Abstract
Temporally and spatially controlled master regulators drive the Caulobacter cell cycle by regulating the expression of >200 genes. Rapid clearance of the master regulator, CtrA, by the ClpXP protease is a critical event that enables the initiation of chromosome replication at specific times in the cell cycle. We show here that a previously unidentified single domain-response regulator, CpdR, when in the unphosphorylated state, binds to ClpXP and, thereby, causes its localization to the cell pole. We further show that ClpXP localization is required for CtrA proteolysis. When CpdR is phosphorylated, ClpXP is delocalized, and CtrA is not degraded. Both CtrA and CpdR are phosphorylated via the same CckA histidine kinase phospho-signaling pathway, providing a reinforcing mechanism that simultaneously activates CtrA and prevents its degradation by delocalizing the CpdR/ClpXP complex. In swarmer cells, CpdR is in the phosphorylated state, thus preventing ClpXP localization and CtrA degradation. As swarmer cells differentiate into stalked cells (G1/S transition), unphosphorylated CpdR accumulates and is localized to the stalked cell pole, where it enables ClpXP localization and CtrA proteolysis, allowing the initiation of DNA replication. Dynamic protease localization mediated by a phospho-signaling pathway is a novel mechanism to integrate spatial and temporal control of bacterial cell cycle progression.
Keywords: Caulobacter, ClpXP, phosphorylation, proteolysis, temporal control
The regulation of cell cycle pathways requires the precise timing of both production and degradation of regulatory proteins. Bacterial cells use several families of multicomponent proteases to eliminate proteins from the cell (1). Adaptor proteins that connect the targeted proteins to the proteolytic machinery provide substrate specificity (2). A recently identified degradation factor, RcdA, mediates proteolysis of the Caulobacter CtrA cell cycle master regulator by the dynamically localized ClpXP protease at specific times in the cell cycle (3). Here, we report the identification of a protein, CpdR, that directs ClpXP localization to the cell pole and show that CpdR function is under the control of a phospho-signaling pathway, thus establishing a mechanism for providing temporal and spatial specificity to bacterial proteases.
Each Caulobacter cell division is asymmetric, producing two morphologically distinct daughter cells with different cell fates (see Fig. 1B). The swarmer daughter cell has a single polar flagellum and polar pili. It cannot initiate DNA replication until it differentiates into a stalked cell. This differentiation process includes the loss of the flagellum and the polar chemotaxis receptors, retraction of the pili, construction of a stalk at the cell pole previously occupied by the flagellum, and initiation of chromosome replication. In contrast, the stalked daughter cell immediately initiates chromosome replication, followed by elongation into a predivisional cell and biosynthesis of a new flagellum and chemotaxis apparatus at the pole opposite the stalk. In the predivisional cell, after chromosome replication and segregation are completed, the cytoplasm is compartmentalized into two distinct chambers before the completion of cell division (4).
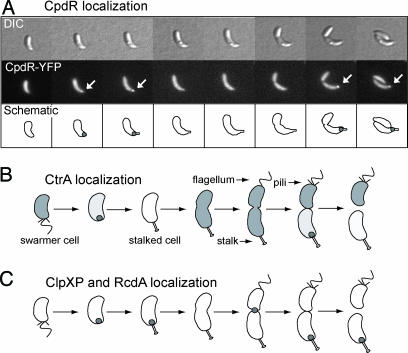
Fig. 1.
The CpdR response regulator is localized to the cell pole. (A) Swarmer cells of a strain expressing cpdR-yfp from a low copy-number plasmid in a wild-type background were harvested, resuspended on an agarose pad, and, thereafter, imaged by DIC and fluorescence microscopy every 45 min. Arrows indicate CpdR-YFP foci. (B and C) Schematic of CtrA localization (B; ref. 8) and localization of the ClpXP protease and the RcdA degradation factor as a function of the cell cycle (C; ref. 3).
The essential response regulator CtrA is centrally involved in regulating the progression of the Caulobacter cell cycle (Fig. 1B). In its role as a DNA-binding regulatory protein, the active phosphorylated form of CtrA, CtrA∼P, directly controls the transcription of at least 95 cell cycle-regulated genes (5, 6). In swarmer cells, CtrA∼P also represses initiation of chromosome replication by binding to five sites in the origin of replication (7). Clearance of CtrA∼P from the stalked cell at the swarmer-to-stalked cell transition, or from the stalked cell compartment at cell division, by simultaneous dephosphorylation and degradation enables initiation of chromosome replication. In a strain in which active CtrA is forced to be present in stalked cells, the replication origin remains repressed, and the culture exhibits a G1/S block (5). Thus, a critical component of cell cycle regulation in Caulobacter is the temporally controlled proteolysis of CtrA.
In the presence of RcdA, the ClpXP protease degrades CtrA (3). ClpXP and its CtrA substrate are simultaneously localized to the stalked cell pole both at the swarmer-to-stalked cell transition and in the stalked cell compartment of the predivisional cell (3, 8). We identify a single-domain response regulator, CpdR, that in its unphosphorylated state directs CtrA proteolysis by controlling ClpXP polar localization and activity. In the absence of CpdR, or in the presence of phosphorylated CpdR, the ClpXP complex fails to localize to the cell pole, and CtrA is not degraded. Thus, CpdR is a central component in the regulation of CtrA proteolysis and, consequently, cell cycle progression.
