Abstract
Hearing in mammals depends upon the proper development of actin-filled stereocilia at the hair cell surface in the inner ear. Whirlin, a PDZ domain-containing protein, is expressed at stereocilia tips and, by virtue of mutations in the whirlin gene, is known to play a key role in stereocilia development. We show that whirlin interacts with the membrane-associated guanylate kinase (MAGUK) protein, erythrocyte protein p55 (p55). p55 is expressed in outer hair cells in long stereocilia that make up the stereocilia bundle as well as surrounding shorter stereocilia structures. p55 interacts with protein 4.1R in erythrocytes, and we find that 4.1R is also expressed in stereocilia structures with an identical pattern to p55. Mutations in the whirlin gene (whirler) and in the myosin XVa gene (shaker2) affect stereocilia development and lead to early ablation of p55 and 4.1R labeling of stereocilia. The related MAGUK protein Ca2+-calmodulin serine kinase (CASK) is also expressed in stereocilia in both outer and inner hair cells, where it is confined to the stereocilia bundle. CASK interacts with protein 4.1N in neuronal tissue, and we find that 4.1N is expressed in stereocilia with an identical pattern to CASK. Unlike p55, CASK labeling shows little diminution of labeling in the whirler mutant and is unaffected in the shaker2 mutant. Similarly, expression of 4.1N in stereocilia is unaltered in whirler and shaker2 mutants. p55 and protein 4.1R form complexes critical for actin cytoskeletal assembly in erythrocytes, and the interaction of whirlin with p55 indicates it plays a similar role in hair cell stereocilia.
Keywords: mutant analysis, stereocilia development
Actin cytoskeleton remodeling is fundamental to a variety of cellular processes, including morphological alterations at the cell surface. In the mammalian inner ear, hair cells develop highly organized bundles of actin-filled stereocilia whose most prominent feature is an extraordinary staircase arrangement. The anatomical process of stereocilia development from microvilli on the surface of hair cells in the vicinity of the kinocilium has been well described both in vivo and in organotypic culture (1–5). At around embryonic day (E)17.5 in mice, microvilli begin to elongate. As stereocilia thicken and continue to elongate, the staircase pattern emerges from around postnatal day (P)1 in mice, with morphological development complete around P5–7. Stereocilia in adjacent rows are connected by tip links that attach the tips of shorter stereocilia to the sides of neighboring taller stereocilia. Tip-link attachment sites are thought to harbor mechanotransduction channels, such that sound stimulation and stereocilia deflection lead to channel opening, cation influx, hair cell depolarization, and auditory transduction (6).
Until recently, the critical molecules for development of the stereocilia bundle were unknown. However, recent work has established that whirlin, a PSD-95/SAP90 Discs-large ZO-1 homologous (PDZ) protein, localizes to the stereocilia tips and, by virtue of mutations in the whirlin gene, has been shown to play an important role in stereocilia development (7). Myosin XVa interacts with whirlin and is responsible for localizing whirlin at the stereocilia tip (8, 9). We have recently shown that whirlin expression is dynamic during stereocilia growth, further underlining its key role in stereocilia development (10). Whirlin demonstrates an ordered appearance and fadeout across the stereocilia rows, beginning with the tallest stereocilia. However, identifying the mechanism by which whirlin mediates actin polymerization and stereocilia growth requires the identification of interacting partners and their localization within developing hair cells.
We have identified an interacting partner to the whirlin protein, the membrane-associated guanylate kinase (MAGUK) protein, erythrocyte protein p55 (p55), and explored its expression in developing stereocilia. We have also explored the expression of the related MAGUK protein, Ca2+-calmodulin serine kinase (CASK), and the protein 4.1 isoforms that interact with p55 and CASK. To explore the function of complexes at the stereocilia tip, comprising whirlin, p55, and protein 4.1 (4.1), we have investigated the expression of these proteins in two mutants, whirler and shaker2, which affect stereocilia development.
Results
Yeast Two-Hybrid Screen.
We carried out a yeast two-hybrid screen by using the short isoform of whirlin as a bait to screen a mouse 17-day embryonic cDNA library. The short isoform contains the PDZ3 and proline-rich domains and is sufficient to rescue the stereocilia growth defect in whirler mutant mice (7). We identified 17 interacting clones, of which three were identified as p55 (data not shown). p55 is a member of the MAGUK family of adaptor proteins. Whirlin has been shown to interact with a related MAGUK protein, CASK, mediated by whirlin’s PDZ3 domain and the guanylate kinase-like (GUK) domain of CASK (11). Moreover, CASK interacts with a brain-enriched isoform of protein 4.1, 4.1N, and the transmembrane protein neurexin to form a tripartite complex that links cell-surface proteins to the actin cytoskeleton and promotes local assembly of actin/spectrin filaments in neurons (12). Both p55 and CASK carry HOOK domains that mediate interaction with protein 4.1 as well as a PDZ domain that interacts with the cytoplasmic domain of cell-surface molecules. In erythrocytes, p55 forms an analogous tripartite complex interacting with isoform 4.1R and the cell-surface molecule, glycophorin C (13). On the basis of the yeast two-hybrid data, we hypothesized that whirlin forms a complex with p55 in growing stereocilia.
