1272 (class B) or IS431 (class C). The class D complex found in Staphylococcus caprae JA186 has a deleted mecR1, but adjacent to the deletion point neither IS1272 nor IS431 was identified. The region abutting the deletion point of ΔmecR1 was identical to a stretch of sequences in the type II SCCmec element in the region distant from mecA gene complex, and we speculate that a homologous recombination might have caused the deletion (21). Type I SCCmec carries a type 1 ccr (ccr-1) and a class B mecA gene complex, type II SCCmec carries a type 2 ccr (ccr-2) and a class A mecA gene complex, type III SCCmec carries a type 3 ccr (ccr-3) and a class A mecA gene complex, and type IV carries a type 2 ccr (ccr-2) and a class B mecA gene complex. Among the three types of ccr genes, only the ccrB1 gene of type I SCCmec is inactive because of a frame-shift mutation (thus, the gene is generally designated ccrB1∗). Because both ccrA and ccrB genes are required for the excision and integration events of SCCmec (20), the ccrB1 gene must have been intact prior to its integration into S. aureus chromosome (18). However, the search for the intact ccrB1 in MRSA strains as well as in strains of methicillin-resistant coagulase-negative staphylococci (C-NS) has been unsuccessful (20).
Since the class B mecA gene complex found in MRSA strains contains a truncated copy of IS1272 that is prevalent in Staphylococcus haemolyticus but not in S. aureus (2, 4), Archer et al. (2) and others (44, 48) proposed that the direction of interspecies transfer of the mecA gene complex is from C-NS to S. aureus. Various types of SCCmec are indeed widely distributed among staphylococcal species other than S. aureus. SCCmec elements are typically present in methicillin-resistant C-NS, and we have observed several methicillin-susceptible strains that harbor staphylococcal cassette chromosome (SCC)-like elements on their chromosomes. Structural comparison of extant SCCmec elements revealed that SCCmec is made up of two mutually independent genetic components (the ccr and mecA gene complexes) (18). We hypothesized, therefore, the existence of a family of mobile genetic elements (SCCs) that serve for interspecies transfer of any useful genetic information among staphylococcal species and that SCCmec is one of the SCCs that is specialized for the transfer of methicillin resistance (17). In this study, in order to prove this hypothesis, we looked for a staphylococcal strain carrying ccr genes but lacking the mecA gene. By screening methicillin-susceptible staphylococcus type strains, we identified a type I SCC element that carries an intact set of type 1 ccr genes (ccrA1 and ccrB1) but no mecA gene in the genome of Staphylococcus hominis type strain GIFU12263.
MATERIALS AND METHODS
Bacteria and growth conditions.
The S. hominis type strain GIFU12263 (equivalent to ATCC 27844) used in this study was a kind gift from Takayuki Ezaki (22). Bacteria were cultivated in brain heart infusion (BHI) broth as described previously (20). The antibiotics tetracycline (Sigma Chemical Co., St. Louis, Mo.) and ceftizoxime (Fujisawa, Pharmaceutical Co. Ltd., Osaka, Japan) were used at concentrations of 10 and 25 μg/ml, respectively. MRSA strain 85/1940 carrying a type I SCCmec and susceptible to tetracycline was used for the transformation experiment (10).
DNA preparation, PCR amplification, and nucleotide sequencing.
The DNA extraction and PCR, long-range PCR, and nested PCR amplification steps were performed as previously described (17, 20). To detect ccr genes among staphylococcus type strains, we carried out PCR amplifications with a set of primers (αc and βc) that we designed by using the sequences common to the three ccr genes. The ccr genes and their surrounding chromosomal regions were amplified by long-range PCR (20), using seven primers (see Fig. 2b), followed by nucleotide sequence determination. The seven primers were designed based on the nucleotide sequences of type I SCCmec in strain NCTT10442 (DDBJ/EMBL/GenBank accession no. AB033763 [see reference 18]). By an inverse PCR (IPCR) procedure, DNA fragments corresponding to the regions upstream and downstream of the ccr genes were amplified, and the products were then sequenced by primer walking. The experimental procedure for IPCR was as follows. The chromosomal DNA of GIFU12263 was digested with EcoRI for cloning the right side and with HindIII for cloning the left side of the SCC12263 element (Takara Shuzo Co., Ltd., Shiga, Japan). Self-ligation of the EcoRI- and the HindIII-digested DNA was performed (DNA concentrations of 1 to 2 μg/ml) for 10 min with a ligation kit (Boehringer Mannheim Biochemica, Mannheim, Germany). The ligation mixtures were then used as the template DNAs for PCR amplification. The primer sets were hR-2 and hR-3 for the EcoRI self-ligation and hL-2 and hL-3 for the HindIII self-ligation (see Fig. 2b). Sequence analysis was performed with the big dye terminator cycle sequencing kit (Applied Biosystem Inc., Foster City, Calif.) on a DNA analyzer (model 3100; Applied Biosystem Inc.). The nested PCR procedure was performed as described previously (17). We used two sets of primers: hL1 and hR1 for the first round of PCR amplification and hL-4 and hR4 for the second round of PCR amplification. All primers used in this study are listed in Table 1.
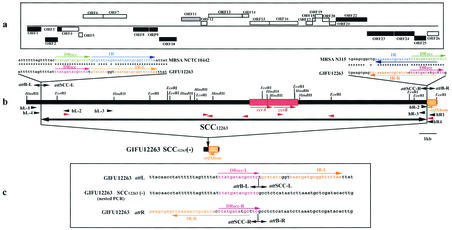
FIG. 2.
Genomic structure of SCC12263. (a) ORFs in and around SCC12263. The ORFs corresponding to sequences more than 90 amino acids in size and twenty-six possible reading frames are indicated by squares. Those above and those below the line are the ORFs whose transcription is directed to the right and those whose transcription is directed to the left, respectively. The white squares indicate the ORFs that were conserved among three SCCmec elements. The black squares indicate the ORFs that showed no identity to the four SCCmec elements. The gray square indicates the ORF with identity to type II SCCmec. (b) The essential structure of SCC12263. Locations of the essential genes are illustrated. The only restriction sites indicated are those of of HindIII and EcoRI. orfXhom is shown in orange, the ccr1 genes are shown in red, and the arrow indicates the direction of transcription. Nucleotide sequences of the left and right boundaries of attL and attR in SCC12263 were aligned with those of attL in NCTC10442 and attR in N315, respectively. The orange arrows indicate inverted complementary repeats, IR-L and IR-R, at both extremities of SCC12263, and the blue arrows indicate those of MRSA. The direct repeats in SCC12263 are indicated by magenta arrows, and those of MRSA are indicated by green arrows. The sequences that are colored are identical to each other. (c) Boundary of SCC12263. Nucleotide sequences around the attL and attR, respectively, are aligned with the sequence of the presumptive integration site, attB, on the GIFU12263 SCC12263(−) chromosome. The orange arrows indicate inverted complementary repeats, IR-L and IR-R, at both extremities of SCC12263. The red arrows indicate direct repeats. The orange and red sequences are identical to each other.
TABLE 1.