A phospho-signaling pathway mediated by the CckA histidine kinase converts CtrA to its CtrA∼P activated form (9, 10). Here, we show that CckA also converts CpdR to its CpdR∼P form, which leads to delocalization and consequent deactivation of the ClpXP proteolytic machinery. The result of these two reinforcing actions is a sharp increase in the level of CtrA∼P in late stalked cells after the initiation of DNA replication. Cell division results in ClpXP localization only to the pole of the daughter stalked cell and the consequent degradation of CtrA, dependent on the differential phosphorylation state of CpdR in the two daughter cells. CpdR is phosphorylated in the predivisional cell and remains phosphorylated in the daughter swarmer cell, preventing the polar localization of the ClpXP protease and the degradation of CtrA. In the stalked cell compartment and at the swarmer-to-stalked cell transition, CpdR is in the unphosphorylated state, promoting polar localization of ClpXP and CtrA proteolysis. Thus, the dynamic control of the localization of a critical protease by a phospho-signaling pathway leads to the temporal and spatial regulation of cell cycle progression.
Results
The CpdR Response Regulator Transiently Localizes to the Cell Pole.
CpdR is a single-domain response regulator consisting of a single CheY-like receiver domain. The cpdR gene (CC0744) encodes a 118-aa protein with a predicted molecular mass of 12.7 kDa and is conserved among the α-proteobacteria.
A plasmid-borne cpdR-yfp fusion under the control of the cpdR promoter was examined in a wild-type background by time-lapse fluorescence microscopy. In a synchronized cell population, CpdR-yellow fluorescent protein (YFP) was observed to transiently localize to the incipient stalked pole during the swarmer-to-stalked cell transition and again to the stalked pole in predivisional cells (Fig. 1A). An identical localization pattern was observed when cpdR-gfp replaced the chromosomal copy of cpdR. The dynamic localization pattern of CpdR to the stalked pole parallels that of the proteolysis complex composed of the ClpXP protease, the RcdA degradation factor, and the CtrA substrate of ClpXP (Figs. 1 B and C; ref. 3). In addition, ClpXP and RcdA also exhibit a separate transient localization to the cell division plane.
CpdR Is Required for CtrA and McpA Proteolysis.
CtrA localizes to the stalked pole at the swarmer-to-stalked cell transition and later to the stalked compartment of the predivisional cell (refs. 8 and 11; Fig. 1B), where it is degraded by the ClpXP protease (3–5, 8, 11, 12). Because CpdR exhibits the same transient localization pattern as CtrA, we asked whether CpdR mediates CtrA proteolysis. To address this question, we performed immunoblots with anti-CtrA antibodies on samples taken from synchronized populations of wild-type and cpdR deletion strains (Fig. 2A). The levels of CtrA remained nearly constant throughout the cell cycle in the ΔcpdR::Ω strain in contrast to its periodic clearance in wild-type cells, suggesting that CpdR is required for the degradation of CtrA. A ΔcpdR::Ω strain with a plasmid bearing a copy of cpdR exhibited the normal cell cycle pattern of CtrA accumulation, demonstrating that the loss of CtrA degradation was due to the absence of CpdR.
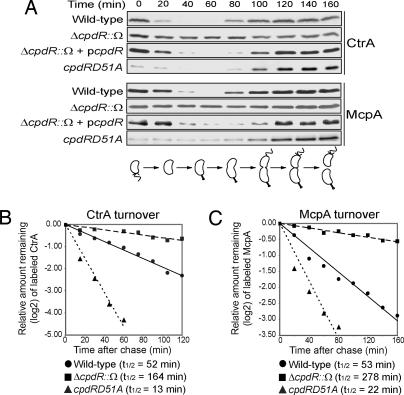
Fig. 2.
Two ClpXP substrates, CtrA and the polar McpA chemoreceptor, depend on CpdR for their proteolysis. (A) Immunoblots of synchronized populations of wild-type, ΔcpdR::Ω, ΔcpdR::Ω with the complementing plasmid pcpdR, and cpdRD51A strains by using antibodies to CtrA and McpA. A cell cycle schematic is shown. (B and C) Pulse–chase experiments showing the half-lives of CtrA (B) and McpA (C) in wild-type, ΔcpdR::Ω, and cpdRD51A strains.
Another ClpXP substrate, the integral membrane McpA chemoreceptor (13), is located at the same stalked pole and also is degraded at the swarmer-to-stalked cell transition (14). To determine whether CpdR function is CtrA-specific or has a general role in the proteolysis of ClpXP polar substrates, we performed the same set of immunoblot experiments described for CtrA, but with anti-McpA antibodies. The loss of McpA proteolysis in the ΔcpdR::Ω strain and its complementation with a plasmid-borne cpdR gene were parallel to that observed with CtrA (Fig. 2A).
