Abstract
Cell adhesion is a crucial step in proliferation, differentiation, migration, apoptosis, and metastasis. In previous works we have shown that cell adhesion is modulated by ouabain, a highly specific inhibitor of Na+,K+-ATPase, recently found to be a hormone. In the present work we pursue the investigation of the effect of ouabain on a special type of cell–cell interaction: the rescue of ouabain-sensitive MDCK cells (W) by ouabain-resistant cells (R). In cultured monolayers of pure W cells, ouabain triggers the “P→A mechanism” (from pump/adhesion) consisting of a cascade of phosphorylations that retrieves adhesion-associated molecules occludin and β-catenin and results in detachment of the cell. When W cells are instead cocultured with R cells, the P→A reaction is blocked, and W cells are rescued. Furthermore, in these R/W cocultures ouabain promotes cell–cell communication by means of gap junctions by specifically enhancing the expression of connexin 32 and addressing this molecule to the plasma membrane. Ouabain also promotes the internalization of the β-subunit of the Na+,K+-ATPase. These observations open the possibility that the crucial processes mentioned at the beginning would be under the control of the hormone ouabain.
Keywords: β-catenin; cell communication; connexin expression; Na+,K+-ATPase; occludin
Cardiotonic steroids started in the 18th century as unknown active principles when William Withering used foxglove tea (Digitalis purpurea) to treat dropsy (congestive heart failure). Once ouabain was isolated, the demonstration that it specifically inhibits the Na+,K+ pump (1) transformed ouabain into a crucial tool to identify the pump with the membrane enzyme Na+,K+-ATPase (2) and unravel its intrinsic mechanisms. Ouabain was recently recognized to be a hormone (3) whose blood level increases in physiological (e.g., exercise) as well as pathological (e.g., arterial hypertension, chronic cardiac and renal failure, preeclampsia, etc.) conditions (4–7). Furthermore, we found that ouabain specifically decreases cell adhesion by triggering a P→A mechanism that involves the activation of protein tyrosine kinases and extracellular signal-regulated kinases, decreases the cell content of the small GTP-binding protein RhoA, and changes the degree of phosphorylation, internalization, and degradation of adhesion molecules such as occludin, E-cadherin, etc. (8, 9) (D. Flores-Benítez, personal communication). We have also shown that detachment may not be ascribed to the ensuing decrease of K content (8) and that a decrease of K content provoked by incubation of R or W cells in media with only 0.1 mM K+ (instead of the regular 4.0 mM) does not cause cell detachment (8). This finding is in keeping with the fact that changes in reduced/oxidized glutathione modify the degree of ouabain resistance without reducing the cell K (10, 11).
Because in the meanwhile ouabain was shown to be a hormone (3), in the present work we pursue the study of its effect on a special type of cell–cell interaction: metabolic cooperation (12). A typical example of cooperation is provided by R cells that have low affinity for ouabain (13, 14) and rescue neighboring cells whose pumps have been blocked (15). In a previous work we also measured the ion content of individual cells in cocultures of R and W cells using an electron probe and found that, in fact, both cell types maintain a similar Na+, K+, and Cl− balance when treated with ouabain (14). However, using the dye Lucifer yellow to probe cell communication, we observed that it only diffuses to neighboring W cells during a brief period of 5–15 h that follows plating at confluence, but not in mature monolayers when rescuing is at its peak (16), and in cocultures of R and W cells where only 13% of the cells communicate (14).
In the present work we first discard that R rescuing of W cells would be achieved through a putative substance secreted by R cells that would destroy ouabain or block its effect. Second, we show that rescuing involves cell–cell contacts and that ouabain increases the number of connections through an enhancement of the specific expression of connexin 32, as well as its displacement toward the cell membrane. Finally, we show that R cells block the internalization of cell adhesion molecules of W cells in coculture.
Results
Rescuing Is Not Elicited Through Humoral Factors.
A priori, the possibility exists that R cells would protect W cells by secreting a substance that would destroy ouabain. The first pair of bars in Fig. 1A corresponds to W cells under control conditions (open bar) and treated with 1.0 μM ouabain for 24 h (shaded bar), showing the detaching effect of ouabain. The second pair refers to W cells that were cultured with media conditioned by bathing monolayers of W cells for 24 h. As expected, this conditioned medium does not impair detaching. Actually, the small amount of protein measured corresponds to secretion of extracellular material. The last pair of bars in Fig. 1A shows the protein remaining in dishes bathed with medium conditioned in monolayers of R cells. This medium does not contain substances that would block or prevent the effect of ouabain because addition of ouabain detaches the W cells. It may be argued that a reputed ouabain-blocking or ouabain-hydrolyzing substance may be quickly inactivated and that by the time conditioned medium is transferred to another monolayer it can no longer protect W cells. To check this possibility we plated W cells only on the filter part of a Transwell support and plated W or R cells in the well (Fig. 1B). The first pair of bars in Fig. 1C is a control to verify ouabain effect on filters. In this case we plated W cells in the well. The second pair of bars in Fig. 1C shows that R cells in the well do not protect W cells on the filter; i.e., rescuing may not depend on humoral factors.
Fig. 1.
