Abstract
Alkoxy side-chain-flanked diarylsulfonamide serves as a reliable structural motif for constructing macrocyclic aromatic tetrasulfonamides. This 90° structural motif is persistent both in solution and in the solid state, which allows the one-step formation of tetrasulfonamide macrocycles. These macrocycles adopt a cone-shaped conformation in solution and in the solid state. For each molecule, an interior cavity surrounded by the aromatic residues is formed. The cavity sizes of the macrocycles can be tuned by incorporating aromatic residues of proper sizes. Guest (solvent) molecules are found in the cavities and bound by side chains. In solution, 1H NMR shows that the cone conformations undergo rapid interconversion at room temperature. The alkoxy side chains are found to be indispensable for maintaining the cone conformation. In addition, these porous molecules self-assemble into hollow tubular structures in the solid state. A variety of host molecules and building blocks for constructing nanoporous solid-state structures can be expected from these molecules.
Keywords: macrocycle, nanocavity, nanotube, oligomer, sulfonamide
Macrocyclic molecules have served as the basis for designing various receptors of organic molecules (1) and as building blocks for constructing nanoporous structures (2–4). Of particular interest are cavity-containing molecules such as calixarenes (5–12) and cyclodextrins (13). One common feature shared by many macrocycles is their multiple, dynamic conformations (7, 10, 11). For example, among the four different conformations of the well studied calix[4]arenes, the cone conformation leads to an internal cavity for hosting neutral guest molecules of complementary sizes. The conformations of larger calixarenes are even more complicated because of increased flexibility (14). As a result, various strategies to limit the conformational freedom of calixarenes have been reported. It was found that calixarenes with rigid cavities could not only serve as hosts that bind guest molecules with enhanced specificity and strength (15–18) but also provide scaffolds for the presentation of chemical functionality, which lead to efficient catalysts and precise control of intermolecular assemblies (19). Covalent modification of resorcinarenes has also led to rigid, deep cavitands that completely encapsulate sizable molecules (20).
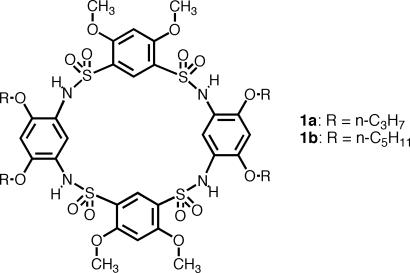
Over the last several years, we have developed folded helical oligomers based on a backbone-rigidification strategy (21, 22). In a recent attempt to design folded aromatic oligosulfonamides, we discovered cyclic aromatic tetrasulfonamides 1a-b, which adopt a cone conformation reminiscent of that of calix[4]arenes in the solid state (23). Results from two-dimensional (2D) NMR (NOESY) studies suggested that such a cone conformation also prevailed in solution. The cavity of these macrocycles was found to accommodate small solvent molecules such as N,N′-dimethylformamide (DMF). These newly discovered macrocycles raised several interesting questions as follows. (i) Can the observed cone conformation be extended to the creation of analogous cone-shaped molecules of different sizes? (ii) How dynamic is the cone conformation in solution? (iii) Are the ether side chains flanking the sulfonamide groups necessary for the adoption of the cone conformation?
In this work, we describe that the diarylsulfonamide moiety flanked by phenolic ether side chains, which can be viewed as the basic structural motif of 1a-b (see Structure 1), adopts a well defined conformation that is predisposed to forming four-residue, macrocyclic sulfonamides. By incorporating naphthalene residues, cone-shaped macrocycles with a cavity larger than that of 1a-b were obtained in one step. The enlarged cavity was found to host guest molecules such as pyridine in the solid state. Although evidence from both x-ray crystallography and 2D NMR indicated that the cone conformation was persistent both in the solid phase and in solution, variable-temperature NMR indicated that such a conformation was highly dynamic, undergoing rapid inversion between cone conformations. Ab initio calculations were carried out on a cone conformation and its partially inverted conformations. Removing some of the ether side chains leads to a macrocycle adopting a 1,3-alternative conformation, demonstrating the importance of the side chains in maintaining the cone conformation. In addition, solid-state structures indicate that these macrocycles, regardless of their cavity sizes, form nanotubular assemblies with pore diameters that are defined by the cavity sizes of the constituent molecules.