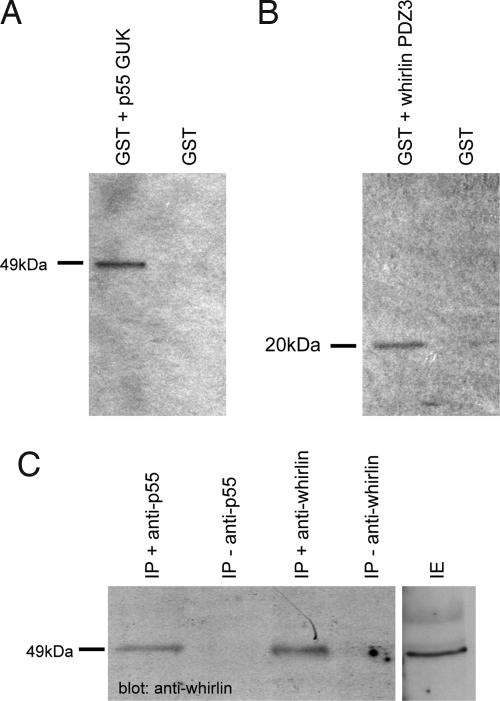
We demonstrated, using in vitro GST pull-down assays, that whirlin interacts with p55 (Fig. 1A and B). Because the whirlin PDZ3 domain had already been shown to interact with the GUK domain of the related protein CASK (11), we focused on exploring interactions between the whirlin short isoform (containing the PDZ3 domain) and the GUK domain of p55. We found that a GST fusion with the GUK domain of p55 was able to pull down the whirlin short isoform (PDZ3 + proline-rich domain). Conversely, a GST fusion with the whirlin short isoform pulled down the p55 GUK domain.
Fig. 1.
In vitro (A and B) and in vivo (C) pull-down assays between whirlin and p55. (A and B) GST-tagged GUK domain of p55 or GST-tagged whirlin PDZ3 domain were expressed in E. coli K12 ER2508 cells using pGEX-4T1. Immobilized proteins were incubated with in vitro translated 35S-labeled short whirlin isoform (A) and the C-terminal region (Lys-323 through Tyr-466), including the GUK domain of p55 (B). GST protein alone was used as a negative control. (C) Cochlear proteins were incubated with p55 or the short whirlin isoform-specific antibodies. Immunoprecipitates from each were then subjected to immunoblot analysis with the short isoform of whirlin. IE, cochlear protein extract.
To confirm the interaction of whirlin and p55 in vivo, we also carried out co-immunoprecipitation (IP) of cochlear extracts from P7–8 mice using both whirlin and p55 antibodies (Fig. 1C). IP using antibodies to p55 pulled down the 49-kDa short isoform of whirlin from cochlear proteins.
Localization of p55 and CASK Protein in the Inner Ear Hair Cells of Mouse.
Given the observed interactions between whirlin and p55, we proceeded to investigate the localization of p55 in the developing stereocilia bundle and at the apical hair cell surface. From E17.5, mouse stereocilia differentiate from microvilli on the apical hair cell surface. Around P0, the developing bundles in apical turns of the cochlea are only partly differentiated and hemispherical and consist of poorly organized rows of thickly clustered stereocilia gradually increasing in height toward the kinocilium (5). From P1–2, the developing bundle is still hemispherical with many microvilli-like structures, within which, nearest to the kinocilium, highly organized rows of stereocilia have begun to emerge with well defined height increments. This structure is fated to develop into the typical highly differentiated and regular staircase arrangement of the stereocilia bundle found in the adult. The surrounding shorter microvilli structures will ultimately regress.
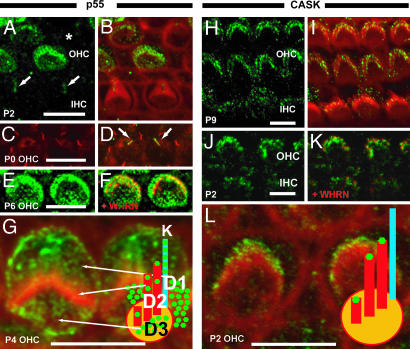
First, we explored p55 labeling of hair cells across a wide range of time points and found that p55 is expressed at the apical hair cell surface of outer hair cells (OHCs) from E17.5 (Fig. 2 and data not shown). In all of the developmental stages examined, there was no significant p55 staining in inner hair cells (IHCs; data not shown). Moreover, labeling of OHCs was patchy, with some cells strongly labeled and some weakly, and others were not labeled at all (Fig. 2 A and B).