R cells may not protect W cells through humoral factors. (A) Effect of 1.0 μM ouabain for 24 h on MDCK adhesion assayed as the amount of protein of W cells remaining attached to the multiwell plates. Open bars correspond to control conditions, and gray bars correspond to ouabain-treated monolayers. The first pair of bars shows detachment produced by added ouabain. The second pair of bars shows the effect of ouabain in W cells incubated in media that had been bathing W monolayers for 24 h (COND. MEDIUM W). The third pair of bars shows detachment of W cells bathed in media that had been conditioned in R cells for 24 h. (B) Cells (small cylinders) were cultured in Transwell permeable supports. The filter compartment always contained W cells, and the well part contained either W or R cells as indicated. Ouabain was always added to the well compartment. (C) W cells in the filter are not protected from ouabain; regardless, the well compartment contains a monolayer of W cells (first pair of bars) or R cells (second pair of bars). (D and E) Pictures correspond to W cells cultured on the filter in the absence and presence of ouabain, respectively. (F and G) Cocultures in the well, where R cells are stained in red in the absence and presence of ouabain, respectively. The plasma membrane was stained with an antibody against the β-subunit of Na+,K+-ATPase (green). Despite detaching W cells cultured on the filter (E), ouabain does not detach them in the R/W coculture in the same monolayer (G).
Rescuing Depends on Cell–Cell Contacts.
To investigate this possibility we stained R cells (red) and prepared mixed monolayers on glass coverslips placed on the well (Fig. 1 F and G), whereas in the filter we plated only W cells (Fig. 1 D and E). Fig. 1 D and F illustrates control conditions, and Fig. 1 E and G shows ouabain-treated (1.0 μM for 24 h) cells. To verify that cells remained in intimate contact, we stained their borders with an antibody against the β-subunit of Na+,K+-ATPase (green in Fig. 1 D–G). Ouabain treatment detaches W cells of pure monolayers (Fig. 1E) but does not affect W or R cells that are members of the same monolayer (Fig. 1G). Some W cells do not contact R cells directly but are nevertheless rescued through intermediate W cells. This finding suggests that gap junctions may be involved.
Are Neighboring MDCK Cells Connected Through Gap Junctions?
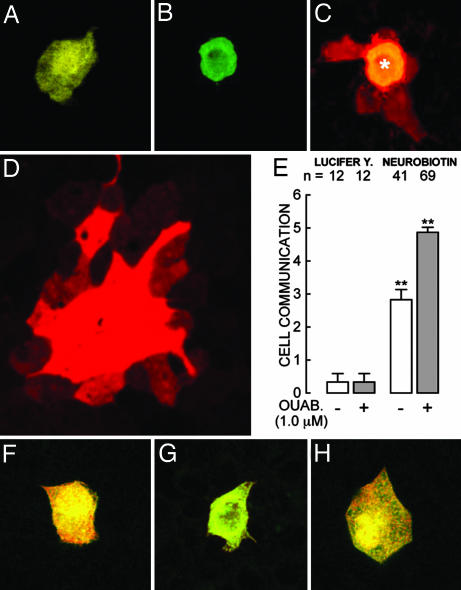
Pictures in Fig. 2A–E belong to monolayers of mixed R and W cells. A given MDCK cell is surrounded by 6.1 ± 0.14 (n = 55) nearest neighbors. Lucifer yellow remains within the injected cell (Fig. 1A). The first pair of bars indicates that the degree of communication, measured with this dye, is nevertheless different from zero presumably because of recordings in cells that were completing a mitotic process and were scored as two neighbors but still had sufficient connection to allow the passage of the dye. Because neurobiotin is not visible under the fluorescent optics used to inject it, impalement was gauged through the simultaneous injection of FITC-dextran (Fig. 2B), which, because of its large size (20 kDa), does not diffuse to neighboring cells. Under confocal microscopy (Fig. 2C) the cell injected with neurobiotin and stained with TRITC-conjugated streptavidin (asterisk) is seen to communicate with three neighboring cells, in keeping with the fact that, whereas Lucifer yellow weighs 457 Da and is negatively charged, neurobiotin weighs only 323 Da and bears a positive charge. As observed by Elfgang et al. (17) in HeLa cells, this kind of selectivity depends on the type of connexin expressed. The incubation with 1.0 μΜ ouabain during 24 h increases the number of nearest neighbors connected to a cell in a mixed monolayer from three to five (Fig. 2E). Scores of cells placed at a distance of the injected cell become secondarily stained (Fig. 2D). Diffusion of the probe to neighboring cells does not occur in monolayers of a single cell type, such as R cells under control (Fig. 2F) and ouabain treatment (Fig. 2G) or W cells under control (Fig. 2H) conditions. W cells treated with ouabain are not shown because they detach upon ouabain treatment. Therefore, these results would indicate that one of the roles of ouabain would be the promotion of cell–cell communication through gap junctions. The most conspicuous molecular components of gap junctions are connexins, and the degree of cell–cell connection depends in part on their nature and abundance (17, 18). Therefore, in the next series of experiments we analyzed ouabain effect on the expression of connexins.
Fig. 2.