Synthetic oligonucleotide primers
| Genetic element(s) and primer designation | Sequencesa | Nucleotide positions | Source or reference |
|---|---|---|---|
| ccr genesb | |||
| βc(β2) | 5′-ATTGCCTTGATAATAGCCTTCT-3′ | 25987-25967 | Okuma et al. (35) |
| αc | 5′-ATCTATTTCAAAAATGAACCA-3′ | 25424-25444 | Okuma et al. (35) |
| SCC12263c | |||
| hL-1 | 5′-TCACCTTCACATACTTTTCTCA-3′ | 12-33 | This study |
| hL-2 | 5′-TCGTATGCGGTATAACCTCCGTCT-3′ | 3361-3338 | This study |
| hL-3 | 5′-ACCTTTATCGATAAACTCAT-3′ | 4851-4870 | This study |
| hL-4 | 5′-AAGTTCCTTCTTGTGTAAATGGA-3′ | 507-529 | This study |
| hR-1 | 5′-ATGAAGATTACTATTTTAGCT-3′ | 22820-22800 | This study |
| hR-2 | 5′-TTACTTTGCTTCCAATCTATTAAT-3′ | 22140-22163 | This study |
| hR-3 | 5′-ATGTAGAATATAAAGAAGGCAA-3′ | 21795-21774 | This study |
| hR-4 | 5′-AACGTATGACACAAGGACAAA-3′ | 22540-22520 | This study |
| Hom1 | 5′-AAAAGGATCCTTGAAGAAATTAATCATGCTGA-3′ | 11940-11951 | This study |
| Hom2 | 5′-AAAAGGATCCTCATTGTTAGATAGTTGGAATA-3′ | 15584-15563 | This study |
Computer analysis of nucleotide and protein sequences.
An initial set of ORFs likely to encode proteins was identified with the GAMBLER computer program, and those shorter than 30 codons were eliminated. ORF prediction and gene homology search and identification had been reported by Kuroda et al. previously (26). A total of 26 ORFs including ccr genes were compared to those of three types of SCCmecs (type I in strain NCTT10442 [DDBJ/EMBL/GenBank accession no. AB033763], type II in strain N315 [DDBJ/EMBL/GenBank accession no. D86934], and type III in strain 85/2082 [DDBJ/EMBL/GenBank accession no. AB037671] [see reference 18]). The homologies between the nucleotide sequences of SCC12263 and SCCmec types I, II, III, and IVa in strain CA05 (DDBJ/EMBL/GenBank accession no. AB063172) and IVb in strain 8/6-3P (DDBJ/EMBL/GenBank accession no. AB063173) (29) were studied as follows. First, the homologous regions were computed using b12seq, i.e., BLAST analysis for two sequences (45), for each pair of sequences. Then, the BLAST results were processed by a script written in Perl (Larry Wall, script in Perl, version 5, 1999, available at website http://www.perl.com/), and visualized with commercial software (Mathematica, version 4, 1999; Wolfram Research, Inc. Champaign, Ill.).
Finally, major matching regions were extracted by combining neighboring shorter matching regions as follows. First, for a pair of nucleotide sequences of SCCs, fragments of homologous regions were enumerated by using bl2seq (45). Second, neighboring fragments were connected to each other to form homologous regions. For this, for each segment A, a segment B was defined to be in its right neighbor if (i) the segments were matches in the same direction; (ii) the left end point of B was to the right of the right end point of A, but within 1,000 bp; (iii) the vertical distance between the two parallel lines including A and B, respectively, was at most 100 bp; and (iv) among such segments, B had the closest left end point to the right end point of A. The distance between end points was measured as the sum of the difference between the horizontal and vertical axes. Finally, maximal collections of neighboring segments with slopes between the two very end points determined to be between −1.1 and −0.9 or between 0.9 and 1.1 were chosen as homologous regions.
Several types of BLAST analyses were carried out with software available at the NCBI site (http://www3.ncbi.nlm.nih.gov/BLAST/). TransQ analysis was carried out with software available at brown.ims.u-tokyo.ac.jp:8118/html/transq.html.
Construction of recombinant plasmid and excision assay.
Two recombinant plasmids, pSR1hom and pSR1aur, harboring ccrA and ccrB genes of GIFU12263 and NCTC10442, respectively, were constructed by cloning the BamHI-digested DNA fragments containing ccr genes into the unique BamHI site of plasmid vector pYT3 (7), as described previously (20). The two primers Hom1 and Hom2 were used for the amplification and cloning of the ccrAB gene complexes from the chromosomal DNAs of the above-mentioned strains. The fidelity of the nucleotide sequences of the ccr genes harbored by the two plasmids pSR1hom and pSR1aur was confirmed before use. Electroporation was performed as described previously (20).
Nucleotide sequence accession number.
The sequence of SCC12263 of strain GIFU12263 has been assigned DDBJ/EMBL/GenBank accession number AB063171.
RESULTS
Identification of ccr genes among methicillin-susceptible Staphylococcus type strains.
Using primers common to three types of ccr genes, we performed PCR amplification, with DNAs extracted from 27 C-NS type strains as the templates (18). Positive PCR results were obtained with S. hominis type strain GIFU12263 (38), Staphylococcus arlettae GIFU10765, and Staphylococcus auricularis GIFU10395. The 0.5-kb amplified PCR fragment from S. hominis type strain GIFU12263 was sequenced and compared with three types of ccr gene in an S. aureus strain. The partial nucleotide sequences of ccr genes in the S. hominis type strain were identical to the corresponding regions of type 1 ccr genes. Then the whole nucleotide sequences of the ccr genes were determined, and the deduced amino acid sequences were compared to those of ccr1∗ of NCTC10442 (Fig. 1a). The Ccr proteins encoded by the ccr genes in S. hominis GIFU12263 were highly basic proteins with 99 and 84% amino acid identities to CcrA1 and CcrB1* of NCTC10442, respectively. For the sake of comparison, we reconstituted the putative intact CcrB1 polypeptide of NCTC10442 by adding back an adenine to the deletion point of the mutated ΨccrB1 gene (Fig. 1a). The N-terminal domain of about 20 residues, containing presumptive catalytic active sites of CcrA and CcrB (6, 18, 19, 21), were well conserved between the two polypeptides.
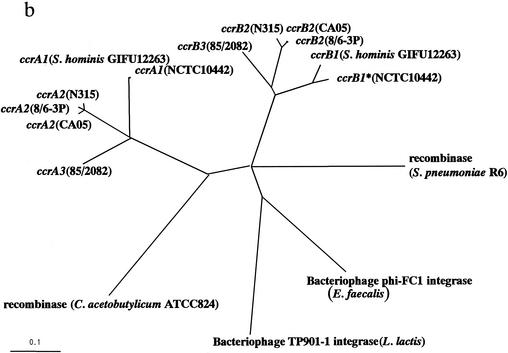
FIG. 1.