Structure 1.
Results and Discussion
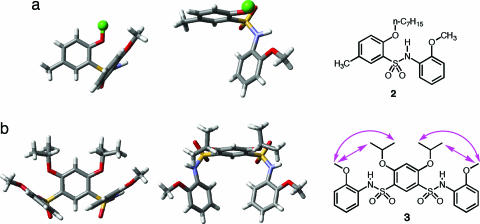
To probe the origin and possible generality of the stable cone conformations adopted by 1a-b, noncyclic aromatic sulfonamide 2 and disulfonamide 3, which can be regarded as fragments of 1a or 1b, were investigated by x-ray crystallography and 2D 1H NMR.
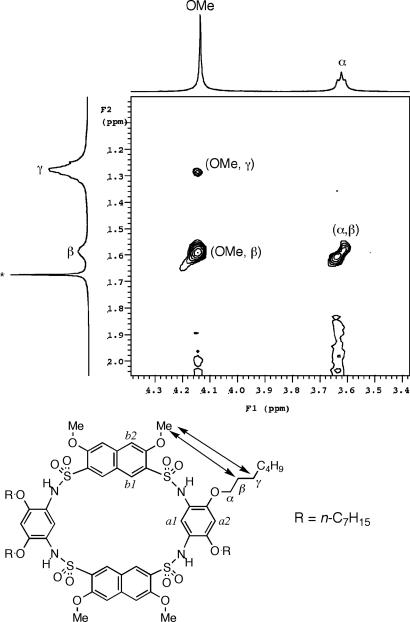
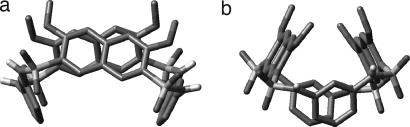
The crystal structure of 2 (Fig. 1a) reveals a conformation involving two adjacent benzene rings with their planes being nearly perpendicular to each other. This conformation is very similar to that of the diarylsulfonamide motif in the crystal structure of 1a. The crystal structure of 3 (Fig. 1b) reveals a partial cone conformation that is similar to that of 1a. Similar to 2, the adjacent benzene rings of 3 are nearly perpendicular to each other, leading to a partially enclosed cavity that is defined by the aromatic rings from the three residues. 2D NMR [rotating-frame Overhauser effect (ROE) spectroscopy] studies of 3 revealed ROEs between the protons of the methoxy and n-butyl side chains (see structure in Fig. 1b, indicated by arrows) (see Figs. 8–10, which are published as supporting information on the PNAS web site). These ROEs are consistent with a solution conformation in which all of the benzene rings and their side chains point to the same side. That no ROEs corresponding to other alternative conformations were detected suggests that the solution conformation of 3 is consistent to that revealed by its crystal structure.
Fig. 1.
Side and top views of the crystal structure of 2 (a) and 3 (b). The side-chain ROEs (arrows) revealed by ROE spectroscopy (2 mM in CDCl3, 500 MHz, 295 K; mixing time: 0.3 s) spectrum of 3. For clarity, the heptyl group of 2 is replaced with a dummy atom.
These results clearly demonstrate that the cone conformations adopted by 1a-b are not due to the cyclization of the oligosulfonamide backbones. Instead, the diarylsulfonamide moiety, with its conformation involving nearly perpendicular adjacent aromatic rings, is solely responsible for the formation of the four-residue macrocycles and their observed shapes. Thus, noncyclic precursors for 1a or 1b must have adopted a convergent conformation that facilitated the final cyclization step.