Gap junctions and their sensitivity to ouabain. (A–E) Cocultures of R and W cells. (A) Lucifer yellow can be observed directly under the same microscope used to impale the cells. (B) Neurobiotin does not fluoresce under this optics; therefore, to gauge the injection this probe was coinjected with fluorescent (FITC)-dextran of 20 kDa (green) that does not diffuse to neighboring cells. Monolayers were then fixed and stained with TRITC-conjugated streptavidin (red). (C) Confocal micrograph of a cell injected with dextran (∗) plus neurobiotin that diffuses to several nearest neighbors. (D) In ouabain-treated monolayers neurobiotin propagates to secondarily connected cells, and the area of stained cells becomes considerably larger. This picture was taken at one-half of the magnification of the other six to show the whole area of stained cells. (E) The first pair of bars corresponds to cells injected with Lucifer yellow, and the second pair of bars corresponds to cells injected with neurobiotin both in the absence (open bars) and presence (gray bars) of ouabain. Neurobiotin does not diffuse to neighboring cells in confluent monolayers of pure cell types. (F) An R cell (of 10) showing that the probe remains within the injected cell. (G) Another R cell (of 10) treated with ouabain for 24 h. (H) A W cell (of 10) cultured under control conditions. This test cannot be performed in W cells treated with ouabain because they detach and abandon the monolayer.
Ouabain Specifically Enhances the Expression of Connexin 32.
Western blots in Fig. 3A show that connexins 26, 32, and 43 are present in R/W monolayers, albeit in slightly different amounts. Ouabain does not affect the amount of Cx43 or Cx26 but elicits a significant enhancement of Cx32 (P = 0.0356) (Fig. 3B). These connexins differ in their pattern of distribution as well. Cx43 is found mainly near the plasma membrane (Fig. 3C), Cx32 is found in the cytoplasm and in cytoplasmic spots (Fig. 3E), and Cx26 preferentially accumulates in spots at the nucleus (Fig. 3G). Under the effect of ouabain, part of Cx43 shifts to the cytoplasm (Fig. 3D), Cx32 accumulates mainly in the vicinity of the membrane (Fig. 3F), and Cx26 does not exhibit an obvious change (Fig. 3H). Because connexin 26 is not placed close to the plasma membrane, it may not play a relevant role in cell–cell communication. In this respect, recent works indicate that connexins have other functions besides forming gap junctions, such as suppressing cell growth (19) and protecting cells from apoptosis (20). Interestingly, the change in connexin 32 content does not take place in monolayers of single R cells with 1.0 μΜ ouabain (Fig. 3I). Results in Fig. 3 indicate that ouabain does perturb the expression of connexins in a way that agrees with the enhancement of metabolic cooperation shown in Fig. 2 D and E. This finding stresses the fact that adhesions between cells are sites of highly sophisticated exchange of molecules and signals, which alters gene expression, the behavior of participating cells, the nature of the linkage, etc. (21, 22).
Fig. 3.
Ouabain specifically up-regulates the expression of connexin 32. (A) Western blot of connexins 43, 32, and 26 in R/W monolayers with or without 1.0 μM ouabain for 24 h. (B) By using the band of actin to normalize the density of connexin bands, ouabain was found to enhance the expression of connexin 32 without affecting the expression of Cx43 and Cx26. Immunofluorescence images of mixed monolayers show that each connexin distributes according to a particular pattern (C, E, and G under control condition), which is disarranged by 1.0 μM ouabain (D, F, and H). (I) Expression of connexin 32 in monolayers of R cells.
We have previously found a P→A mechanism that operates in W cells, but not in R cells, because in these cell pumps have low affinity for ouabain (8, 9). Yet the α-subunit of Na+,K+-ATPase of rescued W cells does bind this substance and, in cultures of pure W cells, does trigger the P→A mechanism and retrieves membrane molecules accordingly. Therefore, the observation that when W cells are cocultured with R cells they remain attached raises the possibility that the P→A response is somehow blocked by the presence of R cells. This possibility is tested in the next series of studies.
Metabolic Cooperation and Cell Attachment.
We gauged the P→A mechanism with the β-subunit of Na+,K+-ATPase (23), occludin, and the attachment-associated β-catenin (21). Under control conditions all these molecules give a typical chicken-fence pattern in W cells (Fig. 4A, D, and G). The same molecules, but in monolayers treated with ouabain, are pictured in Fig. 4 B, E, and H. These photographs were taken at 8 h (Fig. 4H) and 12 h (Fig. 4 B and E) of ouabain treatment and not at 24 h, because W cells would not have withstood ouabain for this long incubation time and would have been totally detached. The β-subunit of Na+,K+-ATPase (Fig. 4B) and occludin (Fig. 4E) start to disappear from the plasma membrane because of an intense process of internalization revealed by a multitude of cytoplasmic vesicles loaded with them, a process already described (24, 25). Also, β-catenin is gradually removed from the plasma membrane and is switched to the nucleus (red in Fig. 4H). After prolonged ouabain treatments (1.0 μM for 24 h) W cells completely detach, and adhesion-associated proteins cannot be measured (Fig. 4J, second track). On the contrary, when W cells are cocultured with R cells (stained in red beforehand) they withstand ouabain and remain attached. Therefore, we used cocultures to investigate the status of the P→A mechanism during rescuing for 24 h and using three different markers (β-Na+,K+-ATPase, occludin, and β-catenin). The pattern of these molecules in W cells does not seem to be disarranged by ouabain (Fig. 4 C, F, and I), indicating that the presence of R cells blocks the P→A mechanism of W cells. In keeping with these images, Fig. 4J (last four lanes) shows that in this condition the abundance of these adhesion proteins does not decrease in R cells and in R and W mixtures. In summary, ouabain does not distort the pattern of adhesion proteins of R cells because their Na+,K+-ATPase has little affinity for the hormone, and ouabain does not distort the pattern of W in coculture either because contact with R cells nullifies their P→A mechanism.