Fig. 2.
Localization of p55 and CASK at the apical surface of mouse hair cells. (A and B) Cochlear whole mounts were stained with antibody to p55 (green) and phalloidin (red). (A) p55 labeling of P2 hair cells. (B) Merge of p55 and phalloidin. Some OHCs are strongly labeled, others weakly, and some are not labeled (asterisk). In postnatal stages, no significant levels of p55 staining were detected in stereocilia of IHCs (data not shown). (C) β-Tubulin (red) staining highlights microtubules in P0 OHCs. Arrows indicate kinocilia. (D) Merged image with p55. The merged image shows that p55 and β-tubulin colocalize in the kinocilia. (E and F) p55 (green) colocalizes with whirlin (red) at the stereocilia tips in hair cells. (E) p55 localization in P6 OHC. (F) Merged image with whirlin. (G) More highly magnified image of p55 labeling in P4 OHCs; p55 is detected in stereocilia (domain, D2) as well as strial (D1), neural (D3) domains of the OHCs, and kinocilia (k). (H–L) Cochlear whole mounts were stained with antibody to CASK (green) and phalloidin (red). (H) CASK labeling of P9 OHCs and IHCs. (I) Merge of CASK and phalloidin. CASK was localized at stereocilia tips of both OHCs and IHCs. (J and K) CASK (green) colocalizes with whirlin (red) at the stereocilia tips in hair cells. (K) Merged image with whirlin localization. (L) More highly magnified image of CASK labeling in P2 OHCs. CASK labeling is detected at stereocilia tips. (Scale bar, 5 μm.)
We have previously shown that whirlin localizes to the tips of mouse hair cell stereocilia (10). The signals of p55 also can be seen in the stereocilia tips, which are partially colocalized with whirlin at the stereocilia tips (domain 2, Fig. 2 E–G). However, we also observed significant scattered labeling at the apical hair cell surface outside of the longest stereocilia, both strial and neural (domains 1 and 3; Fig. 2G), which appeared to represent labeling of shorter microvilli structures (5) that will ultimately regress. This pattern of staining continued until P17, where labeling of both longer and shorter structures decreased significantly (data not shown). On average, from E17.5 to P17, 76% of cells showed labeling distributed relatively evenly between the three domains. Whirlin expression appears at P1 and fades out at P14 (10). Thus, p55 expression mirrors aspects of whirlin’s temporal pattern of expression. However, p55 shows a differing spatial pattern. p55 labeling does not obviously appear to traverse the rows of the stereocilia bundle in a temporally ordered fashion, as has been observed for whirlin (10). Also, whirlin does not show both the strial and neural domains of labeling in shorter microvilli that we have observed for p55 and is confined to the longer stereocilia that form the mature stereocilia bundle (10). Thus, it would appear that p55 is expressed in stereocilia structures that do not harbor whirlin, which indicates that p55 occupies specific domains within the hair cell in the absence of interactions with the whirlin protein.
We also explored the localization of the CASK protein in hair cells. Unlike p55, CASK labeling is seen only in the main stereocilia bundle structure but not microvilli (Fig. 2 H and I). The CASK signal is also detected in both OHC and IHC stereocilia bundles. As with whirlin, the labeling is predominantly localized at the stereocilia tips, and dual-labeling experiments with whirlin indicate significant colocalization of whirlin and CASK (Fig. 2 J and K).
Localization of Protein 4.1 Isoforms in the Inner Ear Hair Cells of Mouse.
Different MAGUK proteins are coupled to the actin cytoskeleton through different members of the protein 4.1 family (see above; refs. 12–16). Four isoforms of 4.1 protein, namely 4.1B, 4.1G, 4.1N, and 4.1R, have been identified (17, 18). We proceeded to investigate the expression of isoforms 4.1R and 4.1N that interact with the MAGUK proteins p55 and CASK, respectively.