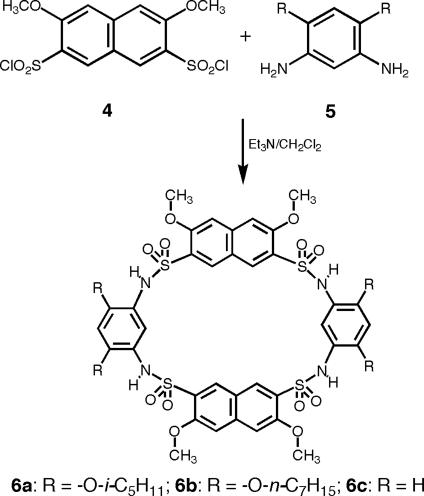
If the diarylsulfonamide moiety is indeed a convergent structural motif, aromatic oligosulfonamide macrocycles with similar conformations, but larger cavities than those of 1a and 1b, should be available by including large aromatic rings into the design (see Scheme 1). To probe this possibility, naphthalenedisulfonyl chloride 4 was added to diamines 5a and 5b in methylene chloride in the presence of triethylamine, followed by refluxing the solution for 12 h. Indeed, MALDI-TOF indicated that 6a-b formed as the major product from these one-step reactions. After removal of solvent, the remaining residues were washed with a small amount of methylene chloride, followed by washing with water and acetone and, in the case of 6a, tetrahydrofuran (THF), and then recrystallized from pyridine–methanol, which led to the isolation of pure 6a or 6b as a white solid.
Scheme 1.
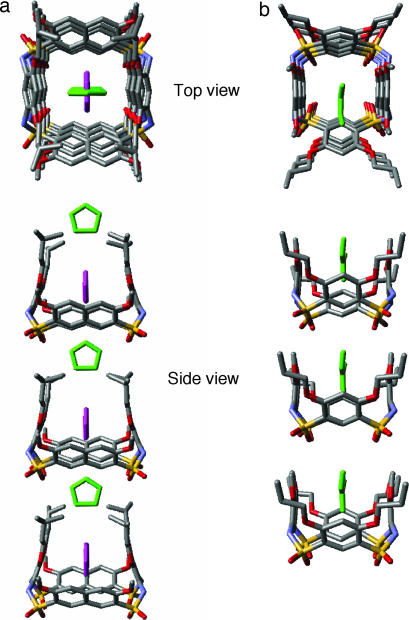
Single crystals of 6a were grown by slow cooling from pyridine. As Fig. 2 shows, in the solid state, macrocycle 6a adopts a cone conformation. This conformation has a rough C2 symmetry and a cavity surrounded by the four aromatic residues. The neighboring benzene and naphthalene rings are nearly perpendicular to each other. A dihedral angle of ≈100° exists between the two naphthalene rings, while the two benzene rings of the diamine residue slightly tilt toward the C2 axis. The ether side chains are attached to the wider rim with the sulfonamide oxygens defining the narrower rim. The four N–H groups of 6a point away from the center of the cavity and are not intramolecularly H-bonded with the two flanking ether oxygens (average NH…O distances of 2.73 and 2.90 Å).
Fig. 2.
The crystal structure of 6a shown in side (a and b) and top (c) views. The pyridine and THF molecules are shown in space-filling model. Except for the amide hydrogens, all other hydrogens are removed for clarity. The THF molecule was from the purification step.
As expected, incorporating naphthalene residue into 6a leads to a cavity (8.47 × 8.89 Å, measured by distances between the centers of nonadjacent aromatic rings) that is larger than that of 1a (6.98 × 7.97 Å). In contrast to the cavity of 1a, which could only accommodate small solvent molecules such as DMF, a pyridine molecule is found in the cavity of 6a. The pyridine molecule is sandwiched between the two benzene rings of the diamine residues, with its plane being perpendicular to those of the naphthalene rings. The benzene rings tilt slightly inward to achieve optimum contact distances (≈3.6 Å) with the pyridine ring (Fig. 2 b and c). This observation suggests that this class of macrocycles have a sufficiently large, well defined cavity with the potential ability to undergo conformational fine-tuning for binding organic molecules. Even more interesting is the presence of a THF molecule that is “holding up” by the four isopentyl side chains. The THF molecules interact with the host molecule via van der Waals contacts between its methylene groups and the methyl groups on the isopentyl side chains. This result suggests the possibility of designing sophisticated hosts by incorporating different side chains into the macrocycle.
NOESY experiment was carried out with the more soluble 6b. Nuclear Overhauser effects (NOEs) between the protons of the methoxy and the n-heptyl groups were observed (Fig. 3) (see Figs. 11 and 12, which are published as supporting information on the PNAS web site), suggesting that the solution conformation of 6b is indeed similar to that revealed by the solid-state structure of 6a.
Fig. 3.
Partial NOESY spectrum of 6b in CDCl3 at 273 K (500 MHz, 2 mM; mixing time: 0.35 s).