Fig. 4.
Effect of ouabain on the P→A mechanism. (A, D, and G) Immunofluorescence images of the β-subunit of the Na+,K+-ATPase, occludin, and β-catenin in W cells under control conditions. (B, E, and H) The same markers as affected by 1.0 μM ouabain for 12 h (B and E) or 8 h (H). Nuclei in H were stained in red with propidium iodine. (C, F, and I) The same markers in monolayers of mixed W and R cells (red) treated with ouabain for 24 h. Note that in B, E, and H we used a shorter incubation time to observe the distribution of markers in W cells because this cell type would not have withstood this concentration of ouabain for 24 h. (J) Western blots of occludin and β-catenin in pure monolayers of W or R cells or in cocultures of W/R cells under control and 24 h of ouabain treatment. (K) TER under the same experimental conditions.
Of course, retrieval of adhesion-associated molecules has functional consequences, such as a drop of transepithelial electrical resistance (TER) because of opening of the tight junction (TJ) (8, 9). The value of TER reflects very closely the sealing of the TJ (26). Fig. 4K demonstrates that ouabain causes a drastic decrease of TER across monolayers of W cells because of disassembling of the TJ, but this decrease does not occur in the monolayers of R cells. Mixed monolayers do not open their TJs in the presence of ouabain, stressing the conclusion that coculture of W cells with R cells blocks the P→A mechanism of contacting W cells. A simple circuit analysis (data not shown) indicates that the opening of a mere 2% of the TJs would have sufficed to almost completely vanish TER (27).
Discussion
Except for synapses, contacts between cells were regarded as mere nonspecific and neutral reinforcements of tissue architecture. Yet today cell contacts are known (i) to consist of dozens of different molecular species that are highly specific to each type of junction; (ii) to undergo phosphorylation changes in response to a multitude of physiological and pathological conditions; (iii) to influence the differentiation so that the fate of a cell depends on the type of cells it contacts, the presence of ions (notably Ca2+), etc.; and (iv) to cause shuttling of signal molecules between the junctions and the nucleus (e.g., ZO-1 and β-catenin) and activate or silence specific genes (21). The present demonstration that ouabain affects cell–cell interaction by enhancing the expression of connexin 32 and suppressing the P→A mechanism indicates that one of the physiological roles of this hormone is to determine the fate of a cell through cell adhesion. These results may afford clues to why ouabain plays an important role in health and disease (3, 4, 28).
Ouabain binds to the α-subunit of Na+,K+-ATPase, blocks ion pumping, and distorts ion balance. However, at concentrations low enough to cause negligible inhibition of the pump, it triggers the transference from the plasma membrane to the nucleus of junction-associated molecules (unpublished observations) without detaching or causing the apoptosis observed at high concentrations or long incubation times (>24 h) (29). R cells instead do not bind ouabain or exhibit these changes. Furthermore, they protect W cells in cocultures via gap junctions. We also show that ouabain causes an increase in cell–cell communications (Fig. 2D), and this increase is accompanied by an enhancement of the expression of connexin 32. Whereas Lucifer yellow is a negatively charged probe, neurobiotin is a positive one, and Cao et al. (18) have demonstrated that connexin 32 facilitates the passage of cations. This finding is even more remarkable if we take into account the fact that Ledbetter and Gatto (30) have shown that, in the presence of ouabain, 3T3 fibroblast communication is decreased.
Our present results support the possibility that cell detachment depends on the inhibition of Na+,K+-ATPase activity by ouabain and that rescue of W cells is exerted by maintenance of their ion balance through gap junctions. The first possibility is in keeping with previous results by Contreras et al. (8) using a variety of inhibitors of the pump. Furthermore, using a “Ca-switch” technique developed in our laboratory (27, 31–33), Rajasekaran et al. (34) have shown that blocking the Na+,K+-ATPase of MDCK cells, either with ouabain or through the removal of K+ from the incubating media, blocks junction formation and polarization that normally ensue after the addition of Ca2+. The authors were also able to decrease TER across monolayers of retinal pigment epithelial cells indistinctly by using ouabain or depleting K+ (35). However, there is a growing body of evidence indicating that the pump may act through signaling pathways that do not depend on ion changes (10, 36–39). Therefore, the possibility exists that the relationship between pump inhibition and cell adhesion, being so crucial to essential functions of the cell (i.e., proliferation, differentiation, apoptosis, metastasis, etc.), would be controlled by a whole set of mechanisms, including ion imbalance and cell signaling.
Therefore, ouabain should be listed among hormones that influence cell–cell communication, such as luteinizing hormones, which decrease the passage of cAMP from follicle cells to the oocyte (40); steroid hormones, which reduce cell communication in endometrial carcinoma (41); and FGF, which up-regulates intercellular dye transfer in primary cultures of embryonic chick lens cells (42). Moreover, the Na+,K+-ATPase can be assembled with four different isoforms of α-subunits that differ precisely in their sensitivity to ouabain (22, 43). It is therefore conceivable that a hormone like ouabain, which influences cell–cell interaction, would also play a role in the architecture of tissues during fetal and adult tissues depending on the α-subunit expressed.