(a) The deduced amino acid sequences of CcrB1 of GIFU 12263 S. hominis were aligned with those of NCTC 10442 MRSA. A black arrowhead indicates the locus of the first amino acid residue changed by an addition of adenine in the nucleotide sequence of ccrB1∗ as described in the text. (b) Phylogenetic relationships among ccrA genes, ccrB genes, and four site-specific recombinases. The four site-specific recombinases that show high similarity to ccr genes and that were selected to investigate phylogenetic relationships are the integrase (int) of bacteriophage phi-FC1 found in E. faecalis (1,216 bp; DDBJ/EMBL/GenBank accession no. AF124258), the integrase of bacteriophage TP901-1 found in L. lactis (1,458 bp; DDBJ/EMBL/GenBank accession no. X85213), the site-specific recombinase found in C. acetobutylicum ATCC 824 (1,635 bp; DDBJ/EMBL/GenBank accession no. AE007636), and the site-specific recombinase found in S. pneumoniae R6 (1,680 bp; DDBJ/EMBL/GenBank accession no. AE008466). The nucleotide sequences of six ccrA genes, six ccrB genes, and four site-specific recombinases were aligned by using the PileUp program with a Genetics Computer Group default scoring matrix. Phylogenetic relationships were examined with the Paupsearch program by the neighbor-joining method by creating 1,000 bootstrap replicates and by selecting 70% of consensus. The tree was visualized with Tree View software, which was obtained from the Tree View website (http://taxonomy.zoology.gla.ac.uk/rod/treeview.htm). The branch length indicates the distance, which is expressed as the number of substitutions per 100 bases. (c) The alignment of deduced amino acid sequences between OrfXaur representative from NCTC 10442 MRSA strain and OrfXhom in GIFU12263.
To obtain a further insight about the evolutionary relationship among ccr1 genes and some related site-specific recombinases in the databases, we constructed a phylogenetic tree with CcrA and CcrB proteins, the integrases of Enterococcus faecalis bacteriophage phi-FC1 and Lactococcus lactis bacteriophage TP901-1 (5), and site-specific recombinases of Clostridium acetobutylicum ATCC 824 (34) and Streptococcus pneumoniae (15), all of which have substantial amino acid similarities to the Ccr recombinases (Fig. 1b). Figure 1 clearly shows that GIFU12263 Ccr and NCTC10442 Ccr constitute the type 1 Ccr subfamily.
S. hominis ccr genes are carried by an element similar to SCCmec.
Figure 2 shows the S. hominis chromosomal region around the ccr genes bounded by the characteristic direct repeats (DRscc) of 14 bases (with 12 identical bases) found 11.3 kb upstream and 7.3 kb downstream of ccr genes, (Fig. 2b and c). Incomplete inverted repeats of 28 bases, IR-L (left) and IR-R (right), also found in typical SCCmec elements were localized at both extremities. The region designated SCC12263 was 21,589 bp in length and contained a total of 26 ORFs whose characterization is given in Table 2. Furthermore, the homologue of S. aureus orfXaur, designated orfXhom, was identified to the right of IR-R. orfX is an ORF of unknown function located near the origin of replication of the S. aureus chromosome, which contains the integration site (attBscc) of SCCmec (17, 26). The orfXhom was 82% identical to orfXaur in nucleotide sequences, and their deduced amino acid sequences were 91% identical to each other (Fig. 1c).
TABLE 2.
ORFs in and around S. hominis SCC12263 with deduced products showing similarities to extant proteins
| ORFa | Value for CDSb
|
Gene | Product | Data for homologue in the database
|
NCTC10442
|
Data indicating homology to ORF of straina: N315
|
85/2082
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starting nucleotide | Ending nucleotide | Size (bp) | Length (aa) | % Identityc | Description of gene product (size [bp])d | % Identityc | Corresponding ORF (size [bp]) | % Identityc | Corresponding ORF (size [bp]) | % Identityc | Corresponding ORF (size [bp]) | |||
| (ORF1) | 708 | 52 | 657 | 218 | Hypothetical protein | 40 | Hypothetical protein, i.e., partial ORF 59 (216) of Staphylococcus aureus bacteriophage phl PVL | |||||||
| ORF2 | 1519 | 974 | 546 | 181 | Hypothetical protein | 52 | Hypothetical protein YdhK (205) of Bacillus subtilis | |||||||
| ORF3∗ | 1716 | 1537 | 180 | 59 | Hypothetical protein | 83 | Partial copper-transporting ATPase CopB (745) of Enterococcus hirae | |||||||
| ORF4 | 2676 | 1918 | 759 | 252 | Hypothetical protein | 92 | CE014 (252) | 71 | CN030 (21) | 71 | CZ021 (214) | |||
| ORF5 | 2915 | 2673 | 243 | 80 | Hypothetical protein | 97 | CE015 (80) | 88 | CN031 (88) | 88 | CZ022 (88) | |||
| ORF6 | 3072 | 4145 | 1074 | 357 | Hypothetical protein | RI | 95 | E023 (355) | 69 | N052 (354) | 69 | Z024 (354) | ||
| ORF7 | 4164 | 5492 | 1329 | 442 | Hypothetical protein | RI | 96 | E024 (442) | 74 | N054 (131) | 74 | Z025 (286) | ||
| ORF8 | 6744 | 5815 | 930 | 309 | Hypothetical protein | 68 | N053 (287) | 68 | Z026 (396) | |||||
| ORF9 | 7230 | 6760 | 471 | 156 | Hypothetical protein | |||||||||
| ORF10 | 8239 | 7319 | 921 | 306 | Hypothetical protein | |||||||||
| ORF11 | 8585 | 9610 | 1026 | 341 | Hypothetical protein | 54 | N029 (348) | |||||||
| ORF12 | 9802 | 10098 | 297 | 98 | Hypothetical protein | 91 | E025 (98) | 77 | N030 (98) | 53 | Z003 (95) | |||
| ORF13 | 10098 | 11867 | 1770 | 589 | Hypothetical protein | 98 | E026 (589) | 75 | N031 (597) | 65 | Z004 (522) | |||
| ORF14 | 11937 | 12152 | 216 | 71 | Hypothetical protein | 98 | E027 (70) | 50 | N033 (61) | 55 | Z008 (70) | |||
| ORF15 | 12055 | 13404 | 1350 | 449 | ccrA1 | Cassette chromosome recombinase A1 | 99 | ccrA1 (449) | 78 | ccrA2 (448) | 78 | ccrA3 (448) | ||
| ORF16 | 13426 | 15054 | 1629 | 542 | ccrB1 | Cassette chromosome recombinase B1 | 84 | ccrB1 (542) | 80 | ccrB2 (542) | 85 | ccrB3 (542) | ||
| ORF17 | 15520 | 15870 | 351 | 116 | Hypothetical protein | 93 | E031 (116) | 87 | N0410 (116) | 53 | Z011 (116) | |||
| ORF18 | 15863 | 15955 | 93 | 30 | Hypothetical protein | |||||||||
| ORF19 | 15957 | 16268 | 312 | 103 | Hypothetical protein | 91 | E032 (108) | 97 | N042 (103) | 48 | Z013 (131) | |||
| ORF20∗ | 16285 | 16518 | 234 | 77 | Hypothetical protein | 90 | E033 (169) | 90 | N043 (168) | 63 | Z014 (173) | |||
| ORF21∗ | 16583 | 16789 | 207 | 68 | Hypothetical protein | 92 | E033 (169) | 95 | N043 (168) | 58 | Z014 (173) | |||
| ORF22 | 17135 | 19078 | 1944 | 647 | M.StsI | Modification methylase | 58 | StsI methylase (653) of Streptococcus sanguis | ||||||
| ORF23 | 20489 | 19086 | 1404 | 467 | Hypothetical protein | |||||||||
| ORF24 | 21398 | 20523 | 876 | 291 | Hypothetical protein | |||||||||
| ORF25 | 22105 | 21470 | 636 | 211 | 5′-Methylcytosine-specific restriction enzyme | 35 | 5′-Methylcytosine-specific restriction enzyme A of Methanosarcima mazei | |||||||
| (ORF26) | 22820 | 22341 | 480 | 159 | orfXhom | Conserved hypothetical protein OrfX | 91 | orfX (159) | 91 | orfX (159) | 91 | orfX (159) | ||
ORFs shown in parentheses were located outside of SCC12263. Incomplete ORFs that are potentially defective genes or pseudogenes containing frame-shift mutations are annotated with asterisks.