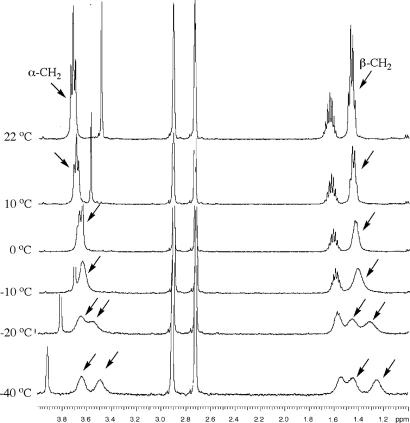
Variable-temperature 1H NMR experiments were performed on 6b to probe the dynamics of its solution conformation. In deuterated N,N-dimethylformamide (DMF-d7), the diastereotopic α- and β-methylene protons of the heptyl side chains appear at ≈3.7 and ≈1.5 ppm at room temperature. Upon lowering temperature, each of these two groups of signals first started to broaden and then split into two separate signals between −10°C and −20°C (Fig. 4). In chloroform-d (see Fig. 13, which is published as supporting information on the PNAS web site), the α- and β-methylene protons of 6b similarly split into two signals but at much lower temperatures (below −35°C). In both solvents, no other 1H signals showed any splitting within the temperature ranges examined. These results indicate that, at room temperature, the observed signals for the α- and β-methylene protons were the result of rapid interconversion of the cone conformations, in a way similar to the ring-flipping of cyclohexane. The low coalescence temperatures suggest that the energy barrier for the interconversion of the cone conformations is very small. The solvent-dependence of the coalescence temperature is probably due to stabilization of the cone conformation by differential guest binding (DMF vs. chloroform) or by enhanced intermolecular interaction between the macrocycles.
Fig. 4.
Partial 1H NMR spectra of 6b in DMF-d7 (2 mM, 500 MHz) at different temperatures. The signals of the α- and β-methylene protons are indicated by arrows.
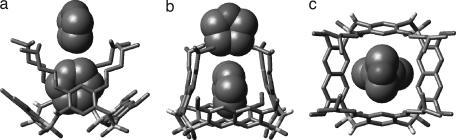
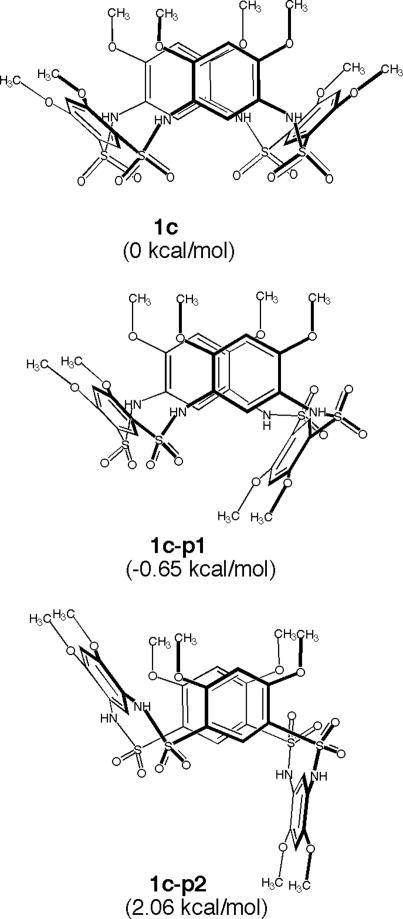
The interconversion of two cone conformations requires the inversion of the aromatic walls of the system, which should go through partial cone conformations. To investigate the stability of the cone and partial cone conformations, ab initio calculation was performed on cyclic tetrasulfonamide 1c, an analog of 1a and 1b. In addition to the cone conformation, two partial cone conformations, 1c-p1 and 1c-p2, are the possible intermediates for the interconversion of the cone conformations of 1c. The partial cone conformations 1c-p1 and 1c-p2 are created by inverting one of the benzenedisulfonyl residues or one of the benzenediamine residues (Fig. 5). Results from the ab initio study revealed that the stabilities of cone conformation 1c and one of the partial cone conformations, 1c-p1, are nearly the same, whereas a difference of 2.06 kcal/mol, was revealed between 1c and the other partial cone conformation, 1c-p2. These results suggest that the interconversion of the cone conformations can readily happen by going through the partial cone conformations, most likely via 1c-p1.