Actually, results in Fig. 4B suggest that ouabain can play a biological role far more important than to influence cell–cell communication, proliferation, differentiation, migration, apoptosis, and metastasis and that stems from the following argument: Na+,K+-ATPase has an α-subunit, the catalytic one, that binds ouabain on its extracellular side, and a β-subunit that has properties of a cell–cell adhesion molecule (Fig. 5A). We have previously shown that when dog β-subunit is transfected into CHO (Chinese hamster ovary) cells these cells become adhesive (23). Furthermore, MDCK cells only express Na+,K+-ATPase in their borders contacting another MDCK cell, but not in their free, apical, or basal sides, as observed in Fig. 5 B and C (23, 44–46). This lateral distribution is even achieved when the enzyme may have previously delivered to the apical domain and its lateral diffusion in the plane of the membrane has been prevented by the gasket constituted by the TJ (27). The contact between Na+,K+-ATPases expressed at contacting membranes of epithelial cells seems to be a specific one because MDCK cells cannot express Na+,K+-ATPase at heterotypic borders (MDCK/CHO) (23). Only when CHO cells are transfected with dog β-subunit and cocultured with MDCK cells can these cells express Na+,K+-ATPase in heterotypic MDCK/CHO contacts (23). β- and α-subunits of Na+,K+-ATPase become firmly bound to each other right after their synthesis in the endoplasmic reticulum, and this union is so firm that when CHO cells express transfected dog β-subunit this subunit forces the coexpression of endogenous α-subunit from CHO cells as well (23). This attachment between α and β ensures that the interaction between β-subunits that traps them at the borders of the paracellular spaces would also determine the expression of the whole Na+,K+-ATPase at this location.
Fig. 5.
Role of Na+,K+-ATPase and its modulation by ouabain. (A) The enzyme consists of a catalytic α-subunit and a β-subunit that has a long extracellular domain with adhesive properties (23). (B) Because of β/β adhesiveness the enzyme stabilizes at the intercellular space, which is sealed at the outermost end by the TJ and opens toward the interstitial side. Because of the firmness of the α/β union, the whole enzyme resides at the borders of the intercellular space. (C) Monolayers of MDCK in transverse optical sections show the Na+,K+-ATPase (green) at the cell–cell borders but not at the apical or basal domains. Nuclei were stained with propidium iodine (red). (D) Pumps located in the lateral cell border facing the intercellular space lower the Na+ concentration in the cytoplasm, and the gradient of this ion across the apical border acts as a driving force for cotransporters and countertransporters (brown and gray circles) translocating H+, K+, Ca2+, sugars, amino acids, etc. (E) Ouabain (pink circles) causes endocytosis of pumps as in Fig. 4B, thereby diminishing the driving force for translocating mechanisms and decreasing the flux of nutrients. (F) When one of the cells is a ouabain-resistant one (R), cell–cell attachment molecules are not retrieved from its membrane or from the membrane of its neighbor W, ouabain-binding pumps of the W cell are not endocytosed (Fig. 4C), and cotransporters remain in operation because the Na+ gradient across the apical membrane does not dissipate. Ionic balance of the W cells is maintained by the ouabain-resistant pumps of R cells (14) as well as the presence of gap junctions, which, because of the enhancement of connexin 32 (Fig. 3), increases cell–cell communication (Fig. 2).
All metazoan from Cnidaria up exist because their cells exchange substances with an internal milieu that, despite its incredible small volume (compared with the ocean it substitutes), acts as a constant reservoir whose stability is maintained by transporting epithelia that exchange substances with the environment. The star of such exchange is Na+,K+-ATPase because it not only transports Na+ and K+ vectorially but is secondarily responsible for the driving force that operates cotransporters and countertransporters of glucose, amino acids, ions, etc. (Fig. 5D) (22). Therefore, we may speculate that, when ouabain determines the retrieval of the β-subunit from the plasma membrane as shown in Fig. 4E and suggested in Fig. 5E, it down-regulates the expression of Na+,K+-ATPase in the cell membrane. In other words, hormone ouabain may regulate the vectorial transport of Na+ across epithelia, accompanied by the down-regulation of glucose, amino acids, ions, and other biologically relevant substances. Even when the overall concentration of ouabain may be on the order of 1–10 nM, some tissues may be exposed to concentrations 100 times higher. This may be the situation, for instance, of the distal part of the nephron. It is conceivable that under these circumstances cooperation with a ouabain-resistant cell may rescue a ouabain-sensitive one.
Materials and Methods
Cell Culture.
MDCK cells were obtained from American Type Culture Collection. Ouabain-resistant MDCK cells were kindly donated by Daniel Louvard (Curie Institute, Paris). All cells were grown as described elsewhere (8, 26, 47). Cells were plated on Transwell permeable supports (Costar, catalog no. 3470), glass coverslips, or multiwell plates. Because ouabain is used as an Na+,K+-ATPase inhibitor and as a pharmaceutical agent, it was studied over a wide range of concentrations (14–16). Accordingly, we have explored its effects on ion pumping and the P→A mechanism from 10 nM to 10 μM (8, 9), and for the present work we chose 1.0 μM.
Mixed Monolayers.
R cells were incubated with 6.3 μM Cell Tracker Orange (CMTMR, Molecular Probes, catalog no. C-2927) for 1 h at 36.5°C. Then cells were washed three times with PBS solution (GIBCO) and reincubated for 1 h in DMEM supplemented with 10% calf serum (GIBCO, catalog nos. 436-110 and 200-6170). The cells were trypsinized and mixed with W cells in a 50:50 proportion to be cocultured on the desired substrate. Cocultures were incubated the next day with ouabain (1.0 μM for 8–12 or 24 h as indicated in the text) and subsequently used for immunofluorescence, protein, or TER assays (44, 47, 48).
