Abstract
A longstanding paradox in the activation of cytotoxic T lymphocytes (CTL) arises from the observation that CTL recognize and rapidly destroy target cells with exquisite sensitivity despite the fact that cytokine production requires sustained signaling at the immunological synapse. Here we solve this paradox by showing that CTL establish sustained synapses with targets offering strong antigenic stimuli and that these synapses persist after target cell death. Simultaneously, CTL polarize lytic granules toward different cells without discrimination regarding antigenic potential. Our results show that spatiotemporal uncoupling of immunological synapse and lytic granule secretion allows multiple killing and sustained signaling by individual CTL. This unique mechanism of responding to multiple contacts provides remarkable efficiency to CTL function.
Keywords: confocal microscopy, immunological synapse, sustained signaling
An intriguing feature of CTL biology is that, although cytotoxicity is a rapid and low-threshold response, activation to interleukin production requires a prolonged time and an adequately strong antigenic stimulation (1, 2). We have previously shown that individual CTL interacting with cognate target cells exhibit a dual activation threshold reflecting the formation of two distinct immunological synapses (IS) at the CTL/target cell contact site: the lytic synapse and the stimulatory synapse (3). The term “lytic synapse” was used to refer to the polarization of the lytic machinery detectable at both low and high antigen concentrations, and the term “stimulatory synapse” was used to refer to the large-scale molecular segregation of surface molecules and signaling components characteristic of a mature IS and occurring only with target cells providing strong antigenic stimuli (3). The formation of a lytic synapse corresponded to full activation of CTL to cytotoxicity (whereas IFN-γ production was marginal, and calcium mobilization was low and erratic). The formation of a stimulatory synapse corresponded to activation to cytokine production (3).
A large amount of information on the molecular dynamics occurring at the CTL/target cell contact site is now available; however, CTL activation to biological responses is still an enigmatic process because of three major unresolved questions.
The first question is how can CTL behave as efficient and rapid killers and at the same time gather the sustained signals required for cytokine production on the surface of their targets (2, 4)? Cytotoxicity is a very efficient phenomenon characterized by a high degree of sensitivity and rapidity. It has been previously estimated that target cells displaying as few as 1–10 specific peptide/MHC complexes on their surface can trigger cytotoxicity (5). In a recent study Purbhoo et al. (6) measured the number of peptide/MHC complexes present at the cell–cell contact site in living CTL/target cell conjugates. They showed that CTL could indeed be activated to lethal hit delivery by as few as two to three specific peptide/MHC complexes present at the cellular interface. Lethal hit delivery requires a very short time. Upon conjugation with cognate target cells, CTL rapidly polarize their lytic machinery toward the opposing cells (7). Moreover, CTL can kill outnumbering target cells (8), indicating that they may rapidly detach and recycle from one target to another (9–12). How the process of CTL activation is compatible with rapid low-threshold cytotoxic responses and transient CTL/target interactions is still unknown.
The second unresolved question is how is the polarization of lytic machinery regulated in CTL interacting with multiple targets simultaneously? We have recently shown that CD4+ helper T cells simultaneously interacting with different antigen-presenting cells (APC) rapidly polarize their secretory machinery toward the APC providing the strongest antigenic stimulus. This rapid polarization allows CD4+ T cells to provide their help in a selective fashion (13). Although selective help delivery is instrumental for the development of adaptive immune responses, selective polarization of CTL lytic machinery toward the targets offering strong antigenic stimuli could be detrimental for indiscriminate immune surveillance against all potentially dangerous cells. Whether CTL selectively polarize against defined targets or conversely are able to behave as indiscriminate killers is still unknown.
A third question concerns the functional relation between the lytic and stimulatory synapses in individual CTL. The mature IS was originally described as a specialized signaling domain formed at the contact site between T cells and APC, characterized by large-scale molecular clustering and segregation of surface molecules and signaling components (7, 14, 15). Current research has led to an expansion of this term, where IS now indicates a multitude of structures that are mediators of intercellular communication (3). It has been suggested that in CTL the IS may have the role of polarizing secretion of lytic granules toward target cells (7). However, results from our laboratory and other laboratories have recently shown that polarization of CTL lytic machinery toward targets can occur in the absence of the large-scale molecular clustering and segregation characteristic of a mature IS (3, 6, 16). Together these results suggest that lethal hit delivery and molecular rearrangement at the synapse are rather independent events that may possibly take place separately in different areas of the cell and at different time points of interaction.