To confirm that the absence of CpdR affects proteolysis of CtrA and McpA, we performed pulse–chase experiments and compared the half-lives of CtrA and McpA in the wild-type and ΔcpdR::Ω strains. Both strains contained a high-copy number plasmid carrying either ctrA or mcpA transcribed from the xylose-inducible xylX promoter. The measured half-lives of CtrA and McpA in cells lacking CpdR are significantly longer (3.2-fold and 5.3-fold, respectively) than in wild-type cells (Fig. 2 B and C).
In most Gram-negative bacteria, two alternative ATPase subunits, ClpA or ClpX, can assemble with the proteolytic subunit ClpP (15). One example of a substrate degraded by the ClpAP protease in Caulobacter is the flagellar MS ring protein, FliF. This protein anchors the flagellar basal body in the inner membrane and is degraded coincident with flagellar release during the swarmer-to-stalked cell transition (16, 17). The cell cycle pattern of FliF proteolysis is not altered in the ΔcpdR::Ω strain, suggesting that CpdR function is specific for ClpXP substrates (see Fig. 6A, which is published as supporting information on the PNAS web site).
CpdR Directly Interacts with ClpXP and Is Required for Its Polar Localization.
The temporally regulated proteolysis of CtrA by the ClpXP protease depends on the localization of CtrA to the cell pole (8) mediated by the degradation factor RcdA (3). ClpXP transiently localizes to the same cell pole and at the same time as CtrA and RcdA, and both of these proteins interact in vivo with ClpX (3). To determine the role of CpdR in the degradation of ClpXP’s polar substrates, we asked whether CpdR is necessary for polar localization of the ClpXP protease by examining the cellular positioning of ClpX in absence of CpdR. Wild-type and ΔcpdR::Ω strains containing a xylose-inducible clpX-gfp fusion were imaged by using differential interference contrast (DIC) and fluorescence microscopy (Fig. 3A). In wild-type cells, ClpX-GFP localizes to the pole during the swarmer-to-stalked cell transition and to the pole of the stalked compartment of the predivisional cell. ClpX also is transiently present at the cell division plane just before cell division (Fig. 3A; ref. 3). In a ΔcpdR::Ω strain, ClpX-GFP no longer localizes to the cell pole. However, it does retain its transient appearance at the division plane. Thus, CpdR is required specifically for the localization of ClpX only to the cell pole. We reported that RcdA, which is required for the polar localization of CtrA and its degradation by the localized ClpXP protease, is not required for the polar localization of ClpXP (3). Together, these experiments show that the loss of ClpXP localization results in the loss of both CtrA and McpA proteolysis. As expected, neither CtrA nor RcdA localize to the cell pole in the absence of CpdR (Fig. 7, which is published as supporting information on the PNAS web site). The requirement of CpdR for CtrA and RcdA localization is most likely a consequence of CpdR’s role in the polar localization of ClpXP. RcdA is specific for CtrA degradation, whereas CpdR is necessary for both CtrA and McpA degradation. Therefore, the absence of RcdA should not interfere with the localization pattern of CpdR. Indeed, CpdR localized to the cell pole in ΔrcdA cells during the swarmer-to-stalked cell transition and at the stalked pole in predivisional cells (data not shown), demonstrating that RcdA is dispensable for CpdR localization.
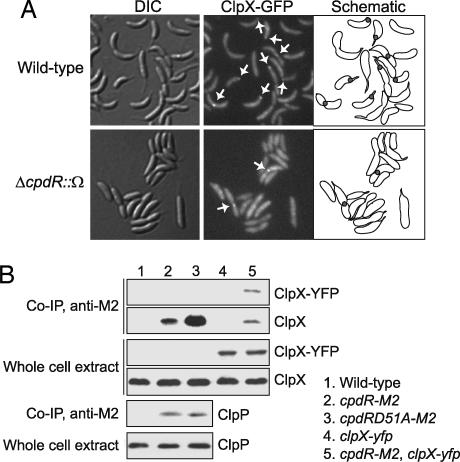
Fig. 3.
CpdR directly interacts with ClpXP and is required for its polar localization. (A) Wild-type (Upper) or ΔcpdR::Ω (Lower) strains, containing a chromosomal xylose-inducible clpX-gfp fusion were treated for 1 h with xylose and were observed by DIC and fluorescence microscopy. Arrows indicate ClpX-GFP foci. (B) Samples from coimmunoprecipitation experiments performed with anti-M2 antibody and whole-cell extract were probed with anti-ClpX and anti-ClpP antisera. The strains used in these experiments are indicated at the right of the figure.
To determine whether CpdR and the ClpXP complex physically interact, we performed coimmunoprecipitation experiments. We created a strain with the cpdR gene translationally fused to an M2-epitope tag-encoding sequence at its native chromosome locus in place of the wild-type gene. We then used M2 antibodies to immunoprecipitate protein complexes from a cell lysate of this strain. Immunoblots of the immunoprecipitated sample then were probed with anti-ClpX or anti-ClpP antibodies (Fig. 3B). The anti-ClpX antibody recognized a protein that migrates at the predicted size of ClpX in the whole-cell extracts (Fig. 3B, lanes 1 and 2) and in the CpdR-M2 immunoprecipitated sample (Fig. 3B, lane 2). The same result was obtained with anti-ClpP antibody (Fig. 3B, lanes 1 and 2). To confirm that ClpX was indeed present in the M2 immunoprecipitate, we repeated these experiments on cell lysates taken from strains that contained a xylose-inducible clpX-yfp integrated at the chromosomal xylX promoter (Fig. 3B, lanes 4 and 5). Immunoblot analysis with the anti-ClpX antibody revealed a protein identical in size to ClpX and an additional protein that migrated at the predicted size of the ClpX-YFP only in the sample containing CpdR-M2 (Fig. 3B, lane 5). These experiments demonstrate that CpdR interacts with ClpX and ClpP within the cell.
