Abstract
VEGF, nitric oxide (NO), inflammation, and vascular- and extravascular remodeling coexist in asthma and other disorders. In these responses, VEGF regulates angiogenesis. VEGF also induces inflammation and remodeling. The mechanisms of the latter responses have not been defined, however. We hypothesized that VEGF-induces extravascular tissue responses via NO-dependent mechanisms. To evaluate this hypothesis, we compared the effects of transgenic VEGF165 in lungs from normal mice, mice treated with pan-NO synthase (NOS) or endothelial NOS (eNOS) inhibitors, and mice with null mutations of inducible NOS (iNOS) or eNOS. These studies demonstrate that VEGF selectively stimulates eNOS and iNOS. They also demonstrate that VEGF induces pulmonary alterations via NO-dependent and -independent mechanisms with angiogenesis, edema, mucus metaplasia, airway hyperresponsiveness, lymphocyte accumulation, dendritic cell hyperplasia and S-nitrosoglutathione reductase stimulation being NO-dependent and dendritic cell activation being NO-independent. Furthermore, they demonstrate that eNOS and iNOS both contribute to these responses. NO/NOS-based interventions may be therapeutic in VEGF-driven inflammation and remodeling.
VEGF is a critical regulator of angiogenesis in physiologic responses such as reproduction, development, and wound healing and pathologic responses as diverse as those in tumors, obesity, retinopathies, and ischemic vascular disorders (1–6). In these settings, VEGF induces the proliferation, sprouting, and migration of endothelial cells (EC), regulates EC survival, induces vasodilatation, and regulates vascular permeability (1, 2). Recent studies have demonstrated, however, that VEGF also has prominent inflammatory, immune, and remodeling effects on nonvascular tissues (7, 8). The mechanisms that VEGF uses to exert its EC effects have been intensely investigated. In contrast, very little is known about the mechanisms that VEGF uses to induce extravascular responses in the lung or other tissues.
Exaggerated Th2 inflammation and airway remodeling are cornerstones in the pathogenesis of asthma (9). Increases in vessel number, vessel size, vessel surface area, and vascular leak are prominent features of these remodeling responses (10–14). In keeping with these findings, exaggerated levels of VEGF that correlate directly with disease activity (12) and inversely with airway caliber and airway hyperresponsiveness (AHR) (11, 15–17) have been detected in biologic samples from patients with asthma (7, 11, 15–17). VEGF was originally postulated to contribute to asthma via its effects on vascular permeability (10, 18). However, recent studies from our laboratory refined this concept by demonstrating that the overexpression of VEGF in the murine lung induces an asthma-like phenotype with inflammation, parenchymal and vascular remodeling, edema, mucus metaplasia, myocyte hyperplasia, AHR, dendritic cell (DC) hyperplasia and activation, enhanced respiratory antigen sensitization, and augmented Th2 inflammation (7). These studies also demonstrated that VEGF is required for antigen-induced Th2 inflammation and IL-4 and -13 elaboration (7). The mechanisms of these VEGF-induced vascular and extravascular pulmonary alterations have not been adequately defined, however.
Nitric oxide (NO) is an essential gaseous regulator of mammalian physiology that is produced by the NO synthase (NOS) family of enzymes (19). Over the last few years, intimate relationships between asthma and NO and between VEGF and NO have been appreciated. The former studies demonstrated that elevated levels of exhaled breath NO, Eno, is a signature of asthma and that the induction of inducible NOS (iNOS) can be readily appreciated in asthma tissues (20–22). The latter studies demonstrated that VEGF is a powerful stimulator of NOS and NO production (23, 24) and that VEGF-induced vascular permeability (25), calcium mobilization (endothelial cells) (26), and angiogenesis (27) are mediated by NO. However, although exaggerated levels of VEGF, NOS, and NO coexist in tissues from asthmatics, the roles of NO in the pathogenesis of the inflammatory, immune, mucus, DC, and physiologic effects of VEGF have not been addressed, and the NOS genes that contribute to these responses have not been defined.