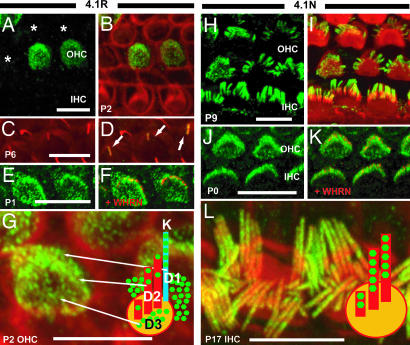
By immunohistochemical analysis, both 4.1R and 4.1N are expressed in hair cells. Interestingly, 4.1R shows a nearly identical pattern of labeling to that of p55 (Fig. 3A–G). Under high magnification (Fig. 3G), the labeling of 4.1R is detected in the stereocilia bundle (domain 2) and partially colocalizes with whirlin at the stereocilia tip (Fig. 3 E and F). As with p55, we also observed significant labeling at the apical hair cell surface outside of the longest stereocilia, both strial and neural (domains 1 and 3), which similarly appeared to represent labeling of microvilli structures (5) that will ultimately regress. We detected no significant labeling of IHCs by 4.1R (data not shown). Moreover, there are some cells that are not labeled (Fig. 3A). 4.1R labeling declines significantly around P14, followed by fadeout of 4.1R expression in all hair cells at P17 (data not shown). In contrast to 4.1R, expression of 4.1N is confined to the stereocilia bundles in both OHC and IHC (Fig. 3 H–L). At P0 and P5, 4.1N is localized in the developing bundle, including many immature microvilli-like structures on the cuticular plates (Fig. 3J). After P9, 4.1N is detected in the stereocilia bundles (Fig. 3 H and I), where it demonstrates colocalization with whirlin at the stereocilia tip (Fig. 3 J and K). 4.1N is constitutively expressed at the stereocilia bundles until adulthood (data not shown).
Fig. 3.
Localization of 4.1R (A–G) and 4.1N (H–L) at the apical surface of mouse hair cells. (A and B) Cochlear whole mounts were stained with antibody to 4.1R (green) and phalloidin (red). (A) 4.1R labeling of P2 hair cells. (B) Merge of 4.1R and phalloidin. Some cells are strongly labeled, others weakly, and some are not labeled (asterisks). In postnatal stages, no significant 4.1R staining was detected in IHCs (data not shown). (C and D) 4.1R labeling was also observed in the kinocilia. (C) β-Tubulin (red) staining highlights microtubules in P6 OHCs. Arrows indicate kinocilia. (D) Merged image with 4.1R labeling. The merged image shows that the 4.1R and β-tubulin colocalize in the kinocilia. (E and F) 4.1R (green) colocalizes with whirlin (red) at the stereocilia tips in hair cells. (E) 4.1R localization in P1 OHC. (F) Merged image with whirlin. (G) More highly magnified image of 4.1R labeling in P2 OHCs. 4.1R is detected in stereocilia (domain, D2) as well as neural (D3), strial (D1) domains of the OHCs, and kinocilia (k). (H–L) Cochlear whole mounts were stained with antibody to 4.1N (green) and phalloidin (red). (H) 4.1N labeling of P9 OHCs and IHCs. (I) Merge of 4.1N and phalloidin. 4.1N was localized at stereocilia bundles of both OHCs and IHCs. (J and K) 4.1N (green) colocalizes with whirlin (red) at the stereocilia tips in hair cells. (K) Merged image with whirlin. (L) 4.1N localization in P17 hair cells. (L) More highly magnified image of 4.1N labeling in P17 IHCs. 4.1N labeling was not detected along the whole length of stereocilia but was concentrated at the apical regions of stereocilia bundles, including the tips. (Scale bar, 5 μm.)
p55 and 4.1R Labeling of Kinocilia.
We also detected labeling of hair cell kinocilia with both p55 (Fig. 2 C and D) and 4.1R (Fig. 3 C and D) at stages P0–6. To confirm kinocilia labeling, we carried out double staining using β-tubulin antibody. We demonstrated colocalization of both p55 and 4.1R with β-tubulin signals at the kinocilia (Figs. 2 C and D and 3 C and D). Interestingly, p55 and 4.1R are specifically localized to the kinocilium, with no labeling observed in other microtubule structures in the organ of Corti, such as the primary cilia of Dieters cells and tubuler belts. Moreover, kinocilia signals were detected in both OHCs and IHCs.
Functional Analysis of p55, CASK, and 4.1 in Stereocilia Using Whirler and Shaker2 Mutants.
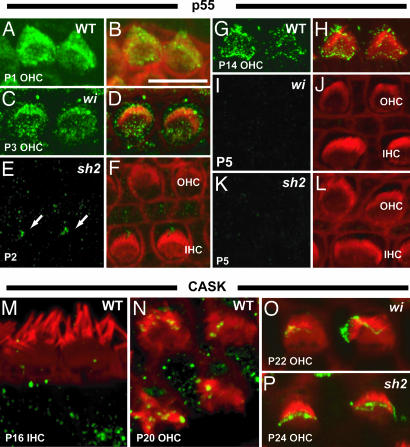
We proceeded to explore further the interaction of whirlin, p55, and 4.1 proteins by investigating the labeling of p55 in the whirler mutant, which lacks whirlin, and the shaker2 mutant, which lacks myosin XVa (7, 18). We discovered that expression of p55 is ablated in the absence of whirlin from P5 onwards (Fig. 4I and J). Up to P5, the labeling pattern appears similar to WT. This result supports the interaction of p55 and whirlin and suggests that whirlin is required for localization of p55 in stereocilia, as well as other sites at the apical hair cell surface. In addition, we found that expression of p55 is completely ablated in shaker2 mutant hair cells at all time points (Fig. 4 E–L). However, p55 was still expressed in kinocilia (Fig. 4 E and F).