Fig. 5.
The relative energies of the cone conformation 1c and its corresponding partial cone conformations 1c-p1 and 1c-p2 based on ab initio calculation at the B3LYP/6-311G(d,p) level.
To probe the role of the alkoxy side chains on maintaining the cone conformations of this class of molecules, compound 6c was prepared by treating 1,3-phenylenediamine with naphthalenedisulfonyl chloride 4. Macrocycle 6c can be regarded as being derived from removing the alkoxy side chains on the diamino benzene residues of 6a-b. X-ray quality crystals of 6c were obtained from a solvent mixture containing DMSO:EtOH:THF (10:0.5:0.5) by slow cooling. The crystal structure of 6c is shown in Fig. 6. Similar to macrocycles 1 and 6a-b, the adjacent aromatic rings of 6c are nearly perpendicular to each other. However, in contrast to the cone conformations observed for the other tetrasulfonamide macrocycles, the solid-state structure of 6c involves the inversion of the benzene rings, leading to a conformation reminiscent of the 1,3-alternate conformation of calix[4]arenas (Fig. 6; see also Fig. 14, which is published as supporting information on the PNAS web site). Such a conformation of 6c demonstrates the critical role of the alkoxy side chains in maintaining the cone conformations of macrocycles 1 and 6a-b. We believe that the repulsive interaction between the ether and sulfonamide oxygens are responsible for maintaining the observed cone conformation of macrocycles 1 and 6a-b.
Fig. 6.
The crystal structure of macrocycle 6c viewed from the side of one of the two naphthalene residues (a) and from the side of one of the two benzene residues (b).
Finally, examining the solid-state structures of macrocycles 6a and 1a indicated tubular assemblies consisting of molecules stacked above one another (Fig. 7). In each case, the pore size of the porous column is defined by the cavity size of the macrocyclic molecules. For both 6a and 1a, solvent molecules are observed in the macrocyclic cavities. The 2D NMR and x-ray results imply that, both in solution and in the solid state, tubular assembly may be a general phenomenon associated with these tetrasulfonamide macrocycles. More detailed studies could lead to the creation of nanotubular structures with a variety of pore sizes based on this predictable assembling pattern.
Fig. 7.
Top and side views of the solid-state tubular assemblies of 6a (a) and 1a (b). (a) Three molecules of 6a are shown. The solvent molecules (pyridine, purple; THF, green) in the macrocyclic cavity are also shown. (b) Three molecules of 1a are shown. The solvent molecules (DMF, green) in the cavity are also shown. For clarity, all H atoms are removed.
Conclusion
In summary, we have demonstrated that the diarylsulfonamide moiety with flanking alkoxy groups is a reliable structural motif for designing cone-shaped macrocyclic aromatic tetrasulfonamides. This motif adopts a well defined conformation with a 90° dihedral angle, based on which macrocyclic aromatic tetrasulfonamides with stable cone conformations containing cavities of various sizes are formed in one step. The diameter of a cavity is determined by the size of the incorporated aromatic rings. Guest molecules can fit into the cavity of a macrocycle with the proper size. The tubular assemblies of both 1a and 6a in the solid state, and very likely in solution, suggest an exciting possibility of developing a general system that allows the design of nanotubular structures with predictable assembly and tunable pore sizes. Thus, these readily available, cone-like macrocycles represent a previously undescribed class of shape-persistent (6, 7), cavity-containing molecules that should serve as useful building blocks for developing various hosts and nanoporous structures.
Materials and Methods
NMR analyses were carried out on Varian INOVA 500 spectrometer (500 MHz) in CDCl3 or DMF-d7. Tetramethylsilane (TMS) was used as the internal standard for 1H NMR and 13C NMR. Chemical shifts are reported in ppm values downfield from TMS, and J values are reported in Hz.
Compound 2.