The Phosphorylation State of CpdR Regulates CtrA and McpA Stability.
In bacterial two-component signal transduction pathways, response regulators are phosphorylated at an aspartate site through a histidine kinase (18). The CpdR single-domain response regulator has an aspartate residue at position 51 that is highly conserved in all α-proteobacterial CpdR homologues. We generated a mutant allele of cpdR, cpdRD51A, with an alanine at position 51 instead of an aspartic acid (see Supporting Text, which is published as supporting information on the PNAS web site). In vivo phosphorylation experiments showed that, as expected, CpdRD51A is not phosphorylated (Fig. 4A, lane 3). The cpdRD51A mutant forms filamentous cells, when grown in rich media (Fig. 8B, which is published as supporting information on the PNAS web site). However, growth in minimal media resulted in a smaller proportion of filamentous cells, allowing the isolation of swarmer cells and synchronous growth. To determine whether the lack of CpdR phosphorylation affects the cell cycle pattern of CtrA and McpA degradation, we performed immunoblots with anti-CtrA and anti-McpA antibodies on samples taken from a synchronized culture of the cpdRD51A strain (Fig. 2A). There was a striking increase in CtrA and McpA turnover in cpdRD51A swarmer cells. Thus, in the presence of only unphosphorylated CpdR, there is an unusually active degradation of both ClpXP’s substrates at the beginning of the cell cycle, suggesting that the unphosphorylated version of CpdR promotes proteolysis, whereas the phosphorylated version of CpdR inhibits degradation in swarmer cells. Pulse–chase experiments to measure the half-lives of CtrA and McpA in the cpdRD51A strain showed that the half-lives of CtrA and McpA in cpdRD51A cells were significantly shorter, 4-fold and 2.4-fold, respectively, than in wild-type cells (Fig. 2 B and C), confirming that the phosphorylation state of CpdR influences the degradation rate of CtrA and McpA. The reappearance of both CtrA and McpA later in the cell cycle (Fig. 2A) may result from the known active resynthesis of both proteins at that time, thus producing substrate faster than it can be degraded by the localized protease.
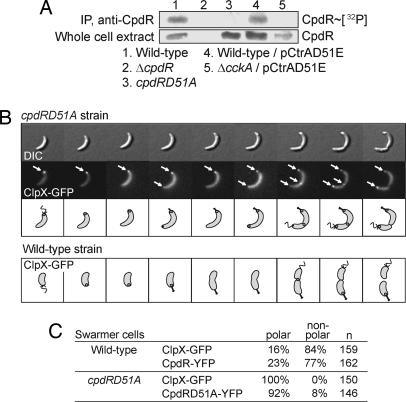
Fig. 4.
The CpdR phosphorylation state affects ClpX localization in a CckA-dependent manner. (A) CpdR and CpdR∼P accumulation in wild-type and mutant cells, revealing that CpdR depends on CckA for its phosphorylation. Cells were labeled with 30 μCi [γ-32P]-ATP per ml of culture for 3 min, lysed, and immunoprecipitated with anti-CpdR antibodies. (B Upper) A cpdRD51A strain with xylose-inducible clpX-gfp expression was treated for 1 h with xylose. Swarmer cells were isolated, resuspended on an agarose pad, and imaged by DIC and fluorescence microscopy at 30-min intervals over the course of the cell cycle. Arrows indicate ClpX-GFP foci. (B Lower) Schematic of ClpX-GFP localization during the wild-type cell cycle (3). (C) Proportion of swarmer cells containing ClpX-GFP or CpdR-YFP polar foci in wild-type and cpdRD51A strains. The total number of observed cells is indicated as “n,” the proportion of cells with polar foci as “polar,” and the proportion of cells with no polar foci as “non-polar.”
The Phosphorylation State of CpdR Regulates ClpX Localization.