Fig. 4.
Localization of p55 and CASK at the apical surface of hair cells in WT, whirler (wi), and shaker2 (sh2) mutant mice during cochlear development. All images were taken from the apical turn of the cochlea. (A–L) Localization of p55 in WT and mutant mice during cochlea development. In WT mice, p55 labeling declines significantly around P14 (G and H) and fades out at P17 (data not shown). In the wi mutant, expression of p55 is normal until the P4 stage (P3 stage illustrated in C and D). However, labeling of p55 fades out from P5 in the wi mutant (I and J). Expression of p55 in stereocilia is completely ablated in the sh2 mutant (E, F, K, and L), although labeling of kinocilia (arrows) is still detected (E and F). (M–O) Localization of CASK in WT and mutant mice in mature hair cells. In WT mice, CASK signals were detected in stereocilia tips into adulthood in OHCs (N), but IHC labeling was not seen after P16 (M). CASK labeling is unaffected in both mutants in OHCs and IHCs (O and P and data not shown).
We also analyzed the labeling of the related MAGUK protein, CASK, in the whirler and shaker2 mutants. There was some diminution of signal in the stereocilia at P22 in the whirler mutant, but otherwise the pattern of labeling was similar to WT in both mutants (Fig. 4 M–O).
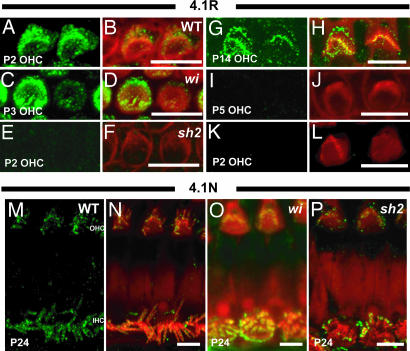
We also investigated the effects of the whirler and shaker2 mutants on the localization of 4.1 isoforms. 4.1R is also ablated in the whirlin mutant from around P5 onward (Fig. 5C, D, I, and J). Again, similar to p55, we found that labeling of 4.1R is completely ablated in the shaker2 mutant at all time points (Fig. 5 E, F, K, and L). However, 4.1R labeling of kinocilia can still be detected in the shaker2 mutant (data not shown). We saw no effect of the whirlin or shaker2 mutation on labeling of the 4.1N isoform (Fig. 5 M–P).
Fig. 5.
Localization of 4.1R and 4.1N at the apical surface of hair cells in WT, whirler (wi), and shaker2 (sh2) mutant mice during cochlear development. All images were taken from the apical turn of the cochlea. (A–L) Localization of 4.1R in WT and mutant mice during cochlea development. 4.1R shows a nearly identical pattern of labeling to that of p55 in the WT. Labeling of 4.1R fades out from P5 in the whirler mutant (C, D, I, and J). Expression of 4.1R in stereocilia is completely ablated in the shaker2 mutant (E, F, K, and L), although labeling of kinocilia is still detected (data not shown). (M–P) Localization of 4.1N in WT and mutant mice in mature hair cells. In WT mice, 4.1N signals were detected in stereocilia into adulthood as with CASK (M and N). 4.1N labeling is unaffected in both mutants (O and P).
Discussion
We present a variety of evidence, including pull-down, localization, and mutant data to support the interaction of whirlin, the MAGUK protein, p55, and protein 4.1R within stereocilia structures in the hair cells of the inner ear. First, in vitro and in vivo pull downs identify an interaction between whirlin and p55. Second, both p55 and 4.1R demonstrate similar labeling patterns in hair cells, with signal detected not only in the longest stereocilia structures that form the stereocilia bundle proper but also in additional domains that include microvilli on the apical hair surface that will ultimately regress. Finally, the whirler and shaker2 mutants show similar effects on p55 and 4.1R stereocilia labeling.
The expression of p55 and 4.1R in the longest stereocilia, the site of whirlin expression, leads us to propose that p55, 4.1R, and whirlin together form a complex responsible for mediating actin polymerization and stereocilia development. This is supported by the pull-down data and the effect of the whirler mutation on p55 and 4.1R expression. In erythrocytes, p55 forms a complex with 4.1R and glycophorin C that serves as an anchor for actin microfilament assembly. It appears that p55 plays a wider role in actin cytoskeletal assembly beyond its known erythrocyte function. In keeping with current models of MAGUK protein complexes that mediate actin cytoskeleton assembly (12, 14), it will be important to determine any cell-surface proteins, analogous to glycophorin C, to which p55 binds in stereocilia and which may mediate extracellular signals controlling actin polymerization and stereocilia organization.
p55 and 4.1R signal is detected in domains at the apical hair cell surface that do not express whirlin (8–10). Thus p55 and 4.1R are able to occupy domains in the absence of whirlin interaction. The functional relevance of the expression of both p55 and 4.1R in domains outside of the longest stereocilia is not yet clear. However, it does suggest that these proteins, mediating as they do actin polymerization, are not only controlling stereocilia elongation but also regulating the growth of microvilli structures fated to regress.