1H NMR (500 MHz, CDCl3) δ: 7.67 (s, 1H), 7.55 (s, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.21 (d, J = 8.2 Hz, 1H), 6.96 (t, J = 7.7 Hz, 1H), 6.86∼6.74 (m, 3H), 4.04 (t, J = 6.7 Hz, 2H), 3.74 (s, 3H), 2.26 (s, 3H), 1.91 (m, 2H), 1.53 (m, 2H), 1.40∼1.30 (m, 6H), 0.90 (t, J = 6.18 Hz, 3H). MS (MALDI-TOF), Calcd for C21H29NO4S 391.18 (M+), found 414.1 (M+Na+). 13C NMR (100.6 MHz, CDCl3) δ: 154.04, 148.86, 135.05, 130.84, 129.52, 126.73, 126.61, 124.16, 121.11, 119.67, 112.58, 110.50, 69.25, 55.65, 31.73, 29.16, 25.89, 22.63, 20.27, 14.08.
Compound 3.
Compund 3 was prepared by treating the corresponding benzenedisulfonyl chloride (0.5 mmol) with o-anisidine (0.11 ml, 1 mmol) in CH2Cl2 in the presence of triethylamine. Yield: 70%, colorless crystal, mp: 174–176°C. 1H NMR (500 MHz, CDCl3) δ: 8.51 (s, 1H), 7.40 (d, J = 8.0 Hz, 2H), 7.38 (s, 2H), 6.96 (t, J = 7.8 Hz, 2H), 6.82 (t, J = 7.5 Hz, 2H), 6.76 (d, J = 8.0 Hz, 2H), 6.32 (s, 1H), 4.62 (m, 2H), 3.78 (s, 6H), 1.39 (d, 12H). MS (MALDI-TOF), Calcd for C26H32N2O8S2 564.16 (M+), found 587.1 (M+Na+). 13C NMR (100.6 MHz, CDCl3) δ: 160.11, 147.95, 134.82, 126.32, 123.93, 121.44, 118.84, 118.00, 110.47, 98.63, 72.65, 55.59, 21.48; MS (ESI) m/z, Calcd for C24H39NO6 437.28 (M+), found 438.3 (M+H+).
2,7-Dimethoxynaphalene-3,6-Disulfonyl Chlorides (4).
The following procedure for preparation of 4 was based on a similar method reported in ref. 24. The commercially available disodium 2,7-dihydroxynaphalene-3,6-disulfonate (4a; technical, 5 g, 13.7 mmol) and NaOH (2.2 g, 55 mmol) were dissolved in water (30 ml), and dimethyl sulfate (5.2 ml, 55 mmol) was added dropwise to the solution under stirring. After maintaining the temperature at ≈65°C for 3 h, NaCl (6 g) was added to the mixture to precipitate 2,7-dimethoxynaphalene-3,6-disulfonate (4b), which was filtered off, washed with saturated NaCl, and dried. Repeating the above steps often can lead to the isolation a small amount of 4b from the filtrate. Half of the obtained 4b (3.14 g) was suspended in dry DMF (5 ml), and thionyl chloride (1.5 ml, 20 mmol) was added dropwise under stirring, along with the other half of the methoxy salt in portions, while the temperature was kept at <15°C. After the mixture was stirred for another 3 h at room temperature, ice and water were added, and the product was removed by filtration, washed with ice-water, and dried in a desiccator. Recrystallization of the crude product from CHCl3–CH3OH gave 2,7-dimethoxynaphalene-3,6-disulfonyl chlorides (4) (1.27 g, 24%), mp 240–242°C.
General Procedures for Preparing 6a–c.
4,6-Dialkoxy-1, 3-diaminobenzene (3) (1.1 mmol) was dissolved in CH2Cl2 (15 ml) to give solution A. Disulfonyl chlorides 4 (0.385 g, 1 mmol) was dissolved in CH2Cl2 (15 ml) to give solution B. Solutions A and B and triethylamine (0.28 ml, 2 mmol) were added simultaneously to a flask at room temperature, followed by heating the reaction mixture under reflux for 12–24 h. After removing the solvent, the crude product was washed with a small amount of methylene chloride, water, and acetone. The product was then recrystallized with pyridine–methanol to give 6a or 6b as white solids. The yields have not been optimized.