We hypothesized that NO is a critical mediator of VEGF-induced vascular and extravascular alterations in the lung and that VEGF induces many of these responses via specific isoforms of NOS. To test this hypothesis, we characterized the effects of transgenic VEGF in WT mice, mice treated with pan-NOS or selective endothelial NOS (eNOS) inhibitors, and mice with null mutant eNOS or iNOS loci. These studies demonstrate that VEGF is a potent inducer of eNOS and iNOS, but not neuronal NOS (nNOS), in the adult murine lung. They also demonstrate that VEGF induces pulmonary alterations via NO-dependent and -independent mechanisms with angiogenesis, edema, mucus metaplasia, AHR, T cell accumulation, DC hyperplasia, and S-nitrosoglutathione reductase (GSNOR) stimulation being NO-dependent with DC activation being NO-independent. Lastly, they demonstrate that eNOS and iNOS contribute to these responses.
Results
Regulation of NOS and Vascular Effects of VEGF.
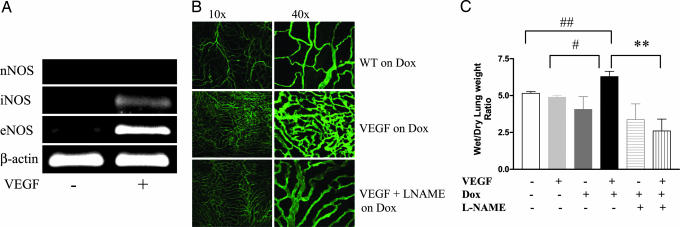
To address the roles of NO in VEGF-induced pulmonary alterations, we evaluated the levels of mRNA encoding the three NOS isoforms in transgene-negative (Tg−) and VEGF transgenic (Tg+) mice. The levels of iNOS, eNOS, and nNOS mRNA in lungs from Tg− mice receiving normal or doxycycline (dox) water and Tg+ mice receiving normal water were near or below the limits of detection our assay (Fig. 1A and data not shown). In contrast, dox administration caused an impressive increase in the levels of eNOS and iNOS, but not nNOS mRNA, in lungs from Tg+ mice (Fig. 1A). In accord with published studies (28), these NOS increases were seen in a variety of cells, with eNOS being most prominent in endothelial cells and iNOS being most prominent in epithelial cells and macrophages (data not shown). Thus, VEGF is a potent and selective stimulator of iNOS and eNOS in the adult murine lung.
Fig. 1.
VEGF regulation of NOS and vascular effects of NO. Tg− and Tg+ mice received dox water for 2 weeks in the presence and absence of l-NAME. The levels of mRNA encoding eNOS and iNOS were assessed (A), pulmonary angiogenesis was evaluated with CD31 staining of the trachea (B), and pulmonary edema was quantitated with wet/dry lung weight ratio(s) (C). The noted values represent assessments in a minimum of four animals. **, P ≤ 0.01; #, P ≤ 0.02; ##, P ≤ 0.05.
To define the role(s) of NO in the pathogenesis of VEGF-induced vascular alterations, we compared the effects of VEGF in mice that produced NO normally and mice treated with the pan-NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME). Neovascularization and edema were not readily appreciated in lungs from Tg− mice receiving normal or dox water and Tg+ mice receiving normal water (Fig. 1 B and C and data not shown). In contrast, transgenic VEGF caused a significant increase in airway vascularity and lung wet/dry ratios (Fig. 1 B and C). NO played a significant role in these responses because VEGF-induced vascularity and edema were decreased with l-NAME treatment (Fig. 1 B and C). Thus, VEGF induces neovascularization and increases vascular permeability in the murine lung via a mechanism(s) that is, at least partially, NOS-dependent.
NOS in VEGF-Induced Inflammation and DC Alterations.