It is intriguing that, although whirlin and myosin XVa expression is confined to the mature stereocilia bundle structure, the absence of these proteins leads to loss of p55 and 4.1R labeling in both the stereocilia bundle and surrounding shorter stereocilia-like structures. Thus both these mutants, although they affect proteins that are localized to the longest stereocilia, generate pleiotropic effects at other regions of the hair cell. The function of myosin XVA appears to be to traffic whirlin to the stereocilia tip (9) and would presumably be fundamental to the establishment of a p55/4.1R/whirlin complex. This is reflected in the observation that the shaker2 mutant shows a complete ablation of expression of p55 and 4.1R. In contrast, in the whirler mutant, the p55 and 4.1R signal fades out from around P5, indicating that whirlin plays an important role in stabilizing the complex but is not pivotal in the trafficking of p55 and 4.1R to the stereocilia tip.
Recognizing the structural and functional similarities between the p55/4.1R and CASK/4.1N complexes, and the interaction between whirlin and CASK in neurons (11, 15), we investigated the expression of CASK and 4.1N in stereocilia. Both the proteins displayed signal that was largely confined to the mature stereocilia bundle structure in both OHCs and IHCs and thus qualitatively different from the p55 and 4.1R patterns. Moreover, the absence of whirlin in the whirler mutant or myosin XVa in the shaker2 mutant showed no major effect on the expression of either CASK or 4.1N.
We demonstrated that both p55 and 4.1R signal localize at the kinocilia of inner ear hair cells. The migration of the kinocilium to one side of the developing stereocilia bundle is a step that defines bundle polarity, and kinocilial links connect the kinocilium to the adjacent tallest stereocilia (19, 20). 4.1R has been shown to interact with interphase microtubules in human T cells, the components of the contractile apparatus in skeletal myofibers, and the tight junctions in epithelial cells (21). 4.1R has also been shown to colocalize with mitotic microtubules during the mitotic stage of the cell cycle, and 4.1R associates with mitotic-spindle proteins such as dynein and dynactin (22). Possibly, 4.1R and p55 may have a role not only in stereocilia development but also in the stereocilia–kinocilium linkage. There is a variety of evidence to indicate that cadherin 23 (CDH23) and a transient receptor potential (TRP) channel 1 (TRPA1) are components of the tip link and the mechanotransduction channel, respectively, the key machinery of the mechanotransduction apparatus (23–25). Interestingly, CDH23 and transient receptor potential channel 1 (TRPN1), another TRP family member, are also localized at the kinocilia of hair cells in rodents and Xenopus, respectively (26, 27). It will be important to explore whether there are any functional interactions among p55, 4.1R, and these other groups of stereocilia proteins. It has been recently reported that whirlin interacts with two components of the usherin proteome, USH2A (28, 29) and VLGR1b (29). USH2A appears to be a component of interstereocilia ankle links (28). USH2A and VLGR1b colocalize with whirlin at the synaptic regions of photoreceptor cells and OHCs (29). It will also be important to establish the relationship, if any, between the interactions reported here and these observations.
Methods
Yeast Two-Hybrid Screen.
The short isoform of whirlin, including the PDZ3 and proline-rich domains, was used as bait to screen a pretransformed mouse E17 cDNA library according to the Matchmaker Two-Hybrid System Protocol (Clontech).
Antibodies.
p55 (T-19), CASK (N-17), 4.1R (C16 or P20), and 4.1N (D-17) antibodies were purchased from Santa Cruz Biotechnology and were tested for specificity by Western and tissue section analysis (see supporting information, which is published on the PNAS web site). All antibodies detected a single polypeptide of the expected size on immunoblots. In addition, the antibodies demonstrated the expected expression patterns by immunohistochemistry on tissue sections.
In Vitro Pull-Down Assays.