Nucleotide positions given are from the nucleotide sequence deposited under DDBJ/EMBL/GenBank accession no. AB063171, and they were measured in the 5′ (starting nucleotide) to 3′ (ending nucleotide) direction. CDS, coding sequence.
Identity to the amino acid sequence of the best match revealed in homology search of the GenBank and EMBL databases with TFastA.
Gene product sizes are numbers of amino acids. PVL, Panton-Valentine leukocidin; RI, region I.
The ORFs similar to those of SCCmec elements (amino acid identities between the corresponding ORFs were equal to or greater than 48%) are shown in Fig. 2a. The ccr gene complex (composed of ccrA and ccrB genes plus surrounding ORFs) was also well conserved in SCC12263. Four ORFs (orf4 to -7) located near the left extremity of the element were well conserved among SCC12263 and three types of SCCmec. The 3.5-kb region, called R-I (17), containing orf6 and -7, was also well conserved among three types of SCCmec. The ORF11 shown in Fig. 2 had homology to ORFN029 of the type II SCCmec (17).
Certain ORFs shown in Fig. 2 were specific to SCC12263. However, most of them did not show homology with any of the extant genes (see “Cluster of restriction-modification enzyme gene homologues in right end of SCC12263”). It was also significant that there was no antibiotic resistance gene or mobile element found in SCC12263. The G+C content of SCC12263 was 29.52%, which, though not in itself significant, was lower than the range of G+C content reported for species S. hominis (30 to 36% [38]). There were nine regions (eight ORFs within SCC12263 and one ORF outside SCC12263) having substantially deviant G+C content values (under 29%). The most notable examples were orf22 (StsI methylase [M.StsI] homologue), orf23, and orf25, whose G+C contents were 23.77, 23.93, and 24.21%, respectively.
Further analysis of GC3, the G+C content of the third codon letter, whose deviation from the average value of the entire chromosome may be associated with genes acquired by horizontal transfer (26), revealed GC3 values lower than 24% for orf2 to -11 and -22 to -25. Especially low were the values of orf22 to -25, which were 13 to 15%. It was also noted that orfXhom, shown as ORF26, had a significantly high G+C content of 37.5%, as in the case with orfXaur in MRSA strains (39.41% [see reference 17]).
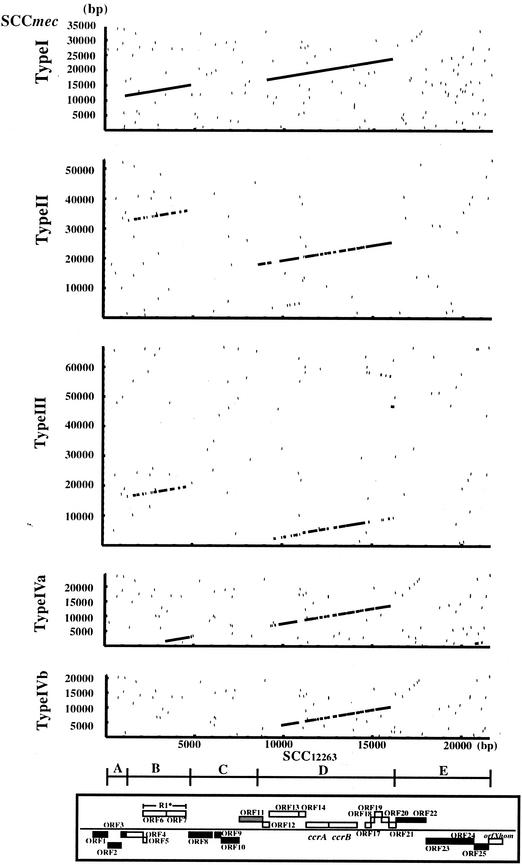
Figure 3 compares nucleotide sequences of SCC12263 and SCCmec types I, II, III, IVa, and IVb. Two regions, B (from nucleotide positions 1,117 to 4,890) and D (from nucleotide positions 8,600 to 16,198), of SCC12263 were found to be highly homologous to the extant SCCmecs. Another three regions, A (from nucleotide positions 1 to 1,116), C (from nucleotide positions 4,891 to 8,599), and E (from nucleotide positions 16,199 to 21,589), did not show significant homology. The extents of homology of the B and D regions with the corresponding regions of the SCCmec elements are indicated in Table 3. Region D contained orf11 to -19 that corresponded to the ccr gene complex (18). On the other hand, region B encompassed orf2 to -7, whose functions are unknown.
FIG.3.
Homologous regions of nucleotide sequences of SCC12263 and SCCmec type I, II, III, IVa, and IVb and their identities. The nucleotide positions are relative to the SCCs. The SCC region of SCC12263 corresponds to the region from nucleotide 770 to 22358 in GIFU12263, the type I SCCmec corresponds to the region from nucleotide 4504 to 38867 in AB033763, the type II SCCmec corresponds to the region from nucleotide 4687 to 57653 in D86934, the type III SCCmec corresponds to the region from nucleotide 899 to 67794 in AB037671, the type IVa SCCmec corresponds to the region from nucleotide 975 to 25222 in AB063172, and type IVb SCCmec corresponds to the region from nucleotide 301 to 21219 in AB063173. The regions A through E (see text for description of location) and how they correspond to the structure presented in Fig. 2a are shown at the bottom.
TABLE 3.