In the present work we visualize molecular dynamics at the CTL/target cell contact site in CTL interacting with individual and multiple targets and provide time-lapse confocal microscopy records that shed light on the dynamics of CTL/target interaction. We show that CTL do not cycle between targets but rather remain attached to annihilated targets. Moreover, CTL adhere to strongly antigenic targets for a prolonged period even after disintegration of these cells and continue to undergo sustained signaling and IS formation. Meanwhile, CTL can kill multiple targets encountered simultaneously by polarizing lytic granules toward different targets with no discrimination of their antigenic potential.
Our results illustrate an unexpected intercellular dynamics that allows both multiple killing and sustained CTL activation. By means of this mechanism CTL rapidly administer lethal hits to various targets encountered simultaneously while establishing preferential liaisons with targets that are still offering activation stimuli even after death.
Results
Polarization of Lytic Machinery Is Intrinsically Very Rapid.
To define, in individual CTL, the dynamics of lethal hit delivery versus the formation of a stable IS, we initially studied the time kinetics of lytic synapse formation. CTL were loaded with LysoTracker red (to visualize lytic granules), and target cells were loaded with calcein, a dye that is lost upon cell death (17). The interaction between living CTL and target cells was studied by using time-lapse laser scanning confocal microscopy. Target cells were either unpulsed or pulsed with 1 nM (a peptide concentration sufficient to saturate cytotoxic response) or 10 μM (a peptide concentration that saturates both cytotoxicity and cytokine production) antigenic peptide (3).
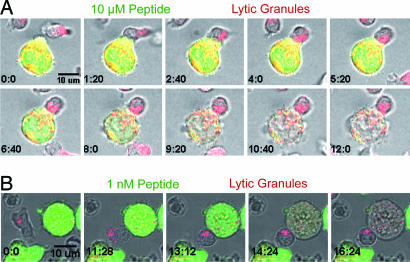
Fig. 1 shows that polarization of lytic granules toward target cells pulsed with antigenic peptide occurred within a few minutes after the initial contact between CTL and their targets. The polarization of CTL lytic granules was followed by the loss of calcein from target cells, showing that lytic synapse formation leads to target death. The time kinetics of lytic granule polarization are better appreciated in Movies 1 and 2, which are published as supporting information on the PNAS web site, showing that lytic granule polarization was detectable within 3 min after cell–cell contact. Interestingly, the time kinetics of lytic machinery reorientation was independent of the strength of antigenic stimulation, because it was similar in CTL interacting with targets pulsed with 10 μM or 1 nM peptide (a quantification of a statistically significant number of conjugates is shown Fig. 6, which is published as supporting information on the PNAS web site). The time kinetics of calcein loss was variable in the different CTL/target conjugates. This finding suggests that, although CTL deliver hits homogeneously rapid, the time required to trigger individual target cell death is variable.
Fig. 1.
The time kinetics of lethal hit delivery does not depend on the strength of antigenic stimulation. Sequences of snapshots depicting lytic granule polarization toward target cells pulsed with 10 μM or 1 nM peptide concentration are shown. (A) A CTL loaded with LysoTracker red (red) is interacting with a target cell pulsed with 10 μM peptide. Targets are loaded with calcein, a probe that is lost upon cell death (green). (B) A CTL loaded with LysoTracker red (red) is interacting with a target cell pulsed with 1 nM peptide and loaded with calcein (green). It should be noted that LysoTracker red is rapidly released into the culture medium and taken up to some extent by the target cells. The snapshot sequence in A corresponds to Movie 1, and the snapshot sequence in B corresponds to Movie 2. The numbers indicate the time points in minutes. (Scale bar: 10 μm.) Data are from two representative experiments of 16 for the two concentrations of peptide.
CTL interacting with unpulsed target cells formed conjugates and crawled on target cell surface in the absence of calcein leakage and of any other evidence of target cell death (Movie 3, which is published as supporting information on the PNAS web site).
Together these results indicate that lytic granule polarization is intrinsically very rapid, allowing CTL to promptly attack targets with no distinction of their antigenic potential.
Stimulatory Synapse and Sustained Signaling Keep Going After Target Cell Annihilation.
The above results support the notion that lytic synapse formation and target cell annihilation are rapid events occurring during the early steps of CTL/target interaction. This observation raises the question: how can a stimulatory synapse be formed and maintained for a prolonged time at the cellular interface while allowing for the sustained signaling that is required for CTL activation to cytokine production (2)?