The in vitro pull-down assays were carried out by using the Promega MagneGST Pull-Down System according to the manufacturer’s instructions. Briefly, the C-terminal region (Lys-323 through Tyr-466), including the GUK domain of p55 or the short whirlin isoform, were radiolabeled with35S by in vitro translation by using the T7-coupled transcription-translation system (Promega). GST fusion proteins containing either the GUK domain of p55, the PDZ3 domain of whirlin, or GST alone were expressed in Escherichia coli K12 ER2508 cells by using pGEX-4T1 and purified and immobilized according to the manufacturer’s instructions. To verify that equivalent amounts of specific GST-fusion proteins and GST protein alone were used, extracts were analyzed by Coomassie blue staining. Purified proteins were then incubated with equal amounts of 35S-labeled whirlin short isoform or the C-terminal region (Lys-323 through Tyr-466), including the GUK domain of p55 for 35 min at room temperature on a rotating wheel. GST alone was used as a negative control for each specific GST-fusion protein. The beads were then washed eight times with wash/binding buffer (Promega) supplemented with 0.05% Nonidet P-40. Bound proteins were resuspended in 20 μl of 2× SDS sample buffer and analyzed on 12.5% SDS polyacrylamide gels that were then dried and autoradiographed.
In Vivo Pull-Down Assays.
Cochleas from mice aged between P7 and P8 were homogenized in PBS containing 0.5 mM PMSF, 1 mM DTT, 0.5 mM EDTA, and a protease inhibitor mixture (Roche Diagnostics). The homogenate was then centrifuged (20 min, 13,000× g) and the supernatant used as the cochlear soluble protein extract in the in vivo pull-down assays (ExtraCruz D from Santa Cruz Biotechnology), according to the manufacturer’s instructions. Briefly, 50 μl of suspended IP matrix was mixed with 10 μg of IP antibody, either p55 or whirlin short isoform specific, and 500 μl of PBS and incubated at 4°C overnight on a rotating wheel. After incubation, the IP antibody–IP matrix complex was pelleted by centrifugation at 13,000 × g for 30 sec at 4°C and the pelleted complex washed twice with PBS containing a protease inhibitor mixture (Roche). The IP antibody–IP matrix complex was then incubated with 100 μg of precleared cochlear soluble protein extract and incubated overnight at 4°C on a rotating wheel. After incubation, the mix was centrifuged and the pellet washed as above four times. The pellet was then resuspended in 2× reducing sample buffer and samples analyzed on a 12.5% SDS–polyacrylamide gel that was then subjected to immunoblot analysis by using the appropriate primary antibody.
Immunohistochemistry.
Rabbit anti-whirlin polyclonal antibody was as described (10). Whole-mount immunostaining for mouse cochlea was performed as described (10). Primary antibodies at the dilutions indicated were: whirlin (1:100), p55 (1:100), CASK (1:200), 4.1R (C16, 1:100; P20, 1:50), and 4.1N (1:150). F-actin and individual cilia were visualized by using phalloidin (Texas red- or Alexa Fluor 633-conjugated; Molecular Probes) and anti-β-tubulin antibody (Cy3-conjugated; Sigma), respectively. Negative controls were performed by using identical protocols, except that primary antibodies were preadsorbed with the relevant epitope peptide (Santa Cruz Biotechnology) at 4°C overnight. Fluorescence images were obtained with a confocal microscope (LSM 510 or LSM 510 META; Zeiss) equipped with Plan Neofluar (100×/1.30 oil immersion objective) or α Plan-Fluar (100×/1.45 oil immersion objective).
Supplementary Material
Acknowledgments
We thank Hilda Tatoessian (Medical Research Council Mammalian Genetics Unit) for technical advice with diaminobenzidine immunohistochemistry. This work was supported by the Medical Research Council; a Grant-in-Aid for Scientific Research (no. 17680034) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and FP6 Integrated Project EUROHEAR, LSHG-CT-2004-512063.
Abbreviations
- 4.1
protein 4.1
- GUK
guanylate kinase-like
- IP
immunoprecipitation
- En
embryonic day n
- Pn
postnatal day n
- IHC
inner hair cell
- OHC
outer hair cell.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tilney L. G., Tilney M. S., DeRosier D. J. Annu. Rev. Cell Biol. 1992;8:257–274. doi: 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- 2.Forge A., Souter M., Denman-Johnson K. Semin. Cell Dev. Biol. 1997;8:225–237. doi: 10.1006/scdb.1997.0147. [DOI] [PubMed] [Google Scholar]