To determine whether the CpdR phosphorylation state affects ClpX localization, we isolated cpdRD51A swarmer cells containing xylose-inducible clpX-gfp and observed live cells by time-lapse fluorescence microscopy. In the cpdRD51A strain, ClpX-GFP was localized at the pole in 100% of the swarmer cells, in contrast to the 16% in wild-type cells (Fig. 4 B and C). Moreover, a second ClpX-GFP focus appeared at the opposite cell pole in 73% of the cpdRD51A predivisional cells, which never occurs in wild-type cells (Fig. 4B). The transient localization of ClpX-GFP to the cell division plane is unaffected in the cpdRD51A strain. The observation that the cell cycle duration of polarly localized ClpX correlates with the relative rate of CtrA or McpA proteolysis suggests that ClpXP proteolysis of these substrates can occur only when localized at the pole. In the absence of ClpX localization in the wild-type swarmer cells, there is a significant accumulation of CtrA and McpA (Fig. 2A). However, when CpdR cannot be phosphorylated, and ClpX is aberrantly localized to the pole of the swarmer cells, the relative accumulation of CtrA and McpA exhibits a dramatic decrease (Fig. 2 B and C). Thus, in the absence of CpdR phosphorylation, the localization and activity of the ClpXP protease is retained for a longer period during the cell cycle, explaining the shorter CtrA and McpA half-lives in cpdRD51A cells. We performed coimmunoprecipitation experiments with a strain containing the cpdRD51A allele translationally fused to a M2-epitope tag at the native cpdR locus as the only cpdR allele within the cell. After immunoprecipitation of CpdRD51A-M2, we performed immunoblots analysis with anti-ClpX and anti-ClpP antibodies showing that unphosphorylated CpdR interacts with ClpX and ClpP (Fig. 3B, lane 3). Together, these results suggest that the phosphorylation state of CpdR determines both the dynamic polar positioning and the activity of the ClpXP proteolysis complex as a function of the cell cycle.
Not surprisingly, when CpdR was kept in its unphosphorylated state by the D51A mutation, we observed that CpdRD51A-YFP was located at the pole in 92% of cpdRD51A swarmer cells, whereas only 23% of the swarmer cells show CpdR-YFP foci in a wild-type background (Fig. 4C). Thus, the CpdR phosphorylation state influences the localization profile of CpdR itself and, consequently, that of ClpXP during the cell cycle.
The CckA Histidine Kinase-Signaling Pathway Mediates CpdR Phosphorylation.
The CckA histidine kinase is known to mediate the phosphorylation of CtrA to create active CtrA∼P (9, 10). Evidence also has been presented that CckA, directly or indirectly, affects CtrA stability (9); the half-life of CtrA was shown to decrease in the absence of CckA. To test the hypothesis that the CckA histidine kinase mediates CpdR phosphorylation, thereby controlling ClpXP-dependent proteolysis of CtrA, we performed in vivo phosphorylation experiments to examine the phosphorylation state of CpdR in the absence or presence of CckA. The CckA histidine kinase is essential for viability, but CckA becomes dispensable in the presence of a phosphorylation-independent version of CtrA (CtrAD51E) (5, 9). Accordingly, the phosphorylation state of CpdR could be assayed in both the wild-type strain bearing a plasmid-borne ctrAD51E allele and a cckA deletion strain bearing the same ctrAD51E allele. Fig. 4A shows that CpdR is not phosphorylated in a strain lacking CckA (Fig. 4A, lane 5), whereas CpdR is phosphorylated in the control strain, in which CckA is present (Fig. 4A, lane 4). The absence of CpdR phosphorylation in the cckA deletion mutant was not due to a lack of CpdR protein (Fig. 4A, lanes 4 and 5 whole cell extract). This result demonstrates that the CckA histidine kinase is required for the phosphorylation of CpdR, creating a redundant regulatory mechanism in which the same histidine kinase mediates CtrA phosphorylation, thereby activating its regulatory functions, and also mediates CpdR phosphorylation, thus preventing the polar localization of ClpXP and, consequently, the proteolysis of CtrA.
CpdR Phosphorylation Is Cell Cycle-Regulated.
We have demonstrated that the phosphorylation state of CpdR controls the polar localization of the ClpXP protease and, thus, its activity. Because the pattern of ClpXP polar localization varies during the cell cycle, these observations predict that the phosphorylation state of CpdR should vary in a parallel manner. Accordingly, we performed in vivo phosphorylation experiments in synchronized cell cultures (Fig. 5A). At the time points shown, aliquots of synchronized cells were pulse-labeled with [γ-32P]-ATP and then immunoprecipitated with anti-CpdR antibody. CpdR∼[32P] was not detected at the transition to stalked cells, coincident with CpdR polar localization, but appeared in predivisional cells at the time of CckA autophosphorylation (Fig. 5A). When CckA is active, CpdR is phosphorylated, and neither CpdR∼P nor ClpXP is localized to the cell pole. It is at this time in the cell cycle that CtrA∼P is active. Because it has been reported that autophosphorylation of CckA cannot be detected in the swarmer cell (9), [γ-32P]-ATP pulse-labeled swarmer cells would not be expected to yield CpdR∼[32P] and, in fact, that was the case (Fig. 5A). To demonstrate that CpdR is maintained in the phosphorylated state in progeny swarmer cells, we grew a mixed population of cells in the presence of [γ-32P]-ATP. We then isolated swarmer cells and allowed them to proceed through the cell cycle. Samples taken at 0, 20, and 40 min were immunoprecipitated with anti-CpdR antibody. CpdR∼[32P] appears in swarmer cells and then is lost at the swarmer-to-stalked cell transition (Fig. 5A). These results demonstrate that the cell cycle-dependent phosphorylation state of CpdR correlates CpdR localization, the localization of the ClpXP protease, and, therefore, the activity of the ClpXP-mediated proteolysis of CtrA and McpA.