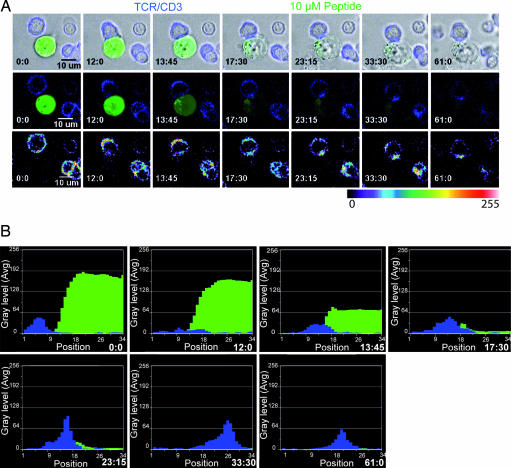
To address this question, we studied the kinetics of stimulatory synapse formation in individual CTL interacting with their targets for a sustained time. Target cells were loaded with calcein to visualize cell death. CTL were stained with Cy5-labeled anti-CD3 Fab antibodies to detect the enrichment of T cell receptor (TCR)/CD3 complexes at the cell–cell contact site, a parameter of mature IS formation. Time-lapse video microscopy showed that initiation of calcein loss in the target cells precedes the clustering of TCR into the IS (Fig. 2A and Movies 4 and 5, which are published as supporting information on the PNAS web site).
Fig. 2.
TCR clustering at the CTL/target cell interface is accomplished after lethal hit delivery and is sustained for a prolonged period. (A) CTL stained with anti-CD3 Fab (blue) are interacting with a target cell pulsed with 10 μM peptide concentration and loaded with calcein (green). The panels depict two CTL interacting with one target cell. (Top) Overlapping of differential interference contrast microscopy images with CD3 and calcein staining. (Middle) Only blue (CD3) and green (calcein) fluorescence are shown. (Bottom) TCR staining intensity using a pseudocolor scale. The snapshot sequence corresponds to Movies 4 and 5. (B) Analysis of CD3 fluorescence intensity at the CTL/target contact site and of calcein fluorescence intensity in target cell. The analysis corresponds to the snapshot sequence presented in A. Data are from Movie 4. A line was drawn at the contact site between the CTL and the target (see Movie 6), and the intensity of green and blue fluorescence was measured on unprocessed images all along this line. The full snapshot sequence is shown in Movie 6. Data are from one representative experiment of six. Treatment with concanamycin A (a selective inhibitor of perforin pathway) inhibited calcein leakage from targets (data not shown).
To quantitatively evaluate the time kinetics of target cell death and of TCR/CD3 enrichment at the synapse we applied the LineScan function of metamorph software to series of snapshots depicting CTL/target cell interaction. This analysis resulted in sequential plots that are presented in Fig. 2B and Fig. 7, which is published as supporting information on the PNAS web site (Fig. 2B is animated in Movie 6, which is published as supporting information on the PNAS web site). This approach shows that, even though the time of enrichment of TCR into the IS varies in individual CTL/target cell conjugates, it is delayed compared with lethal hit delivery. In addition, TCR/CD3 clustering at the cell–cell contact site was sustained long after target annihilation (Fig. 2). When CTL were conjugated with targets pulsed with a 1 nM concentration the targets were readily killed in the absence of the TCR clustering typical of stimulatory IS (Movie 7, which is published as supporting information on the PNAS web site), in agreement with previously reported data (3, 6).
The unexpected observation that stimulatory synapse formation can be sustained even after target cell annihilation suggests that CTL could receive prolonged activatory signals while interacting with fragments of disintegrating targets. We therefore investigated whether CTL maintained sustained signaling for a prolonged time after the death of the opposing target cell.