Corresponding regions of SCC12263 and SCCmeca
| SCCmec type | Region B
|
Region D
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCCmec
|
SCC12263
|
% Identity | SCCmec
|
SCC12263
|
% Identity | |||||||||
| Starting nucleotide | Ending nucleotide | Length (nt) | Starting nucleotide | Ending nucleotide | Length (nt) | Starting nucleotide | Ending nucleotide | Length (nt) | Starting nucleotide | Ending nucleotide | Length (nt) | |||
| Type I | 11508 | 15164 | 3,657 | 1117 | 4787 | 3,671 | 96 | 16921 | 23914 | 6,994 | 9018 | 16018 | 7,001 | 96 |
| Type II | 32763 | 36040 | 3,278 | 1369 | 4644 | 3,276 | 73 | 17968 | 25508 | 7,541 | 8600 | 16017 | 7,418 | 80 |
| Type III | 16364 | 19641 | 3,278 | 1369 | 4644 | 3,276 | 73 | 2395 | 9434 | 7,040 | 9542 | 16198 | 6,657 | 72 |
| Type IVa | 1617 | 3110 | 1,494 | 3411 | 4890 | 1,480 | 97 | 6870 | 13699 | 6,830 | 9306 | 16018 | 6,713 | 79 |
| Type IVbb | 4109 | 10371 | 6,263 | 9876 | 16018 | 6,143 | 80 | |||||||
Nucleotide positions were measured in the 5′ (starting nucleotide) to 3′ (ending nucleotide) direction.
For region B of this SCCmec type, no corresponding sequence was found.
Both regions B and D had the highest similarity (96% identical) to the corresponding regions of type I SCCmec, indicating that not only the ccr gene complex, but also at least one other region of the SCC12263 element, is phylogenetically associated with type 1 SCC mec.
It was also remarkable that the regions similar to B and D regions were located in reverse order in type II and III SCCmec. Furthermore, the region B was very similar to the corresponding region of type IVa (97% identity), whereas the region D was not (79%) (Table 3). These observations suggest that recombination occurred between the two regions during molecular diversification of the SCC elements. Phylogenic relationship was inferred by computing multiple alignments of the core homologous regions, shared by five SCCs for region B and six SCCs for region D (data not shown). The two phylogenetic trees CcrA and CcrB computed as described above were different from each other. As mentioned above, the GC3 of the ORFs in the two regions of SCC12263 was also discrepant: low in region B but not in region D. Therefore even SCC12263 might have been formed by recombination between two evolutionary unrelated DNA fragments. A very weakly homologous region was observed between the rightmost part of type III SCCmec and the region from 14.6 to 16.0 kb of SCC12263 in reverse direction. Another one is between the leftmost part of type VIa SCCmec and the region from 20.7 to 21.2 kb of SCC12263. We know that the rightmost part of the type III SCCmec contains a pseudo-ccr gene complex containing a ccrA2-like gene (18). However, because of the paucity of homology between ccrA gene of the SCC12263 (which should be classified as a ccrA1 gene) and a degenerate ccrA2-like gene, no apparent homology line was visualized with the set for the computation. Regions A, C, and E of SCC12263 did not have corresponding regions in four SCCmec types. However, the nucleotide sequence from positions 771 to 1474 in region A showed significant homology (98% identical) to that in the chromosome of S. epidermidis strain RP62A. Several restriction-modification enzyme gene homologues were identified in region E.
Cluster of restriction-modification enzyme gene homologues in right end of SCC12263.
Several nucleotide sequences homologous to restriction-modification genes were identified in region E of SCC12263. ORF22 (the deduced peptide encoded by orf22) showed high homology to type IIS modification enzymes, M.StsI (the DNA methyltransferase of the StsI restriction-modification system) and M.FokI, that recognize an asymmetric sequence 5′-GGATG-3′ and generate 6-mA on both the strands (23, 24). A motif search with pfam indicated that ORF22 carries two alpha-type domains (motif I [AdoMet binding]-target recognition domain [TRD]-motif IV [DPPY]) in tandem. As for M.FokI (41), this putative protein appears to be formed by fusion of two methyl transferase proteins, each methylating one strand of the recognition sequence.
In the upstream of ORF22, there was a 174-bp nucleotide stretch homologous to type III SCCmec in strain 85/2082. TransQ search revealed weak similarity of this sequence stretch with that encoding a part of HgaI, a type IIS restriction enzyme that recognizes 5′-GACGC-3′ (40). Its two cognate modification enzymes methylate its internal C5 on the two strands (42). This region may represent a remnant of a type IIS restriction enzyme gene. We cannot conclude whether this was part of a once-active gene forming a restriction-modification system with orf22 or not. The carboxy end of ORF25 showed homology with several restriction enzymes (by TransQ and PSI-BLAST analyses). These included McrA, a C5 methyl-specific endonuclease; ml17993 in Mesorhizobium loti found adjacent to a DNA methylase homologue; and HP0052, a gene in a cluster of restriction-modification homologues in Helicobacter pylori (strain 26695) (1). A BLASTx search with ORF23 as a query criterion revealed weak homology with a putative NTPase/helicase of a virus (gi|9631396|ret|NP_048295.1|) and a gene in Sulfolobus solfataricus (gi|13815496|gb|AE006826.1|AE006826). PSI-BLAST analysis detected similarity of the Sulfolobus gene with SAM-dependent O-methyltransferases (eg., gi:13399467) (49). It is not clear whether ORF23 represents a remnant of a novel type of DNA methyltransferase that is homologous to O-methyltransferases.
Spontaneous precise excision of SCC12263.
To test whether SCC12263 behaves like SCCmec as a mobile element, PCR experiments were designed to monitor spontaneous precise excision of SCC12263 occurring in the attL and attR regions. The two combinations of primers, hL-1 and hR-1 and hL-4 and hR-4, were used for the first and second rounds of PCR, as described previously (17). The primers hR-1 and hR-4 were located in orfXhom, and hL-1 and hL-4 were located in the chromosomal region outside the left boundary of SCC12263 (Fig. 2b). It has been reported that the sensitivity of the first round of PCR was such that it could detect attBscc sequence generated in 1 in 10,000 cells of the culture by precise excision of SCC element (17). At this level of sensitivity, no amplified band was detected when GIFU12263 DNA was used as a template for PCR. However, the second round of PCR (nested PCR) did amplify a 0.8-kb DNA fragment. The DNA fragment was purified and was subjected to nucleotide sequence determination. By comparing it with the nucleotide sequences of chromosome-SCC junction sequences, we could confirm occurrence of precise excision of the SCC and infer the precise cutting site as shown in Fig. 2. The result indicated that spontaneous precise excision of SCC12263 presumably mediated by ccr genes occurred in S. hominis strain GIFU12263, though at a low frequency of less than 1 in 104 cells (17).
ccr genes from SCC12263 promote excision of SCCmec.