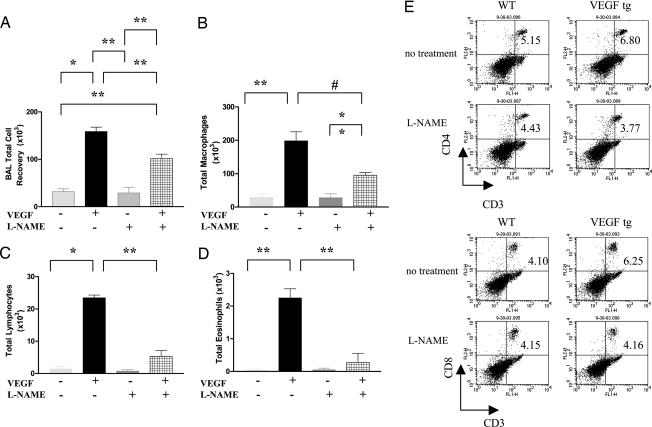
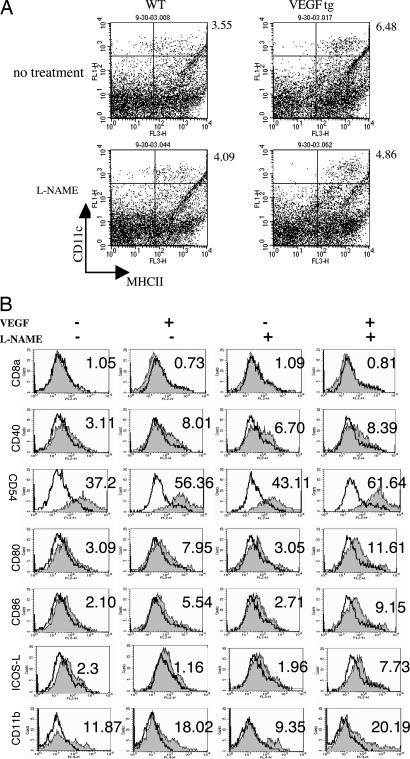
We previously demonstrated that VEGF induces a mononuclear cell, eosinophil, and CD4+ and CD8+ T cell-rich inflammatory response and DC hyperplasia and activation in the murine lung (7). To define the roles of NO in these responses, we compared these parameters in VEGF Tg+ mice treated with l-NAME or its vehicle control. Significant inflammation and alterations in DC number and/or activation were not appreciated in comparisons of Tg− mice receiving normal or dox water or Tg+ mice receiving normal water (data not shown). Nevertheless, bronchoalveolar lavage (BAL) and tissue inflammation and increased numbers of activated DC were seen in mice in which transgenic VEGF was activated via dox water administration (Figs. 2 and 3 and data not shown). In all cases, NO played a significant role in these responses because l-NAME diminished BAL inflammation, tissue inflammation, and macrophage, lymphocyte, eosinophil, CD4+ cell, CD8+ cell, and DC recovery (Figs. 2 and 3 and data not shown). Interestingly, l-NAME did not diminish DC differentiation and activation. In fact, NOS inhibition, while decreasing DC numbers, augmented the levels of expression of CD54, CD80, CD86, and ICOSL, suggesting that NO normally feeds back to inhibit DC expression of these important surface and accessory molecules (Fig. 3B). Collectively, these studies demonstrate that VEGF induces BAL and tissue inflammation and DC accumulation via NO-dependent pathways and inhibits DC activation via a NO-dependent mechanism.
Fig. 2.
NO in VEGF-induced inflammatory and immune alterations. Tg− and Tg+ mice received normal water or dox water in the presence or absence of l-NAME for 2 weeks. BAL total cell (A) and differential cell [macrophages (B); lymphocytes (C); and eosinophils (D)] recovery were then evaluated. CD4+ and CD8+ T cell accumulation was also evaluated with FACS (E). NO inhibition (with l-NAME) abrogated the VEGF induction of the number of T cells (E). The noted values represent assessments in a minimum of four animals. *, P ≤ 0.0001; **, P ≤ 0.01; #, P ≤ 0.02.
Fig. 3.
NO in VEGF-induced DC alterations. Tg− and Tg+ mice received dox water in the presence or absence of l-NAME for 2 weeks. DC number (A) and DC activation (B) were evaluated. The noted values represent assessments in a minimum of four animals.
NO in VEGF-Induced Mucus Metaplasia, AHR, and GSNOR Regulation.