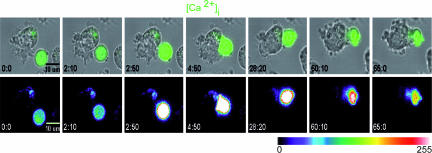
CTL were loaded with Fluo-4 to detect the intracellular Ca2+ concentration ([Ca2+]i) increase (3, 13), and CTL/target interactions were recorded for a sustained time. As shown in Fig. 3 and Movies 8 and 9, which are published as supporting information on the PNAS web site, the [Ca2+]i increase was sustained for 1–2 h after the initial cellular contact, a time considerably longer than the time required for the death of the target. To obtain more quantitative information on the duration of [Ca2+]i increase in CTL after target cell death we measured the duration of calcium signaling and the time required to detect target blebs in 32 conjugates detected in nine different movies. Fig. 8, which is published as supporting information on the PNAS web site, shows that the [Ca2+]i increase is significantly sustained after target cell death. These results show that CTL interacting with target cells providing strong antigenic stimulation do not rapidly disassemble stimulatory synapse upon target annihilation. Rather, they remain in contact with the dead targets and sustain active signal transduction for a long period.
Fig. 3.
In CTL the [Ca2+]i increase is sustained for a period after target cell annihilation. (Upper) A CTL previously loaded with Fluo-4 AM is shown during interaction with a target cell pulsed with 10 μM peptide concentration. The green intracellular staining of the T cell depicts [Ca2+]i increase. (Lower) The Fluo-4 staining intensity using a pseudocolor scale. Data are from one representative experiment of nine. In CTL interacting with unpulsed target cells the [Ca2+]i increase was not sustained. Occasionally CTL underwent a spiky [Ca2+]i increase, as we have described (see ref. 3).
Individual CTL Kill Multiple Targets Simultaneously in a Nonselective Fashion.
The above results show that human CTL remain attached to their targets for a relatively prolonged time rather than rapidly recycling from one target to another. This observation raises the question of how CTL may kill outnumbering heterogeneous targets during an immune response. To address this question we investigated the possibility that CTL may be able to kill multiple targets encountered simultaneously regardless of their antigenic potential.
CTL simultaneous interaction with multiple targets was investigated by time-lapse confocal microscopy. Target cells were pulsed with a high (10 μM) or a low (1 nM) antigenic peptide concentration and were loaded with different dyes to allow their identification. We initially focused on lytic granule secretion. Movies 10 and 11 and Fig. 9, which are published as supporting information on the PNAS web site, and Fig. 4 show that CTL interacting with targets pulsed with different antigenic concentrations polarize their lytic granules toward both targets simultaneously.
Fig. 4.
CTL polarize lytic granules toward different targets encountered simultaneously. Snapshots depict lytic granule polarization in CTL interacting simultaneously with target cells pulsed with 10 μM (blue) or 1 nM (green) peptide concentration. Data are from one representative experiment of nine.
To obtain quantitative information on polarization of lytic granules toward two different contact sites we took several snapshots of living cells by randomly changing the field during movie recording. A total of 136 snapshots depicting CTL in simultaneous contact with two targets were recorded in nine independent sessions. The polarization of lytic granule accumulation was scored by visual inspection in a blind study on registered images. T cells in simultaneous contact with two targets offering different antigenic stimuli were initially scored. Eighty-four triplicates were scored: 44% of CTL exhibited a double polarization, 29% exhibited lytic granule accumulation toward one target cell, and 27% showed an uncertain phenotype. More precisely, they showed a tendency to relocate lytic granules toward the two contact sites without clearly focused polarization of lytic granules. A simultaneous polarization of lytic granules in CTL interacting with two targets offering the same antigenic stimulation was also observed. 52 triplicates were scored: 46% of CTL exhibited a double polarization, 17% exhibited lytic granule accumulation toward one target cell, and 37% showed an uncertain phenotype.
Taken together the above results directly illustrate that individual CTL are indeed killers of multiple targets encountered simultaneously. A recent study showed that, in the case of CTL conjugated with two targets offering similar densities of antigenic ligands, the tubulin cytoskeleton oscillates between the two targets (18). In that study, the dynamics of lytic granule polarization in CTL conjugated with targets offering different densities of antigenic ligands was not investigated. Therefore, our results extend these described findings.
We next focused on TCR/CD3 dynamics on the surface of CTL interacting with different targets. CTL were stained with Cy5-labeled anti-CD3 Fab antibodies, and their interaction with targets pulsed with low (1 nM) or high (10 μM) antigenic concentration was visualized. Target cells were loaded with Fluo-4 to have a rapid evidence of lethal hit delivery in parallel with TCR/CD3 dynamics (10, 19). Fig. 5 and Movies 12 and 13, which are published as supporting information on the PNAS web site, show that, although TCR/CD3 was enriched in the contact site with targets offering strong antigenic stimuli, adjacent targets providing a weak antigenic stimulus concurrently received lethal hits as detected by [Ca2+]i increase.