- 3.Denman-Johnson K., Forge A. J. Neurocytol. 1999;28:821–835. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- 4.Anniko M. Anat. Embryol. (Berl.) 1983;166:355–368. doi: 10.1007/BF00305923. [DOI] [PubMed] [Google Scholar]
- 5.Furness D., Richardson G. P., Russell I. J. Hear. Res. 1989;38:95–110. doi: 10.1016/0378-5955(89)90131-7. [DOI] [PubMed] [Google Scholar]
- 6.Hudspeth A. J. Neuron. 1997;19:947–950. doi: 10.1016/s0896-6273(00)80385-2. [DOI] [PubMed] [Google Scholar]
- 7.Mburu P., Mustapha M., Varela A., Weil D., El-Amraoui A., Holme R. H., Rump A., Hardisty R. E., Blanchard S., Coimbra R. S., et al. Nat. Genet. 2003;34:421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- 8.Delprat B., Michel V., Goodyear R., Yamasaki Y., Michalski N., El-Amraoui A., Perfettini I., Legrain P., Richardson G., Hardelin J. P., et al. Hum. Mol. Genet. 2005;14:401–410. doi: 10.1093/hmg/ddi036. [DOI] [PubMed] [Google Scholar]
- 9.Belyantseva I. A., Boger E. T., Naz S., Frolenkov G. I., Sellers J. R., Ahmed Z. M., Griffith A. J., Friedman T. B. Nat. Cell Biol. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 10.Kikkawa Y., Mburu P., Morse S., Kominami R., Townsend S., Brown S. D. Hum. Mol. Genet. 2005;14:391–400. doi: 10.1093/hmg/ddi035. [DOI] [PubMed] [Google Scholar]
- 11.Yap C. C., Liang F., Yamazaki Y., Muto Y., Kishida H., Hayashida T., Hashikawa T., Yano R. J. Neurochem. 2003;85:123–134. doi: 10.1046/j.1471-4159.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- 12.Biederer T., Sudhof T. C. J. Biol. Chem. 2001;276:47869–47876. doi: 10.1074/jbc.M105287200. [DOI] [PubMed] [Google Scholar]
- 13.Marfatia S. M., Leu R. A., Branton D., Chishti A. H. J. Biol. Chem. 1995;270:715–719. doi: 10.1074/jbc.270.2.715. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Mariscal L., Betanzos A., Avila-Flores A. Semin. Cell Dev. Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- 15.Caruana G. Int. J. Dev. Biol. 2002;46:511–518. [PubMed] [Google Scholar]
- 16.Takakuwa Y. Int. J. Hematol. 2000;72:298–309. [PubMed] [Google Scholar]
- 17.Walensky L. D., Shi Z. T., Blackshaw S., DeVries A. C., Demas G. E., Gascard P., Nelson R. J., Conboy J. G., Rubin E. M., Snyder S. H., et al. Curr. Biol. 1998;8:1269–1272. doi: 10.1016/s0960-9822(07)00536-2. [DOI] [PubMed] [Google Scholar]
- 18.Probst F. J., Fridell R. A., Raphael Y., Saunders T. L., Wang A., Liang Y., Morell R. J., Touchman J. W., Lyons R. H., Noben-Trauth K., et al. Science. 1998;280:1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- 19.Ernstson S., Smith C. A. Acta Oto-Layngol. 1986;101:395–402. doi: 10.3109/00016488609108624. [DOI] [PubMed] [Google Scholar]
- 20.Goodyear R. J., Richardson G. P. J. Neurosci. 2003;23:4878–4887. doi: 10.1523/JNEUROSCI.23-12-04878.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Ferreiro C. M., Luque C. M., Correas I. J. Biol. Chem. 2001;276:44785–44791. doi: 10.1074/jbc.M107369200. [DOI] [PubMed] [Google Scholar]
- 22.Huang S. C., Jagadeeswaran R., Liu E. S., Benz E. J., Jr. J. Biol. Chem. 2004;279:34595–345602. doi: 10.1074/jbc.M404051200. [DOI] [PubMed] [Google Scholar]
- 23.Siemens J., Lillo C., Dumont R. A., Reynolds A., Williams D. S., Gillespie P. G., Muller U. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- 24.Sollner C., Rauch G. J., Siemens J., Geisler R., Schuster S. C., Muller U., Nicolson T., Tubingen 2000 Screen Consortium Nature. 2004;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- 25.Corey D. P., Garcia-Anoveros J., Holt J. R., Kwan K. Y., Lin S. Y., Vollrath M. A., Amalfitano A., Cheung E. L., Derfler B. H., Duggan A., et al. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 26.Lagziel A., Ahmed Z. M., Schultz J. M., Morell R. J., Belyantseva I. A., Friedman T. B. Dev. Biol. 2005;280:295–306. doi: 10.1016/j.ydbio.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Shin J. B., Adams D., Paukert M., Siba M., Sidi S., Levin M., Gillespie P. G., Grunder S. Proc. Natl. Acad. Sci. USA. 2005;102:12572–12577. doi: 10.1073/pnas.0502403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adato A., Lefevre G., Delprat B., Michel V., Michalski N., Chardenoux S., Weil D., El-Amraoui A., Petit C. Hum. Mol. Genet. 2005;14:3921–3932. doi: 10.1093/hmg/ddi416. [DOI] [PubMed] [Google Scholar]
- 29.van Wijk E., van der Zwaag B., Peters T., Zimmerman U., te Brinke H, Kersten F. F. J., Marker T., Aller E., Hoefsloot L. H., Cremers C. W., et al. Hum. Mol. Genet. 2006;15:751–765. doi: 10.1093/hmg/ddi490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.