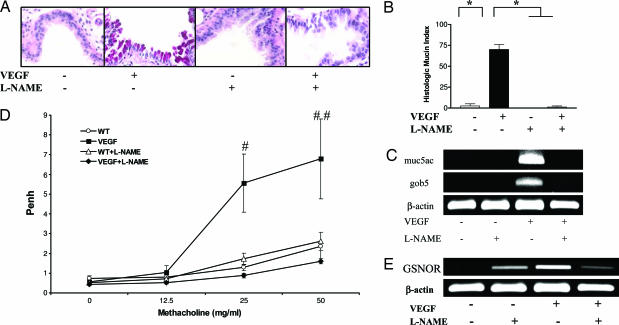
Studies were also undertaken to define the roles of NO in VEGF-induced mucus metaplasia and AHR. In accord with prior observations from our laboratory (7), significant mucus metaplasia and exaggerated airways responses to methacholine were not appreciated in Tg− mice receiving normal or dox water or Tg+ mice receiving normal water (Fig. 4 A–D and data not shown). In contrast, goblet cell hyperplasia enhanced levels of Muc5ac and Gob5 mRNA, and exaggerated airway responses to methacholine were readily appreciated in mice in which transgenic VEGF was activated (Fig. 4 A–D). NOS appeared to play important roles in these responses because goblet cell hyperplasia, mucin gene expression, and AHR were significantly decreased in Tg+ mice treated with l-NAME (Fig. 4 A–D). Thus, VEGF induces mucus metaplasia and AHR via mechanisms that are at least partially NOS-dependent.
Fig. 4.
NO in VEGF-induced mucus metaplasia, AHR, and GSNOR expression. Tg− and Tg+ mice received dox water in the presence and absence of l-NAME for 2 weeks, and mucus metaplasia (periodic acid/Schiff stain) (A), the histologic mucin index (B), mucin-related gene expression (C), methacholine responsiveness (D), and GSNOR mRNA levels (E) were evaluated. The noted values represent assessments in a minimum of four animals. *, P ≤ 0.0001; #, P ≤ 0.02; ##, P ≤ 0.05 vs. others.
The effects of NO on airway tone are regulated, at least in part, by the levels of its down stream metabolite S-nitrosoglutathione (GSNO) and the enzyme that metabolizes GSNO, GSNOR (29). Thus, studies were undertaken to determine whether VEGF regulated the expression of GSNOR, and the importance of NO in this response was evaluated. In these experiments, the levels of mRNA encoding GSNOR in lungs from Tg− mice were near or below the limits of detection in our assays. In contrast, the levels of GSNOR mRNA were markedly increased in lungs from Tg+ mice receiving dox water (Fig. 4E). In the Tg− mice, l-NAME treatment increased the levels of the mRNA encoding GSNOR (Fig. 4E). In contrast, l-NAME abrogated the VEGF-induced increase in GSNOR mRNA (Fig. 4E). These studies suggest that the low levels of NO that are produced at baseline inhibit GSNOR, whereas the high levels that are induced in response to VEGF increase GSNOR mRNA.
Relative Contributions of iNOS and eNOS.
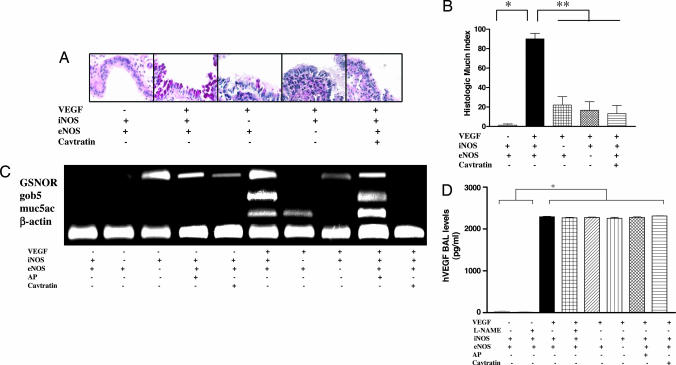
Studies were next undertaken to define the relative contributions of eNOS and iNOS in the VEGF-induced responses. This analysis was done by comparing the phenotypes induced by transgenic VEGF in mice with WT and null eNOS or iNOS loci and mice treated with the selective eNOS inhibitor cavtratin (30, 31). These studies demonstrated that eNOS and iNOS contributed to these responses because each one of these interventions decreased VEGF-induced goblet cell hyperplasia; mucin, Gob-5, and GSNOR gene expression (Fig. 5); and BAL and tissue inflammation (see Fig. 6, which is published as supporting information on the PNAS web site, and data not shown). Interestingly, in many cases, the alterations induced by eNOS abrogation were equal to or exceeded those caused by the ablation of iNOS. This phenomenon can be readily appreciated in comparisons of the effects of these interventions on BAL inflammation, goblet cell hyperplasia, and mucin gene expression (Figs. 5 and 6). In contrast, iNOS was a more important contributor to the induction of GSNOR (Fig. 5C). These studies demonstrate that eNOS and iNOS both make significant contributions to NO-dependent VEGF-induced tissue alterations.