Fig. 5.
Lytic and stimulatory synapses can be uncoupled in individual CTL. Sequences of snapshots depicting TCR/CD3 staining (blue) in CTL interacting simultaneously with target cells pulsed with a high (10 μM; red) or a low (1 nM; green) peptide concentration. To rapidly detect lethal hit delivery in target cells, targets pulsed with 1 nM peptide were loaded with Fluo-4 AM. (Top) Overlapping of differential interference contrast microscopy images and fluorescence staining. (Middle) Only fluorescence staining is shown. (Bottom) TCR staining intensity using a pseudocolor scale. The white arrows in Bottom indicate the TCR/CD3 enrichment at the CTL/target contact site. At the end of the time recording a second target cell receives the lethal hit by a CTL not visible in the movie and undergoes [Ca2+]i increase. The snapshot sequence corresponds to Movies 12 and 13. Data are from one representative experiment of three.
Together these results indicate that, in individual CTL, lytic and stimulatory synapses can be uncoupled. This uncoupling allows individual CTL to gather activation signals on targets offering strong antigenic stimuli while killing other cells with high efficiency.
Discussion
It is known that CTL responses are both very sensitive and efficient. However, the molecular mechanisms that provide the extraordinary efficiency of cytotoxic function are still elusive. Four findings reported in this article provide steppingstones to address this challenging question.
First we show that CTL lytic synapse formation is very rapid. The observation that the lytic synapse is rapidly formed is, in principle, not surprising because it has been thoroughly documented that CTL swiftly kill target cells (7, 10). However, the relation between the strength of antigenic stimulation and the time kinetics of lethal hit delivery was never investigated. Here we show that lytic granule polarization is an intrinsically fast response. Our time-lapse video recordings also show that lytic granule polarization is accomplished before stimulatory synapse formation. This result is in agreement with the notion that activation of the TCR signaling cascade precedes mature IS formation (20) and further shows that CTL can elicit cytotoxic function before large-scale clustering of TCR/CD3.
A second observation provided by our study is that, when target cells display a strong enough antigenic stimulus, CTL remain in contact with their dying targets for a prolonged time while undergoing IS formation and sustained signaling. In some cases we observed that a dying target cell could be bound by a second CTL, which established a new synapse with the annihilated target (Fig. 2A and Movies 4 and 5). This observation indicates that target death does not preclude sustained CTL activation. It is tempting to speculate that during in vivo responses cellular bodies and fragments of targets not yet cleared by scavenger mechanisms could provide platforms to incoming CTL for sustained signaling and serial TCR engagement and therefore contribute to amplify CTL responses (2). We also oberved that human CTL do not rapidly cycle between targets even when they interact with targets pulsed with a low antigenic concentration. This observation indicates that in our cell system the well known efficiency of CTL cytotoxic function (8) is not based on serial killing.
A third observation provided by our study is that CTL can kill multiple targets encountered simultaneously by polarizing lytic granules toward multiple opposing targets with no discrimination of their antigenic potential. This observation can explain why CTL used in the present study do not rapidly recycle from one target to another yet behave as efficient killers (3). Previous studies in which the dynamics of murine CTL/target interaction were investigated by time-lapse video microscopy came to the conclusion that the efficiency of cytotoxic function is due to rapid CTL detachment from annihilated targets and recycling (9–12). Our data are not in contrast with previous reports because our observations and previously reported observations underline the capacity of CTL to encounter multiple targets and preserve their lytic potential to kill an increasing number of opposing cells (12). In other words, it was previously reported that CTL eliminate multiple targets in a row (10); here we show that they mostly kill them simultaneously, although the two processes may be not mutually exclusive.
Our findings are in agreement with the previously reported observation that, in CTL infiltrating the nervous system of lymphocytic choriomeningitis virus-infected mice, lytic granules are not found at one given neuron/CTL contact site but are seen at different cell–cell contact sites (21). Our results extend this previous observation because in that study it was not possible to investigate the time parameters of lytic and stimulatory synapse formation or to modulate the strength of antigenic stimulation.