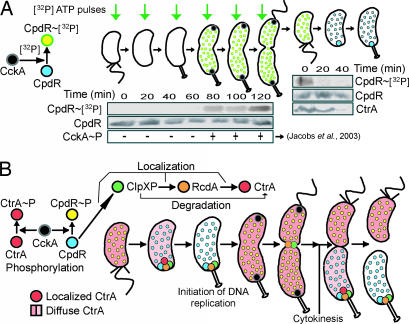
Fig. 5.
The regulation of CtrA proteolysis by cell cycle-dependent phosphorylation of CpdR. (A) Schematic model of CpdR phosphorylation in the presence of [γ-32P] mediated by CckA (Left). Aliquots of a population of synchronized wild-type cells (Center) were labeled with 30 μCi [γ-32P]-ATP per ml of culture for 3 min at the indicated intervals. Samples were lysed, and immunoprecipitated with anti-CpdR antibodies. Green arrows represent the time point of the [γ-32P]-ATP pulse. Phosphorylated CpdR is detected in predivisional cells, coincident with the time when CckA is localized and active (9, 10). As a control, additional samples were taken at 20-min intervals for immunoblot analysis with CpdR antibodies to assess total CpdR accumulation. A mixed population of wild-type cells was grown in 500 ml of M5G media to an OD660 of 0.25, resuspended in 20 ml of M5G, labeled with 15 μCi [32P]-ATP per ml of culture for 45 min, and swarmer cells were isolated and allowed to proceed synchronously through the cell cycle. Samples were taken at 0, 20, and 40 min, lysed, and immunoprecipitated with anti-CpdR antibodies, showing that CpdR∼P is present in the swarmer cells (Right). Immunoblots using antibodies to CpdR and CtrA showed no change in CpdR levels and the expected decrease in CtrA at the swarmer-to-stalked cell transition. (B) Model of CckA phospho-signaling pathway mediated proteolysis of the CtrA master regulator. A detailed description is given in Discussion.
Discussion
The control of proteolytic activity has been shown to be critical for cell cycle progression in Caulobacter, proper sporulation in Bacillus subtilis, and the transition in and out of stationary phase in Escherichia coli (1). In Caulobacter, the essential master regulator CtrA controls the transcription of genes involved in cell division, DNA methylation, flagellar biogenesis, and pili biogenesis (6, 19–21) and also silences the initiation of DNA replication by binding to five sites in the origin of replication (7). Because of its multiple functions, the presence and activity of CtrA is tightly regulated, temporally and spatially, by two redundant mechanisms, synthesis-proteolysis and phosphorylation-dephosphorylation (3–5, 8–12, 22–27). Both of these mechanisms have to be simultaneously blocked to inhibit Caulobacter cell cycle progression at the G1/S transition (3, 5). The selection of the right protein at the correct time in the cell cycle for its degradation by a protease depends on intrinsic recognition signals within the protein, on a tagging system, or, in some cases, on an adaptor or effector protein that participates in delivering the substrate to the protease (1). The regulated proteolysis of CtrA at the swarmer-to-stalked cell transition and in the stalked compartment of the predivisional cell requires multiple steps. These steps include the interaction with the RcdA degradation factor (3), the localization of both RcdA and CtrA to the stalked pole (3, 8, 11), the concomitant polar localization of ClpXP protease complex (3), and the physical interaction among ClpX, RcdA, and CtrA at that cell pole. Here, we have shown that the localization of ClpXP is essential for CtrA proteolysis, the CpdR single-domain response regulator mediates the dynamic polar localization and activity of the ClpXP protease, and the localization function of CpdR depends on its phosphorylation state.
CpdR Controls CtrA Degradation by Positioning of the ClpXP Protease at the Pole.
The CpdR single-domain response regulator is required for CtrA localization and degradation and dynamically localizes to the cell pole together with ClpX, RcdA, and CtrA. In absence of CpdR, the ClpXP protease is not positioned at the pole and, consequently, two substrates of ClpXP, the CtrA and the McpA chemoreceptor, are not degraded. CpdR therefore controls the activity of ClpXP by positioning the protease at the cell pole. Thus, the dynamic positioning of a protein complex controls its function. ClpX and ClpP have been shown to be essential for viability in Caulobacter (12). However, the polar localization and activity of the ClpXP protease at the pole is not essential for viability. We have shown that ClpXP also is transiently positioned at the cell division plane (3), independent of CpdR, where it may carry out other location-specific functions essential for the cell division process.
Phosphorylation of CtrA and CpdR by a Common CckA Histidine Kinase Pathway Reinforces the Maintenance of Active CtrA.
The activation of CtrA depends on phosphorylation by the CckA signal transduction pathway (9, 10). CtrA is more stable in the presence of CckA than in its absence by a mechanism that appears to be independent of the CtrA phosphorylation state. It previously has been proposed that CckA may affect the phosphorylation state of another response regulator that directly controls the proteolysis of CtrA by ClpXP (9). We have shown here that the CpdR single-domain response regulator fits that role, because it functions to enable CtrA proteolysis by localizing and activating the ClpXP protease at the cell pole. Significantly, the protease localization function of CpdR is regulated by phosphorylation via the same CckA-signaling pathway that activates CtrA.
Control of Temporal and Spatial Proteolysis by a Cell Cycle Phospho-Signaling System.