Immunofluorescence Microscopy.
Cells grown on coverslips and in Transwell filters were washed with PBS and then fixed and permeabilized with ice-cold methanol for 10 min. Fluorescence was performed as published elsewhere (8, 26, 47). The following antibodies were used: mouse monoclonal anti-β1-subunit of the Na+,K+-ATPase (donated by M. Caplan, Yale University, New Haven, CT); anti-occludin (Zymed, catalog no. 33-1500); anti-β-catenin (Zymed, catalog no. 13-8400); anti-connexins 43, 32, and 26 (Antibody Sampler Pack, Zymed, catalog no. 90-0500); Alexa Fluor 488-conjugated goat anti-mouse IgG (Molecular Probes); and FITC goat anti-rabbit IgG (Zymed).
Conditioned Media.
MDCK cells (R or W) were plated at confluence in multiwell plates (Costar, catalog no. 3524) and either left as control or treated with 1.0 μM ouabain. Twelve hours later the medium thus conditioned was transferred to monolayers of W cells and left for another 24 h. These monolayers were then rinsed with PBS and suspended in 1% SDS (GIBCO, catalog no. 15525-017) to measure its protein content through the BCA system (Pierce, catalog no. 23225).
Culture in Transwell Systems.
Cells were seeded in the bottom or on the filter of Transwell permeable supports (Fig. 3). Ouabain (1.0 μM for 24 h) was added to the well medium. Transwell supports were then rinsed with ice-cold PBS, and the filter was excised with a scalpel and extracted overnight with RIPA buffer (see Immunoblot for composition). Samples were then taken to measure protein content.
Immunoblot.
All extraction steps were performed at 4°C. Monolayers grown on 3-cm dishes were washed three times with PBS, extracted twice with 200 μl of RIPA buffer [10 mM Pipes, pH 7.4/150 mM NaCl/2 mM EDTA/2 mM Na3OV4/1% Triton X-100/0.5% Na desoxicolate/10% glycerol/protease inhibitors (Complete, Boehringer)], and scraped with a rubber policeman. The cell lysates were then collected into 1.5-ml microcentrifuge tubes, vigorously shaken for 30 min, sonicated for 30 s, and centrifuged for 20 min at 17,000 × g. Supernatants were recovered, and protein content was measured. The SDS/PAGE-resolved proteins were electrotransferred to a poly(vinylidene difluoride) membrane (Hybond-P; Amersham Pharmacia Biotech, catalog no. RPN 303F) and blocked with 5% dry defatted milk and 3% BSA in PBS. Proteins of interest were detected with antibodies against β-subunit of Na+,K+-ATPase (23), occludin, β-catenin, and the connexins 43, 32, and 26, followed by species-appropriate peroxidase-conjugated antibodies (Zymed, catalog nos. 33-1500, 13-8400, 41091224, 62-6120, and 62-6520, respectively) and a chemiluminescent detection system (ECL PLUS; Amersham Pharmacia Biotech, catalog no. RPN2132).
Injection of Lucifer Yellow and Neurobiotin.
Monolayers grown on glass coverslips were deposited on a glass chamber filled with PBS complemented with 1.8 mM Ca2+. Cells were injected intracellularly with either 3% Lucifer yellow (Molecular Probes, catalog no. A-1593) or 1% Lucifer yellow (Sigma, catalog no. L-0259) by using glass micropipettes (Kimax, catalog no. 4500) made with a Flaming-Brown pipette puller (p-87, Sutter Instruments). Pipettes with a tip resistance of 5–10 MΩ were backfilled with 1 μl of dyes and attached to a holder mounted to a piezo-electric micromanipulator (PCS-750; Burleigh Instruments). Cell impaling was monitored with an inverted microscope (Diaphot 300; Nikon) placed on a vibration isolation table (Technical Manufacturing). Dyes were injected with a glass micropipette connected to a pneumatic microinjector (IM300; Narishige). To verify that a cell was effectively injected with neurobiotin, which cannot be directly observed under the Diaphot microscope, we mixed it with 1% FITC-dextran of 20 kDa (Fine Chemical/Amersham Pharmacia). After 15 min of injection cells were fixed for 15 min with 4% paraformaldehyde in PBS, rinsed twice with PBS, permeabilized with 0.25% Triton X-100, and incubated with streptavidin-conjugated TRITC (Zymed, catalog no. 43-4314) diluted 1:200 (30 min at 37°C). Finally, samples were rinsed twice and mounted with VECTASHIELD (Vector Laboratories, catalog no. H-1000).
TER.
Sealing of the TJ was assessed through the TER. After incubation, the nitrocellulose filter with the monolayer was measured as in refs. 26 and 47. In the case of Transwell supports, TER was measured with a Endhom device (WPI, Stevenage, England) according to the manufacturer’s instructions.
Results are expressed as mean ± SE, and n indicates the number of observations. Results were analyzed by a one-tailed, unpaired t test with Welsh correction and 95% confidence intervals.
Acknowledgments
We acknowledge the expert advice on gap junctions of Dr. Martha Pérez Armendariz and the efficient and pleasant help of P. Nava, A. Lazaro, and E. del Oso. This work was supported by the Consejo Nacional de Ciencia y Technología of México.