The molecular mechanisms of this peculiar multifocal secretion of lytic granules by CTL are elusive. It is tempting to speculate that the early engagement of TCR at different contact sites between CTL and their targets may generate simultaneous signaling foci of different intensities, each one sufficient to drive polarization of some lytic granules. This finding is compatible with the notion that polarized lytic granule secretion is an extremely sensitive CTL response (3, 6). It has been shown that lytic granules use microtubules as tracks to move inside CTL and that they exhibit bidirectional mobility (22). We suggest that, in the case of multiple CTL/target cell interactions, lytic granules would split and point toward various plasma membrane areas where signaling takes place simultaneously, thus enabling multiple target killing (Fig. 4 and Movies 10 and 11).
A fourth observation provided by our study is that lytic and stimulatory synapses can be spatially and temporally uncoupled in CTL interacting with multiple targets, allowing individual CTL to undergo an activation process while killing several cells. This observation is in agreement with previous reports showing that polarization of lytic granules does not always occur in the context of a mature IS (3, 6, 16). The capacity to simultaneously kill multiple targets with no discrimination of their antigenic potential may enable CTL to efficiently counteract the spreading of a viral infection or tumor growth by rapidly annihilating several contiguous cells.
It is possible that the multiple polarization of lytic granules may contribute to bystander killing of innocent targets. It has been shown that targets sensitized by means of transfer of antigenic peptides through gap junctions among adjacent cells (23) or even innocent bystander target cells are killed during CTL/cognate target interaction (24, 25). We also can detect bystander killing in our cellular model (see Supporting Materials and Methods and Supporting Results in Supporting Text and Fig. 10, which are published as supporting information on the PNAS web site). This observed “side effect” in lytic granule secretion might be instrumental to amplify the effects of CTL immune surveillance. The price to pay for this exquisitely sensitive mechanism of cell killing is that in the course of a viral infection the high antigen load of infected targets may generate bystander killing of contiguous cells and thus increase the inflammatory tissue damage associated with the viral infection (26).
In conclusion, our results highlight a basic difference between helper T cells and CTL. Helper T cells interacting simultaneously with different APC polarize their Golgi apparatus toward the APC offering the strongest stimulus (13). Conversely, CTL polarize their lytic granules toward different targets with no distinction. We propose that these opposite behaviors synergize for an optimal adaptive immune response: on one hand helper T cells provide help in a selective fashion, and on the other hand CTL act as rapid killers of heterogeneous targets.
It has been proposed that efficient cytotoxicity is achieved by rapidly delivering lethal hits and by cycling from one target to another (9–12). Here we show that CTL rather exhibit “multiple killing” of targets encountered simultaneously, yet our cellular model is not fully representative of an in vivo condition. We propose that in the course of an immune response CTL may use both mechanisms to rapidly eliminate targets while moving through pathological tissues (27). The combination of “serial” and multiple killing can amplify cytotoxic responses, allowing CTL to ensure a very sensitive and efficient surveillance against potentially dangerous cells.
Materials and Methods
T Cells and Target Cells.
An HLA-A2-restricted T cell line (CMVpp65) specific for the peptide NLVPMVATV of human cytomegalovirus protein pp65 was used (3). HLA-A2-matched Epstein–Barr virus (EBV)-transformed B cells (JY) were used as target cells (3). T cell line and EBV-B cell lines were generated and maintained as described (2).
Dynamics of Lytic Granules and TCR/CD3 in Living Cells.
To visualize lethal hit delivery T cells were loaded with LysoTracker Red (Molecular Probes) for 45 min at 37°C in RPMI medium 1640/5% FCS. To visualize TCR/CD3 dynamics, T lymphocytes were labeled with Cy5 TR66 Fab at 20 μg/ml in RPMI medium 1640/5% FCS at 4°C for 30 min as described (13).
Target cells were labeled with either 1 μM calcein (Molecular Probes) in RPMI medium 1640/5% FCS for 30 min at room temperature or 0.5 μM Orange CMTMR or 0.5 μM Bodipy 630 (both from Molecular Probes) at 37°C for 15 min. Target cells were previously pulsed with 1 nM or 10 μM peptide for 2 h at 37°C.
Target cells were seeded into microchambers (Lab-Tek Chamber coverglass, Nalge Nunc) previously coated with poly-d-lysine (Sigma). Fluorescence measurements were done on a Zeiss LSM-510 confocal microscope at 37°C and 5% CO2. Image sequences of the time-lapse recording were processed with metamorph software (13).
To have a quantitative evaluation of the time kinetics of target cell death and of TCR/CD3 fluorescence at the synapse we applied the LineScan function of metamorph software to series of snapshots depicting CTL/target cell interaction. This analysis resulted in sequential plots that are presented in Figs. 2B and 7 and are animated in Movie 6.