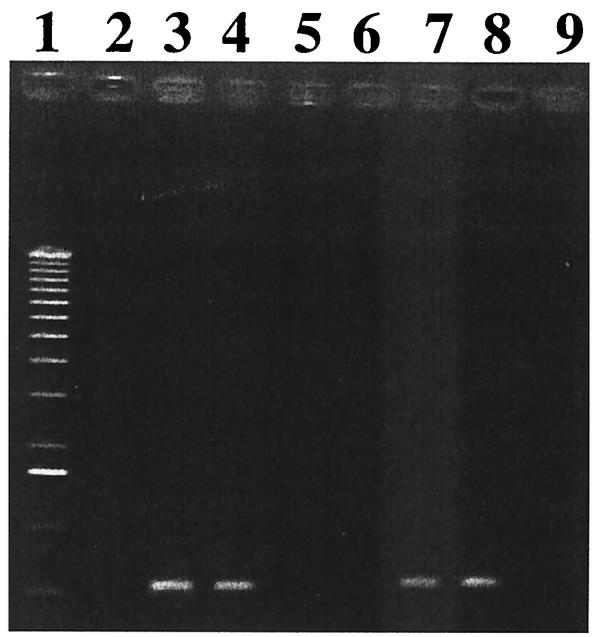
In order to examine if the ccr genes on SCC12263 encode active recombination or not, the ccrA1 and ccrB1 genes of S. hominis GIFU12263 and S. aureus NCTC 10442 were cloned into a shuttle vector, pYT3 (7). The recombinant plasmids obtained were pSR1hom and pSR1aur, respectively. Four plasmids; pYT3 (plasmid vector), pSR1hom, pSR1aur, and pSR2 (pYT3 harboring ccr2 genes was formerly designated pSR [20]) were introduced by electroporation into strain 85/1940, which has a type I SCCmec with a mutated ccrB1 gene that is indistinguishable from that of NCTC10442 (18), followed by selection of transformants on the tetracycline plates. (The reason that we did not use NCTC10442, a representative MRSA strain carrying type I SCCmec (17), was that the strain was tetracycline resistant). The strains 85/1940, 85/1940(pYT3), 85/1940(pSR2), 85/1940(pSR1hom), and 85/1940(pSR1aur) were then cultivated in drug-free broth for 9 days at 30°C with one passage per day, after which the proportion of the cells that had lost SCCmec was evaluated by replicating them onto agar plates with and without ceftizoxime. On the 9th day, the cultures of strains 85/1940, 85/1940(pYT3), and 85/1940(pSR1aur) yielded essentially equal numbers of colonies on the BHI agar plates with or without ceftizoxime (Table 4). On the other hand, the 9 days passage of the strains 85/1940(pSR2) and 85/940(pSR1hom) generated ceftizoxime-susceptible cells, which constituted 6 and 4% of the entire cell population, respectively (Table 4). This demonstrates that the ccrB1 of the S. hominis strain were active in excising the type I SCC mec. To determine the site of excision mediated by the ccr genes of the S. hominis SCC12263, we amplified the attBscc region on the chromosome in 85/1940(pSR1hom) and N315(pSR1hom) by PCR with cR2 and cL3 as the primers (17). As controls, we used the chromosomes of 85/1940, 85/1940(pSR2), 85/1940(pSR1aur), N315, N315(pSR2), and N315(pSR1aur) strains. All strains were cultivated in drug-free broth for 18 h, and then their chromosomal DNAs were extracted. Figure 4 shows that bands were amplified with 85/1940(pSR2), 85/1940(pSR1hom), N315(pSR2), and N315(pSR1hom), but not with 85/1940(pSR1aur) or N315(pSR1aur). The DNA fragments were purified and were subjected to nucleotide sequence determination, and we compared the sequences with those of 85/1940 and N315. The nucleotide sequences in 85/1940(pSR1hom) and N315(pSR1hom) were identical to those in 85/1940(pSR2) and N315(pSR2), respectively. The identity of the excision mediated by pSR2 and pSR1hom indicated that the ccr genes on SCC12263 encoded the recombinases of the same specificities of those of the extant ccr gene products of type I and type II SCC mec.
TABLE 4.
Excision of SCCmec mediated by plasmids carrying ccr genes
| Straina | Day of passage | No. of CFU onb:
|
No. of CZX-resistant colonies (%)c | ||
|---|---|---|---|---|---|
| Master plates | TET plates | TET/CZX plates | |||
| 85/1940(pYT3) | 1 | 159 | 159 | 159 | 100 |
| 9 | 278 | 278 | 278 | 100 | |
| 85/1940(pSR2) | 1 | 188 | 188 | 187 | 99.4 |
| 9 | 188 | 188 | 12 | 6.38 | |
| 85/1940(pSR1hom) | 1 | 218 | 218 | 215 | 98.6 |
| 9 | 294 | 294 | 12 | 4.08 | |
| 85/1940(pSR1aur) | 1 | 225 | 225 | 225 | 100 |
| 9 | 224 | 224 | 224 | 100 | |
Plasmids (pSR2, pSR1hom, pSR1aur, and pYT3) that carried ccr genes from N315, the S. hominis strain, NCTC 10442, and none, respectively, were introduced into strain 85/1940 (SCCmec type I).
Total number of CFU on three plates. Abbreviations: TET, tetracycline; CZX, ceftizoxime.
Values are percentages calculated by the following formula: [(number of CFU on the TET- and CZX-supplemented plates) ÷ number of CFU on the TET-supplemented plates] × 100.
FIG. 4.
PCR for detection of attBscc region on the chromosome. Amplification was carried out with a set of primers cL3 and cR2 specific for the regions to the left and right of the chromosome-SCCmec junction point. Lanes: 1, 1-kb ladder as a molecular weight marker; 2, N315; 3, N315(pSR2); 4, N315(pSR1hom); 5, N315(pSR1aur); 6, 85/1940; 7, 85/1940(pSR2); 8, 85/1940(pSR1hom); 9, 85/1940(pSR1aur).
DISCUSSION
The SCC12263 carried several homologues of restriction-modification genes. Among them, orf22 may encode an active type IIS DNA methyltransferase. Many kinds of mobile genetic elements, such as bacteriophages, integrons, and transposons (4, 19), etc., carry a restriction-modification gene complex (25), which can stabilize maintenance of a plasmid (32) and of genomic islands in the chromosome (8). This is because the cells that have lost the restriction-modification gene complex are supposed to be killed by the attack of the restriction enzyme at the recognition sites of the chromosome that are not protected by a sufficient amount of the methyltransferase. The restriction-modification genes identified on SCC12263 may be responsible at least partially for the stable maintenance of SCC12263 despite its carriage of active ccr genes that encode activity to precisely excise the element from the chromosome. The modification enzyme gene (orf22) appears to be orphan in the sense that it is not adjacent to a cognate restriction enzyme gene in an active form. However, its stabilization effect is expected if its cognate restriction enzyme gene in an active form is present elsewhere in the genome, as has been previously described for the maintenance of the genomic islands in S. aureus chromosome (25, 26). Another likely role of the methyltransferase is defense of SCC12263 DNA upon entry into restricting host bacteria. This role is reminiscent of the role of orphan methylase (not paired with a restriction enzyme) on bacteriophage genomes. The restriction-modification gene homologues were present in a region E that is unique to SCC12263 but lying next to the region D, which is shared by other SCC elements. This type of linkage between macroscopic genome polymorphism with restriction-modification genes has been reported for other genomes as well (1, 33), which is considered supportive of the hypothesis that some restriction-modification systems represent mobile genetic elements (25). The deviation in GC content in this area also supports this hypothesis.