Abbreviations
- TER
transepithelial electrical resistance
- TJ
tight junction.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Schatzmann H. J. Helv. Physiol. Pharmacol. Acta. 1953;11:346–354. [PubMed] [Google Scholar]
- 2.Skou J. C. Biochim. Biophys. Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 3.Schoner W., Bauer N., Muller-Ehmsen J., Kramer U., Hambarchian N., Schwinger R., Moeller H., Kost H., Weitkamp C., Schweitzer T., et al. Ann. N.Y. Acad. Sci. 2003;986:678–684. doi: 10.1111/j.1749-6632.2003.tb07282.x. [DOI] [PubMed] [Google Scholar]
- 4.Bauer N., Muller-Ehmsen J., Kramer U., Hambarchian N., Zobel C., Schwinger R. H., Neu H., Kirch U., Grunbaum E. G., Schoner W. Hypertension. 2005;45:1024–1028. doi: 10.1161/01.HYP.0000165024.47728.f7. [DOI] [PubMed] [Google Scholar]
- 5.Hamlyn J. M., Manunta P. J. Hypertens. Suppl. 1992;10:S99–S111. [PubMed] [Google Scholar]
- 6.Hasegawa T., Masugi F., Ogihara T., Kumahara Y. J. Clin. Hypertens. 1987;3:419–429. [PubMed] [Google Scholar]
- 7.Kajimura S., Seale A. P., Hirano T., Cooke I. M., Grau E. G. Gen. Comp. Endocrinol. 2005;143:240–250. doi: 10.1016/j.ygcen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Contreras R. G., Flores-Maldonado C., Lazaro A., Shoshani L., Flores-Benitez D., Larre I., Cereijido M. J. Membr. Biol. 2004;198:147–158. doi: 10.1007/s00232-004-0670-2. [DOI] [PubMed] [Google Scholar]
- 9.Contreras R. G., Shoshani L., Flores-Maldonado C., Lazaro A., Cereijido M. J. Cell Sci. 1999;112:4223–4232. doi: 10.1242/jcs.112.23.4223. [DOI] [PubMed] [Google Scholar]
- 10.Capella L. S., Gefe M., Silva E. F., Morales M. M., Affonso-Mitidieri O., Lopes A. G., Rumjanek V. M., Capella M. A. Biochim. Biophys. Acta. 2001;1526:293–300. doi: 10.1016/s0304-4165(01)00144-1. [DOI] [PubMed] [Google Scholar]
- 11.Valente R. C., Capella L. S., Monteiro R. Q., Rumjanek V. M., Lopes A. G., Capella M. A. FASEB J. 2003;17:1700–1702. doi: 10.1096/fj.02-0937fje. [DOI] [PubMed] [Google Scholar]
- 12.Ledbetter M. L., Lubin M. J. Cell Biol. 1979;80:150–165. doi: 10.1083/jcb.80.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soderberg K., Rossi B., Lazdunski M., Louvard D. J. Biol. Chem. 1983;258:12300–12307. [PubMed] [Google Scholar]
- 14.Bolivar J. J., Lazaro A., Fernandez S., Stefani E., Pena-Cruz V., Lechene C., Cereijido M. Am. J. Physiol. 1987;253:C151–C161. doi: 10.1152/ajpcell.1987.253.1.C151. [DOI] [PubMed] [Google Scholar]
- 15.Cereijido M., Bolivar J. J., Lazaro A. Pflügers Arch. 1985;405(Suppl. 1):S147–S151. doi: 10.1007/BF00581797. [DOI] [PubMed] [Google Scholar]
- 16.Cereijido M., Robbins E., Sabatini D. D., Stefani E. J. Membr. Biol. 1984;81:41–48. doi: 10.1007/BF01868808. [DOI] [PubMed] [Google Scholar]
- 17.Elfgang C., Eckert R., Lichtenberg-Frate H., Butterweck A., Traub O., Klein R. A., Hulser D. F., Willecke K. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao F., Eckert R., Elfgang C., Nitsche J. M., Snyder S. A., Hulser D. F., Willecke K., Nicholson B. J. J. Cell Sci. 1998;111:31–43. doi: 10.1242/jcs.111.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Dang X., Doble B. W., Kardami E. Mol. Cell. Biochem. 2003;242:35–38. [PubMed] [Google Scholar]
- 20.Lin J. H., Yang J., Liu S., Takano T., Wang X., Gao Q., Willecke K., Nedergaard M. J. Neurosci. 2003;23:430–441. doi: 10.1523/JNEUROSCI.23-02-00430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balda M. S., Matter K. Trends Cell Biol. 2003;13:310–318. doi: 10.1016/s0962-8924(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 22.Cereijido M., Contreras R. G., Shoshani L. Physiol. Rev. 2004;84:1229–1262. doi: 10.1152/physrev.00001.2004. [DOI] [PubMed] [Google Scholar]
- 23.Shoshani L., Contreras R. G., Roldan M. L., Moreno J., Lazaro A., Balda M. S., Matter K., Cereijido M. Mol. Biol. Cell. 2005;16:1071–1081. doi: 10.1091/mbc.E04-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contreras R. G., Lazaro A., Mujica A., Gonzalez-Mariscal L., Valdes J., Garcia-Villegas M. R., Cereijido M. J. Membr. Biol. 1995;145:295–300. doi: 10.1007/BF00232721. [DOI] [PubMed] [Google Scholar]