In additional experiments either CTL (to detect TCR-mediated signaling) or target cells [to detect early cellular damage (10, 19)] were loaded with 1 μM Fluo-4 AM for 30 min at 37°C. The green fluorescence emission of Fluo-4 reflecting the [Ca2+]i was monitored by time-lapse confocal microscopy as described (3).
Supplementary Material
Acknowledgments
We thank Sabina Müller for help and advice and Daniel Coombs, Sylvie Guerder, Jean-Charles Guery, and Loic Dupre for discussion and critical reading of the manuscript. This work was supported by grants from La Ligue Contre le Cancer “Equipe Labellisee 2005.”
Abbreviations
- CTL
cytotoxic T lymphocyte(s)
- TCR
T cell receptor
- IS
immunological synapse
- APC
antigen-presenting cell
- [Ca2+]i
intracellular concentration of Ca2+.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Porgador A., Yewdell J. W., Deng Y., Bennink J. R., Germain R. N. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 2.Valitutti S., Muller S., Dessing M., Lanzavecchia A. J. Exp. Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faroudi M., Utzny C., Salio M., Cerundolo V., Guiraud M., Muller S., Valitutti S. Proc. Natl. Acad. Sci. USA. 2003;100:14145–14150. doi: 10.1073/pnas.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huppa J. B., Gleimer M., Sumen C., Davis M. M. Nat. Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 5.Sykulev Y., Joo M., Vturina I., Tsomides T. J., Eisen H. N. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 6.Purbhoo M. A., Irvine D. J., Huppa J. B., Davis M. M. Nat. Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 7.Stinchcombe J. C., Bossi G., Booth S., Griffiths G. M. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 8.Cerottini J. C., Brunner K. T. Adv. Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson C. J., Thomas J. A. Proc. R. Soc. London B; 1976. pp. 417–429. [DOI] [PubMed] [Google Scholar]
- 10.Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. EMBO J. 1987;6:2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothstein T. L., Mage M., Jones G., McHugh L. L. J. Immunol. 1978;121:1652–1656. [PubMed] [Google Scholar]
- 12.Isaaz S., Baetz K., Olsen K., Podack E., Griffiths G. M. Eur. J. Immunol. 1995;25:1071–1079. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- 13.Depoil D., Zaru R., Guiraud M., Chauveau A., Harriague J., Bismuth G., Utzny C., Muller S., Valitutti S. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Monks C. R., Freiberg B. A., Kupfer H., Sciaky N., Kupfer A. Nature. 1998;395:82–86. [Google Scholar]
- 15.Grakoui A., Bromley S. K., Sumen C., Davis M. M., Shaw A. S., Allen P. M., Dustin M. L. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 16.O’Keefe J. P., Gajewski T. F. J. Immunol. 2005;175:5581–5585. doi: 10.4049/jimmunol.175.9.5581. [DOI] [PubMed] [Google Scholar]
- 17.Lyubchenko T. A., Wurth G. A., Zweifach A. Immunity. 2001;15:847–859. doi: 10.1016/s1074-7613(01)00233-3. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn J. R., Poenie M. Immunity. 2002;16:111–121. doi: 10.1016/s1074-7613(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 19.Keefe D., Shi L., Feske S., Massol R., Navarro F., Kirchhausen T., Lieberman J. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Lee K. H., Holdorf A. D., Dustin M. L., Chan A. C., Allen P. M., Shaw A. S. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 21.McGavern D. B., Christen U., Oldstone M. B. Nat. Immunol. 2002;3:918–925. doi: 10.1038/ni843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poenie M., Kuhn J., Combs J. Curr. Opin. Immunol. 2004;16:428–438. doi: 10.1016/j.coi.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Neijssen J., Herberts C., Drijfhout J. W., Reits E., Janssen L., Neefjes J. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 24.Lanzavecchia A. Nature. 1986;319:778–780. doi: 10.1038/319778a0. [DOI] [PubMed] [Google Scholar]
- 25.Duke R. C. J. Exp. Med. 1989;170:59–71. doi: 10.1084/jem.170.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asquith B., Bangham C. R. J. Theor. Biol. 2003;222:53–69. doi: 10.1016/s0022-5193(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 27.Friedl P., den Boer A. T., Gunzer M. Nat. Rev. Immunol. 2005;5:532–545. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.