This study was done as a part of our pursuit of a molecular predecessor of the SCCmec elements, which are distributed widely among clinical strains of various staphylococcal species (21). Sequencing analysis of the chromosome regions of the S. hominis strain surrounding the ccr genes identified a genomic island at the boundaries of which were found DR and IR sequences and orfXhom having high homology to orfXaur of S. aureus in which an attachment site for SCCmec is located. The structure of the boundaries indicated that the island was integrated at the attachment site attBscc found in the 3′ end of orfXhom, whose sequence was indistinguishable from the attBscc into which SCCmec is integrated site specifically. Thus, we designated the island SCC as the representative of hypothesized SCC family in which the staphylococcal mobile elements share the same structural and functional features with extant SCCmec elements (possession of ccr genes, as well as DR and IR sequences, and site-specific integration into the attBscc) except for carriage of mecA gene complex.
The SCC12263 appears to be a type I SCC element (i.e., it is part of a subgroup of the SCC family as defined by the possession of type 1 ccr gene complex). An SCC belonging to this subfamily should have served as a recipient of class B mecA gene complex in the E region to generate type I SCCmec in the past. However, since strong homologies between SCC12263 and type I SCCmec were confined only to regions B and D, SCC12263 may not be the immediate precursor SCC for the generation of type I SCC mec. It may be that the SCC family is more diverse than we have expected, and continued search may eventually identify an SCC more closely related to the precursor SCC, with a homology extending, for example, to region A as well. The precursor SCCs for type II and type III SCCmec may also be identified in C-NS strains, since some mecA-negative strains that hybridize these types of ccr genes have been identified (Y. Katayama et al., unpublished data).
Besides the structural diversities in SCC elements, there seem to be significant diversities in the biological functions which they carry. We have started to observe that SCC is not confined to the transmission of methicillin resistance alone; that it is a well-developed vehicle for genetic exchange of any useful genes among staphylococcal species is also supported by other recent observations. We have previously described a genetic element, IE25923, integrated at the attBscc in the chromosome of methicillin-susceptible S. aureus type strain ATCC 25923 (18). Although it is considered as a remnant of SCC (it lacks ccr genes), neither the mecA gene complex nor any other antibiotic resistance gene was found among dozens of the ORFs present on it (18). Even extant SCCmec contains some genes whose functions are unrelated to antibiotic resistance: e.g., type I SCCmec contains SD repeat (a repeat domain comprising mainly aspartate and serine)-rich surface proteins that interfere the host cell's capacity to bind to fibronectin and fibrinogen (47), and type II SCCmec contains KDP operons that are involved in the ATP-dependent potassium transfer (43). A recent illustrative case in this line of observation is that found in the genome of an MRSA strain MW2 the sequencing of whose whole genome revealed the presence of an SCC-like structure integrated in tandem with a copy of SCC mec, and in association with this SCC-like structure a staphylococcal enterotoxin gene seh was identified (3). This genetic organization suggests the possibility that the seh gene, which is not present at the corresponding locus in any of the other five sequenced S. aureus chromosomes, might have been acquired by SCC-mediated lateral gene transfer (3). Recently, an SCC without the mecA gene complex has also been identified in S. aureus strain M, which, designated SCCcap1, carries a cap operon instead of antibiotic resistance genes (28). Therefore, it would be quite plausible to consider SCC a ubiquitous mobile genetic element whose function in staphylococcal species is interspecies transfer of genetic information useful to the host cells living in various stressful environments. The environmental selective pressure is not limited to antibiotics alone but could be any number of things, such as competition with other microorganisms, assault from the human immune system, poor or biased nutritional resources, and even pollutants such as cadmium and mercury, against which specific resistance genes in type III SCCmec have been identified (18). In this regard, detection of SCC and identification of the genes it carries would be useful not only for us to learn a bacterium's capability to survive antibiotic chemotherapy but also to track the individual life history of the staphylococcal strain (i.e., the series of environmental stresses that the given organism has undergone).
Acknowledgments
We thank Takayuki Ezaki for the kind gift of 27 staphylococcus type strains; Susumu Ehara, Nobukazu Miyamoto, and Kanae Tsutsumimoto for their excellent technical assistance; and Makoto Kuroda for his help in operating the GAMBLER computer program and technical advice.
This work was supported by Grant-in-Aid for Scientific Research on Priority Areas (13226114) from The Ministry of Education, Science, Sports, Culture and Technology of Japan and the Core University System Exchange Programme under the Japan Society for the Promotion of Science, coordinated by the University of Tokyo Graduate School of Medicine and Mahidol University. The study was also partly supported by a Grant for International Health Cooperation Research (11C-4) from the Ministry of Health and Welfare and by the Research for the future Program of the Japan Society for the Promotion of Science. The work in IMS was supported by grants to I.K. from MEXT, NEDO, and the Uehara Memorial Foundation.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. Jonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Archer, G., J. A. Thanassi, D. M. Niemeyer, and M. J. Pucci. 1996. Characterization of IS1272, an insertion sequence-like element from Staphylococcus haemolyticus. Antimicrob. Agents Chemother. 40:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819-1827. [DOI] [PubMed] [Google Scholar]
- 4.Byrne, M. E., T. G. Littlejohn, and R. A. Skurray. 1990. Transposons and insertion sequences in the evolution of multiresistant Staphylococcus aureus, p. 1303-1311. In R. P. Novick (ed.), Molecular biology of Staphylococci. VCH Publishers, New York, N.Y.
- 5.Christiansen, B., L. Brøndsted, F. K. Vongensen, and K. Hammer. 1996. A resolvase-like protein is required for the site-specific integration of the template Lactococcal bacteriophage TP901-1. J. Bacteriol. 178:5164-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grindley, N. D. F. 2002. The movement of Tn3-like elements: transposition and cointegrate resolution, p. 272-302. In N. L. Craig, R. Craig, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. American Society for Microbiology, Washington, D.C.
- 7.Hanaki, H., K. Kuwahara-Arai, S. Boyle-Vavra, R. S. Daum, H. Labischinski, and K. Hiramatsu. 1998. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 42:199-209. [DOI] [PubMed] [Google Scholar]
- 8.Handa, N., Y. Nakayama, M. Sadykov, and I. Kobayashi. 2001. Experimental genome evolution: large-scale genome rearrangements associated with resistance to replacement of a chromosomal restriction-modification gene complex. Mol. Microbiol. 40:932-940. [DOI] [PubMed] [Google Scholar]
- 9.Hartman, B. J., and A. Tomasz. 1984. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 158:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiramatsu, K., H. Kihara, and T. Yokota. 1992. Analysis of borderline-resistant strains of methicillin-resistant Staphylococcus aureus using polymerase chain reaction. Microbiol. Immunol. 36:445-453. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., K. Asada, E. Suzuki, K. Okonogi, and T. Yokota. 1991. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA). FEBS Lett. 298:133-136. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu, K., L. Cui, M. Kuroda, and T. Ito. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 9:486-493. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu, K., Y. Katayama, H. Yuzawa, and T. Ito. 2002. Molecular genetics of methicillin-resistant Staphylococcus aureus. Int. J. Med. Microbiol. 292:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Hiramatsu, K., N. Kondo, and T. Ito. 1996. Genetic bases for molecular epidemiology of MRSA. J. Infect. Chemother. 2:117-129. [DOI] [PubMed] [Google Scholar]
- 15.Hoskins, J. A., W. Alborn, J. Arnold, L. Blaszczak, S. Burgett, B. S. DeHoff, S. Estrem, L. Fritz, J. Fu, D. W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. Kraft, R. LaGace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. McAhren, M. McHenney, K. McLeaster, K. C. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain, Z., L. Stoakes, V. Massey, D. Diagre, V. Fitzgerald, S. E. Sayed, and R. Lannigan. 2000. Correlation of oxacillin MIC with mecA gene carriage in coagulase-negative staphylococci. J. Clin. Microbiol. 38:752-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito, T., Y. Katayama, and K. Hiramatsu. 1999. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob. Agents Chemother. 43:1449-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of three types off staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, C. 2002. Bacterial site-specific DNA inversion system, p. 230-271. In N. L. Craig, R. Craig, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. American Society for Microbiology, Washington, D.C.