We propose that a phospho-signaling pathway that is intrinsically linked to the dynamic localization of regulatory proteins controls the proteolysis of the CtrA master regulator at specific times in the cell cycle (Fig. 5B). The CpdR response regulator is present in its phosphorylated state in swarmer cells. As swarmer cells transition to stalked cells, CpdR accumulates in its unphosphorylated state and enables the localization of ClpXP to the new stalked cell pole. We have shown that the positioning of ClpXP to the pole is essential for the proteolysis of CtrA and the polarly localized McpA chemoreceptor. The clearance of CtrA from the stalked cell allows the initiation of DNA replication (5, 7). After replication initiation, the CckA histidine kinase localizes to the poles of the predivisional cell where its autophosphorylation correlates with the phosphorylation of newly synthesized CtrA (9) and CpdR (Fig. 5A). Both RcdA and ClpXP transiently localize to the cell division plane, where they likely promote the degradation of cell division proteins (3) in a CpdR independent manner. Upon the completion of compartmentalization, CckA disperses (10). CpdR remains in the phosphorylated state in the swarmer cell, preventing the degradation of CtrA∼P and, therefore, maintaining a block in the initiation of DNA replication. In the stalked cell, CpdR accumulates in the dephosphorylated state, thereby promoting its polar localization and the localization of the ClpXP protease. A protein complex with ClpXP, RcdA, and CtrA is formed at the stalked cell pole, allowing the degradation of CtrA (3). Degradation of CtrA in the stalked compartment again permits the initiation of DNA replication. This model predicts the presence of a CpdR∼P phosphatase located in the nascent daughter stalked cell compartment and in the stalked cell resulting from the swarmer-to-stalked cell transition. The integration of a phospho-signaling pathway and dynamic protein localization to control the transient clearance of a critical cell cycle regulatory protein underscores the fact that the complex circuitry controlling bacterial cell cycle progression must be interpreted in the context of the changing 3D structure of the cell.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
All strains and plasmids used in this study are listed in Tables 1 and 2, which are published as supporting information on the PNAS web site. Caulobacter crescentus strains were grown at 28°C in peptone-yeast extract medium (28), M2G minimal medium (29), or M5G low phosphate media supplemented with glutamate (1 mM) (30). When appropriate, growth medium was supplemented with 0.3% d-xylose/3% sucrose/5 μg·ml−1 kanamycin/25 μg·ml−1 spectinomycin/5 μg·ml−1 streptomycin/1 μg·ml−1 oxytetracycline/1 μg·ml−1 chloramphenicol and/or 10 μg·ml−1 apramycin. Synchronizations of cell cultures were performed as described in refs. 31 and 32. Plasmids were introduced into Caulobacter strains by electroporation (33).
Microscopy.
DIC and fluorescence microscopy were performed as described in ref. 11. For time-lapse microscopy, cells were placed on pads composed of 1% agarose. Fluorescein (Chroma 96170M) and YFP (Chroma 41028) filter sets were used for GFP and YFP fluorescence, respectively. Exposure times were 50 ms for DIC images and 2–3 s for fluorescence images. Images were acquired by using metamorph (Universal Imaging, Downingtown, PA) and processed with photoshop (Adobe Systems, San Jose, CA).
Immunoblots and in Vivo Phosphorylation.
Anti-CpdR serum was diluted 1:1,000 for immunoblotting. Antibodies against CtrA, McpA, FliF, CcrM, ClpX, and ClpP were used as described in refs. 5, 12, 16, 34, and 35, and immunoblotting was performed as described in ref. 36. In vivo phosphorylation experiments were performed as described in ref. 5 with the following modification: Each sample was immunoprecipitated with 10 μl of anti-CpdR antibody.
Pulse–Chase, Immunoprecipitation, and Coimmunoprecipitation.
The determination of CtrA and McpA stability was described in ref. 3 by using 2 μl of anti-CtrA or anti-McpA antibody per sample. The method used for coimmunoprecipitation experiments was as described in ref. 3.
Supplementary Material
Acknowledgments
We thank the members of the Shapiro and McAdams laboratories for advice and support and Martin Thanbichler, Steve Landt, Justine Collier, Leticia Britos, and Patrick Viollier for critical review of the manuscript. This work was supported by National Institutes of Health Grant GM32506 (to L.S.) and Department of Energy, Office of Science Grants DE-FG02-01ER63219 and DE-FG02-04ER63922 (to H.H.M. and L.S.). P.T.M. was supported by Stanford Genome Training Program Grant T32 HG00044 from the National Human Genome Research Institute.