Compound 6a:
Yield: 30%. 1H NMR (500 MHz, 273K, DMF-d7) δ: 7.99 (s, 4H), 7.75 (s, 4H), 7.19 (s, 2H), 7.09 (s, 4H), 5.97 (s, 2H), 4.14 (s, 12H), 3.62 (t, J = 6.2 Hz, 8H), 1.59 (m, 8H), 1.27 (m, 32H), 0.90 (t, J = 7.0, 12H). MS (MALDI-TOF), Calcd C65H92N4O16S4, 1312.54 (M+), found 1335.53 (M+Na+).
Compound 6b:
Yield: 20%. 1H NMR (500 MHz, 273K, DMF-d7) δ: 7.99 (s, 4H), 7.75 (s, 4H), 7.19 (s, 2H), 7.09 (s, 4H), 5.97 (s, 2H), 4.14 (s, 12H), 3.62 (t, J = 6.2 Hz, 8H), 1.59 (m, 8H), 1.27 (m, 32H), 0.90 (t, J = 7.0, 12H). MS (MALDI-TOF), Calcd C65H92N4O16S4, 1312.54 (M+), found 1335.53 (M+Na+).
Compound 6c:
Yield: 28%. 1H NMR (500 MHz, 273K, DMSO) δ: 8.57 (s, 4H), 8.03 (s, 4H), 7.43 (s, 2H), 7.40 (d, J = 7.5 Hz, 4H), 7.01 (s, 2H), 6.95 (t, J = 7.9 Hz, 2H), 6.79 (s, 2H), 4.09 (s, 12H). MS (MALDI-TOF), Calcd C36H32N4O12S4, 840.6 (M+), found 863.5 (M+Na+), 879.4 (M+K+).
X-Ray Data.
Compounds 2 and 3 were crystallized from DMF by slow cooling. Compound 2: space group P-1, a = 8.5122 (12) Å, b = 8.8900 (12) Å, c = 14.309 (2) Å, α = 84.448 (2)°, β = 88.069 (2)°, γ = 75.994 (2)°. Compound 3: space group P212121, a = 9.0348 (7) Å, b = 12.8363 (9) Å, c = 24.4291 (18) Å. Compound 6a was crystallized from pyridine by slow cooling: space group C2/c, a = 32.659 (7) Å, b = 11.398 (2) Å, c = 26.898 (6), β = 103.535 (3)°. Compound 6c was crystallized from a solvent mixture containing DMSO:EtOH:THF (10:0.5:0.5) by slow cooling: space group C2/c, a = 31.333 (6) Å, b = 14.016 (3) Å, c = 14.718 (3) Å, β = 99.59 (3)°.
Ab Initio Calculations.
The ab initio calculations were carried out by using the gaussian 03 package (Revision C.02; ref. 25). The geometry of each conformer was optimized at the B3LYP/6-311G(d,p) level of theory. The frequencies and zero-point energies were also calculated at the B3LYP/6-311G(d) level of theory. No imaginary frequencies were found for the conformers. The total-energy calculations (including the zero-point energy corrections) indicate that the partially flipped conformer 1c-p1 is slightly lower in energy (by 0.65 kcal/mol) than the fully flipped conformer 1c.
Supplementary Material
Acknowledgments
This work was supported by the TransCentury Training Program Foundation for the Talents, Ministry of Education of China, National Natural Science Foundation of China Grant 20372010 (to L.H.), the Changjiang Scholar Program (to B.G.), National Science Foundation Grant CHE-0314577 (to B.G. and X.C.Z.), and Office of Naval Research Grant N000140210519 (to B.G.).
Abbreviations
- DMF
N,N′-dimethylformamide
- DMF-d7
deuterated N,N-dimethylformamide
- ROE
rotating-frame Overhauser effect
- THF
tetrahydrofuran.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference nos. CCDC-288116, -288117, -288118, and -297380).