- 20.Katayama, Y., T. Ito, and K. Hiramatsu. 2000. A new class of genetic element, staphylococcal cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama, Y., T. Ito, and K. Hiramatsu. 2001. Genetic organization of the chromosome region surrounding mecA gene in staphylococcal clinical strains: a role of IS431-mediated mecI deletion in the resistance expression of mecA-carrying, low-level methicillin-resistant Staphylococcus haemolyticus. Antimicrob. Agents Chemother. 45:1955-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura, Y., X. G. Hou, F. Sultana, K. Hirose, M. Miyake, S. E. Shu, and T. Ezaki. 1998. Distribution of Staphylococcus species among human clinical specimens and emended description of Staphylococcus caprae. J. Clin. Microbiol. 36:2038-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kita, K., H. Kotani, H. Sugisaki, and M. Takanami. 1989. The FokI restriction-modification system I. Organization and nucleotide sequences of the restriction and modification genes. J. Biol. Chem. 264:5751-5756. [PubMed] [Google Scholar]
- 24.Kita, K., M. Suisha, H. Kotani, H. Yanase, and N. Kato. 1992. Cloning and sequence analysis of the StsI restriction-modification gene: presence of homology to FokI restriction-modification enzymes. Nucleic Acids Res. 25:4167-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi, I. 2001. Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution. Nucleic. Acids Res. 29:3742-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus, the major hospital pathogen. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 27.Kuwahara-Arai, K., N. Kondo-Hori, E. Tateda-Suzuki, and K. Hiramatsu. 1996. Suppression of methicillin resistance in a mecA-containing pre-methicillin-resistant Staphylococcus aureus strain is caused by the mecI-mediated repression of PBP2′ production. Antimicrob. Agents Chemother. 40:2680-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loung, T. T., S. Ouyang, K. Bush, and C. Y. Lee. 2002. Type 1 capsule genes of Staphylococcus aureus are carried in a staphylococcal cassette chromosome genetic element. J. Bacteriol. 184:3623-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma, X. X., T. Ito, C. Tiensasitorn, M. Jamklang, P. Chongtrakool, S. Boyle-Vavra, R. S. Daum, and K. Hiramatsu. 2002. A novel type of staphylococcal cassette chromosome mec (SCCmec) identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 46:1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuhashi, M., M. D. Song, F. Ishino, M. Wachi, M. Doi, M. Inoue, K. Ubukata, N. Yamashita, and M. Konno. 1986. Molecular cloning of the gene of a penicillin binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J. Bacteriol. 167:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinney, T. K., V. K. Sharma, W. A. Crag, and G. L. Archer. 2001. Transcription of the gene mediating methicillin resistance in Staphylococcus aureus (mecA) is corepressed but not coinduced by cognate mecA and β-lactamase regulators. J. Bacterol. 183:6862-6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naito, T., K. Kisano, and I. Kobayashi. 1995. Selfish behaviour of restriction-modification system. Science 267:897-899. [DOI] [PubMed] [Google Scholar]
- 33.Nobusato, A., I. Uchiyama, S. Ohashi, and I. Kobayashi. 2000. Insertion with long target duplication: a mechanism for restriction-modification-mediated gene mobility suggested from comparison of two complete bacterial genomes. Gene 259:99-108. [DOI] [PubMed] [Google Scholar]
- 34.Nolling, J., G. Breton, M. V. Omelchenko, K. S. Markarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira, D. C., A. Tomasz, and H. D. Lencastre. 2001. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: identification of two ancestral genetic backgrounds and the associated mec elements. Microb. Drug Resist. 7:349-361. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds, P. E., and D. F. J. Brown. 1985. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. FEBS Lett. 192:28-32. [DOI] [PubMed] [Google Scholar]
- 38.Schleifer, K. H. 1986. Gram-positive cocci, p. 999-1002. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology. Williams & Wilkins, Baltimore, Md.
- 39.Song, M. D., M. Wachi, M. Doi, F. Ishino, and M. Matsuhashi. 1987. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 221:167-171. [DOI] [PubMed] [Google Scholar]
- 40.Sugisaki, H. 1993. Nucleotide sequence of the gene of HgaI restriction endonuclease. Bull. Inst. Chem. Res. Kyoto Univ. 71:338-342. [Google Scholar]
- 41.Sugisaki, H., K. Kita, and M. Takanami. 1989. The FokI restriction-modification system II. Presence of two domains in FokI methylase responsible for modification of different DNA strands. J. Biol. Chem. 264:5757-5761. [PubMed] [Google Scholar]
- 42.Sugisaki, H., K. Yamamoto, and M. Takanami. 1991. The HgaI restriction-modification system contains two cytosine methylase genes responsible for modification of different DNA strands. J. Biol. Chem. 266:13952-13957. [PubMed] [Google Scholar]
- 43.Sugiura, A., K. Nakashima, and T. Mizuno. 1992. Clarification of the structural and functional features of the osmoregulated kdp operon of Escherichia coli. Mol. Microbiol. 6:1769-1776. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, E., K. Kuwahara-Arai, J. F. Richardson, and K. Hiramatsu. 1993. Distribution of mec regulator genes in methicillin-resistant Staphylococcus clinical strains. Antimicrob. Agents Chemother. 37:1219-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatusova, T. A., and T. L. Madden. 1999. Blast 2 sequences—a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174:247-250. [DOI] [PubMed] [Google Scholar]
- 46.Utsui, Y., and T. Yokota. 1985. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 28:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaudaux, P. E., V. Monzillo, P. Francois, D. P. Lew, T. J. Foster, and B. Berger-Bächi. 1998. Introduction of the mec element (methicillin resistance) into Staphylococcus aureus alters in vitro functional activities of fibrinogen and fibronectin adhesins. Antimicrob. Agents Chemother. 42:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, S. W., H. de Lencastre, and A. Tomasz. 1998. Genetic organization of the mecA region in methicillin-susceptible and methicillin-resistant strains of Staphylococcus sciuri. J. Bacteriol. 180:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zubieta, C., X. Z. He, R. A. Dixon, and J. P. Noel. 2001. Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nat. Struct. Biol. 8:271-279. [DOI] [PubMed] [Google Scholar]