- 25.Liu J., Liang M., Liu L., Malhotra D., Xie Z., Shapiro J. I. Kidney Int. 2005;67:1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 26.Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. J. Cell Biol. 1978;77:853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras R. G., Avila G., Gutierrez C., Bolivar J. J., Gonzalez-Mariscal L., Darzon A., Beaty G., Rodriguez-Boulan E., Cereijido M. Am. J. Physiol. 1989;257:C896–C905. doi: 10.1152/ajpcell.1989.257.5.C896. [DOI] [PubMed] [Google Scholar]
- 28.Yuan C. M., Manunta P., Hamlyn J. M., Chen S., Bohen E., Yeun J., Haddy F. J., Pamnani M. B. Hypertension. 1993;22:178–187. doi: 10.1161/01.hyp.22.2.178. [DOI] [PubMed] [Google Scholar]
- 29.Pchejetski D., Taurin S., Der Sarkissian S., Lopina O. D., Pshezhetsky A. V., Tremblay J., deBlois D., Hamet P., Orlov S. N. Biochem. Biophys. Res. Commun. 2003;301:735–744. doi: 10.1016/s0006-291x(02)03002-4. [DOI] [PubMed] [Google Scholar]
- 30.Ledbetter M. L., Gatto C. L. Cell Biochem. Funct. 2003;21:363–370. doi: 10.1002/cbf.1036. [DOI] [PubMed] [Google Scholar]
- 31.Balda M. S., Gonzalez-Mariscal L., Contreras R. G., Macias-Silva M., Torres-Marquez M. E., Garcia-Sainz J. A., Cereijido M. J. Membr. Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Mariscal L., Chavez de Ramirez B., Cereijido M. J. Membr. Biol. 1985;86:113–125. doi: 10.1007/BF01870778. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Mariscal L., Contreras R. G., Bolivar J. J., Ponce A., Chavez de Ramirez B., Cereijido M. Am. J. Physiol. 1990;259:C978–C986. doi: 10.1152/ajpcell.1990.259.6.C978. [DOI] [PubMed] [Google Scholar]
- 34.Rajasekaran S. A., Palmer L. G., Moon S. Y., Peralta Soler A., Apodaca G. L., Harper J. F., Zheng Y., Rajasekaran A. K. Mol. Biol. Cell. 2001;12:3717–3732. doi: 10.1091/mbc.12.12.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajasekaran S. A., Hu J., Gopal J., Gallemore R., Ryazantsev S., Bok D., Rajasekaran A. K. Am. J. Physiol. 2003;284:C1497–C1507. doi: 10.1152/ajpcell.00355.2002. [DOI] [PubMed] [Google Scholar]
- 36.Aizman O., Uhlen P., Lal M., Brismar H., Aperia A. Proc. Natl. Acad. Sci. USA. 2001;98:13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akimova O. A., Bagrov A. Y., Lopina O. D., Kamernitsky A. V., Tremblay J., Hamet P., Orlov S. N. J. Biol. Chem. 2005;280:832–839. doi: 10.1074/jbc.M411011200. [DOI] [PubMed] [Google Scholar]
- 38.Saunders R., Scheiner-Bobis G. Eur. J. Biochem. 2004;271:1054–1062. doi: 10.1111/j.1432-1033.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- 39.Tian J., Cai T., Yuan Z., Wang H., Liu L., Haas M., Maksimova E., Huang X. Y., Xie Z. J. Mol. Biol. Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekel N. Mol. Cell. Endocrinol. 2005;234:19–25. doi: 10.1016/j.mce.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Saito T., Tanaka R., Wataba K., Kudo R., Yamasaki H. Oncogene. 2004;23:1109–1116. doi: 10.1038/sj.onc.1207215. [DOI] [PubMed] [Google Scholar]
- 42.Le A. C., Musil L. S. J. Cell Biol. 2001;154:197–216. doi: 10.1083/jcb.200101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lingrel J., Moseley A., Dostanic I., Cougnon M., He S., James P., Woo A., O’Connor K., Neumann J. Ann. N.Y. Acad. Sci. 2003;986:354–359. doi: 10.1111/j.1749-6632.2003.tb07214.x. [DOI] [PubMed] [Google Scholar]
- 44.Contreras R. G., Lazaro A., Bolivar J. J., Flores-Maldonado C., Sanchez S. H., Gonzalez-Mariscal L., Garcia-Villegas M. R., Valdes J., Cereijido M. J. Membr. Biol. 1995;145:305–310. doi: 10.1007/BF00232722. [DOI] [PubMed] [Google Scholar]
- 45.Cereijido M., Shoshani L., Contreras R. G. J. Membr. Biol. 2001;184:299–304. doi: 10.1007/s00232-001-0097-y. [DOI] [PubMed] [Google Scholar]
- 46.Cereijido M., Shoshani L., Contreras R. G., Larre I. Physiol. Mini-Rev. 2005;1:1–10. [Google Scholar]
- 47.Cereijido M., Shoshani L., Contreras R. G. In: Cell–Cell Interactions. Fleming T. P., editor. New York: Oxford Univ. Press; 2002. pp. 71–91. [Google Scholar]
- 48.Contreras R. G., Shoshani L., Flores-Maldonado C., Lazaro A., Monroy A. O., Roldan M. L., Fiorentino R., Cereijido M. Pflügers Arch. 2002;444:467–475. doi: 10.1007/s00424-002-0827-8. [DOI] [PubMed] [Google Scholar]