References
- 1.Purse B. W., Rebek J. Proc. Natl. Acad. Sci. USA. 2005;102:10777–10782. doi: 10.1073/pnas.0501731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vriezema D. M., Aragones M. C., Elemans J. A. A. W., Cornelissen J. J. L. M., Rowan A. E., Nolte R. J. M. Chem. Rev. 2005;105:1445–1489. doi: 10.1021/cr0300688. [DOI] [PubMed] [Google Scholar]
- 3.Hoger S. Angew. Chem. Int. Ed. 2005;44:3806–3808. doi: 10.1002/anie.200500681. [DOI] [PubMed] [Google Scholar]
- 4.Zhao D. H., Moore J. S. Chem. Commun. 2003:807–818. doi: 10.1039/b207442g. [DOI] [PubMed] [Google Scholar]
- 5.Böhmer V. Angew. Chem. Int. Ed. 1995;34:713–745. [Google Scholar]
- 6.Ikeda A., Shinkai S. Chem. Rev. 1997;97:1713–1734. doi: 10.1021/cr960385x. [DOI] [PubMed] [Google Scholar]
- 7.Rebek J. Chem. Commun. 2000:637–643. [Google Scholar]
- 8.Arduini A., Pochini A., Secchi A., Ugozzoli F. In: Calixarenes. Asfari Z., Böhmer V., Harrowfield J., Vicens J., editors. Dordrecht, The Netherlands: Kluwer Academic; 2001. pp. 457–475. [Google Scholar]
- 9.Thondorf I. In: Calixarenes. Asfari Z., Böhmer V., Harrowfield J., Vicens J., editors. Dordrecht, The Netherlands: Kluwer Academic; 2001. pp. 280–295. [Google Scholar]
- 10.Dalla Cort A., Mandolini L. In: Calixarenes in Action. Mandolini L., Ungaro R., editors. London: Imperial College Press; 2000. pp. 85–110. [Google Scholar]
- 11.Shinkai S., Fujimoto K., Otsuka T., Ammon H. L. J. Org. Chem. 1992;57:1516–1523. [Google Scholar]
- 12.Wang D. F., Wu Y. D. J. Theor. Comput. Chem. 2004;3:51–68. [Google Scholar]
- 13.Wenz G., Han B. H., Muller A. Chem. Rev. 2006;106:782–817. doi: 10.1021/cr970027+. [DOI] [PubMed] [Google Scholar]
- 14.Georghiou P. E., Li Z. P., Ashram M., Chowdhury S., Mizyed S., Tran A. H., Al-Saraierh H., Miller D. O. Synlett. 2005:879–891. [Google Scholar]
- 15.Arduini A., McGregor W. M., Pochini A., Secchi A., Ugozzoli F., Ungaro R. J. Org. Chem. 1996;61:6881–6887. doi: 10.1021/jo960937b. [DOI] [PubMed] [Google Scholar]
- 16.van Hoorn W. P., Morshuis M. G. H., van Veggel F. C. J. M., Reinhoudt D. N. J. Phys. Chem. A. 1998;102:1130–1138. [Google Scholar]
- 17.Kusano T., Tabatabai M., Okamoto Y., Böhmer V. J. Am. Chem. Soc. 1999;121:3789–3790. [Google Scholar]
- 18.Vysotsky M. O., Mogck O., Rudzevich Y., Shivanyuk A., Böhmer V., Brody M. S., Cho Y. L., Rudkevich D. M., Rebek J. J. Org. Chem. 2004;69:6115–6120. doi: 10.1021/jo049128f. [DOI] [PubMed] [Google Scholar]
- 19.Rudzevich Y., Vysotsky M. O., Böhmer V., Brody M. S., Rebek J., Broda F., Thondorf I. Org. Biomol. Chem. 2004;2:3080–3084. doi: 10.1039/B410462E. [DOI] [PubMed] [Google Scholar]
- 20.Hooley R. J., Rebek J. J. Am. Chem. Soc. 2005;127:11904–11905. doi: 10.1021/ja052910s. [DOI] [PubMed] [Google Scholar]
- 21.Gong B. Chem. Eur. J. 2000;7:4336–4342. [Google Scholar]
- 22.Sanford A. R., Gong B. Curr. Org. Chem. 2003;7:1649–1659. [Google Scholar]
- 23.He L., An Y., Yuan L. H., Yamato K., Feng W., Gerlitz O., Zheng C., Gong B. Chem. Commun. 2005:3788–3790. doi: 10.1039/b503921e. [DOI] [PubMed] [Google Scholar]
- 24.Bosshard H. H., Mory R., Schmid M., Zollinger H. Helv. Chim. Acta. 1959;42:1653–1658. [Google Scholar]
- 25.Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A., Jr, Vreven T., Kudin K. N., Burant J. C., et al. gaussian 03. Pittsburgh: Gaussian; 2003. Revision C. 02. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.