Abbreviations
- DIC
differential interference contrast
- YFP
yellow fluorescent protein.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Gottesman S. Annu. Rev. Cell. Dev. Biol. 2003;19:565–587. doi: 10.1146/annurev.cellbio.19.110701.153228. [DOI] [PubMed] [Google Scholar]
- 2.Ades S. E. Curr. Biol. 2004;14:R924–R926. doi: 10.1016/j.cub.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 3.McGrath P. T., Iniesta A. A., Ryan K. R., Shapiro L., McAdams H. H. Cell. 2006;124:535–547. doi: 10.1016/j.cell.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 4.Judd E. M., Ryan K. R., Moerner W. E., Shapiro L., McAdams H. H. Proc. Natl. Acad. Sci. USA. 2003;100:8235–8240. doi: 10.1073/pnas.1433105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domian I. J., Quon K. C., Shapiro L. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 6.Laub M. T., Chen S. L., Shapiro L., McAdams H. H. Proc. Natl. Acad. Sci. USA. 2002;99:4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quon K. C., Yang B., Domian I. J., Shapiro L., Marczynski G. T. Proc. Natl. Acad. Sci. USA. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan K. R., Huntwork S., Shapiro L. Proc. Natl. Acad. Sci. USA. 2004;101:7415–7420. doi: 10.1073/pnas.0402153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs C., Ausmees N., Cordwell S. J., Shapiro L., Laub M. T. Mol. Microbiol. 2003;47:1279–1290. doi: 10.1046/j.1365-2958.2003.03379.x. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs C., Domian I. J., Maddock J. R., Shapiro L. Cell. 1999;97:111–120. doi: 10.1016/s0092-8674(00)80719-9. [DOI] [PubMed] [Google Scholar]
- 11.Ryan K. R., Judd E. M., Shapiro L. J. Mol. Biol. 2002;324:443–455. doi: 10.1016/s0022-2836(02)01042-2. [DOI] [PubMed] [Google Scholar]
- 12.Jenal U., Fuchs T. EMBO J. 1998;17:5658–5669. doi: 10.1093/emboj/17.19.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai J. W., Alley M. R. J. Bacteriol. 2001;183:5001–5007. doi: 10.1128/JB.183.17.5001-5007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alley M. R., Maddock J. R., Shapiro L. Science. 1993;259:1754–1757. doi: 10.1126/science.8456303. [DOI] [PubMed] [Google Scholar]
- 15.Porankiewicz J., Wang J., Clarke A. K. Mol. Microbiol. 1999;32:449–458. doi: 10.1046/j.1365-2958.1999.01357.x. [DOI] [PubMed] [Google Scholar]
- 16.Jenal U., Shapiro L. EMBO J. 1996;15:2393–2406. [PMC free article] [PubMed] [Google Scholar]
- 17.Grunenfelder B., Tawfilis S., Gehrig S. M, Osteras M., Eglin D., Jenal U. J. Bacteriol. 2004;186:4960–4971. doi: 10.1128/JB.186.15.4960-4971.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stock A. M., Robinson V. L., Goudreau P. N. Annu. Rev. Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 19.Skerker J. M., Shapiro L. EMBO J. 2000;19:3223–3234. doi: 10.1093/emboj/19.13.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly A. J., Sackett M. J., Din N., Quardokus E., Brun Y. V. Genes Dev. 1998;12:880–893. doi: 10.1101/gad.12.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisenauer A., Quon K., Shapiro L. J. Bacteriol. 1999;181:2430–2439. doi: 10.1128/jb.181.8.2430-2439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quon K. C., Marczynski G. T., Shapiro L. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 23.Domian I. J., Reisenauer A., Shapiro L. Proc. Natl. Acad. Sci. USA. 1999;96:6648–6653. doi: 10.1073/pnas.96.12.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reisenauer A., Shapiro L. EMBO J. 2002;21:4969–4977. doi: 10.1093/emboj/cdf490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtzendorff J., Hung D., Brende P., Reisenauer A., Viollier P. H., McAdams H. H., Shapiro L. Science. 2004;304:983–987. doi: 10.1126/science.1095191. [DOI] [PubMed] [Google Scholar]
- 26.Hung D. Y., Shapiro L. Proc. Natl. Acad. Sci. USA. 2002;99:13160–13165. doi: 10.1073/pnas.202495099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce D. L., O’Donnol D. S., Allen R. C., Javens J. W., Quardokus E. M., Brun Y. V. J. Bacteriol. 2006;188:2473–2482. doi: 10.1128/JB.188.7.2473-2482.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poindexter J. S. Bacteriol. Rev. 1964;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson R. C., Ely B. Genetics. 1977;86:25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs C., Hung D., Shapiro L. Proc. Natl. Acad. Sci. USA. 2001;98:4095–4100. doi: 10.1073/pnas.051609998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alley M. R. Mol. Microbiol. 2001;40:1335–1343. doi: 10.1046/j.1365-2958.2001.02476.x. [DOI] [PubMed] [Google Scholar]
- 32.Evinger M., Agabian N. J. Bacteriol. 1977;132:294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viollier P. H., Thanbichler M., McGrath P. T., West L., Meewan M., McAdams H. H., Shapiro L. Proc. Natl. Acad. Sci. USA. 2004;101:9257–9262. doi: 10.1073/pnas.0402606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alley M. R., Maddock J. R., Shapiro L. Genes Dev. 1992;6:825–836. doi: 10.1101/gad.6.5.825. [DOI] [PubMed] [Google Scholar]
- 35.Stephens C., Reisenauer A., Wright R., Shapiro L. Proc. Natl. Acad. Sci. USA. 1996;93:1210–1214. doi: 10.1073/pnas.93.3.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J. C., Viollier P. H., Shapiro L. Mol. Microbiol. 2005;55:1085–1103. doi: 10.1111/j.1365-2958.2004.04443.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.