Fig. 5.
Relative contributions of iNOS and eNOS and levels of BAL VEGF. Tg− and Tg+ mice with WT (+) or null (−) iNOS or eNOS loci and mice treated with cavtratin or its vehicle control (AP) were incubated with dox water for 2 weeks. Mucus metaplasia (A), the histologic mucin index (B), and the regulation of mucin-related genes and GSNOR (C) and BAL VEGF levels (D) were evaluated. A and C are representative of four similar experiments. The noted values reflect assessments in at least four animals. *, P ≤ 0.0001; **, P ≤ 0.01.
Effects of NOS Interventions on VEGF Production.
NOS-based interventions could alter VEGF effects by altering the production of transgenic VEGF or altering its effector capacity. To differentiate between these options, we compared the levels of transgenic VEGF in BAL fluids from Tg− and Tg+ mice treated with l-NAME, cavtratin, or their vehicle controls and Tg+ mice with WT and null NOS loci. Similar levels of BAL VEGF were seen in Tg+ mice under all conditions (Fig. 5D). Thus, these interventions altered the effects of VEGF by altering VEGF effector pathway activation.
Discussion
NO is produced by a wide variety of cells in the respiratory tract in states of health and disease (19), and in these cells, it regulates a number of central respiratory responses including airway tone, bronchial circulation, mucus and electrolyte secretion, and airway neural activity (reviewed in ref. 19). Studies of NO have also highlighted its ability to mediate pro- and antiinflammatory responses, contribute to tissue injury via the formation of reactive oxidant species such as peroxynitrite and induce local tissue edema (reviewed in refs. 19 and 32). Surprisingly, although increased levels of VEGF and NOS activation are frequently seen at sites of pulmonary pathology (19–22), the role of VEGF in the regulation of NO and the degree to which NO mediates the tissue effects of VEGF in the adult murine lung have not been adequately investigated. Our studies address these issues by demonstrating that VEGF is a selective stimulator of iNOS and eNOS in the murine lung. They also demonstrate that VEGF induces its effects in the lung via NOS-dependent and -independent pathways and highlight the importance of eNOS and iNOS in these responses.
Asthma is characterized by chronic eosinophil-rich and mononuclear cell-rich inflammation, varying degrees of airway remodeling with neovascularization and mucus metaplasia, and AHR on agonist challenge (9). Exaggerated levels of VEGF and exaggerated expression of VEGF receptors is well documented in the asthmatic airway (11–17). Elevated levels of ENO have been documented in comparisons of asthmatics and appropriate controls (21, 22, 33) so frequently that ENO has been proposed to be a biomarker that reflects airway inflammation and/or asthmatic activity (33–35). Despite the reproducibility of this observation, the role that NO plays in the pathogenesis of the asthmatic diathesis is poorly understood. Specifically, it is not known whether the NO is beneficial and acting as a bronchodilator or, alternatively, is a contributor to disease pathogenesis via the induction of inflammation, tissue remodeling, and/or AHR (33, 36). In addition, although there are many lines of evidence that suggest that VEGF plays a critical role in the pathogenesis of the inflammatory, vascular, immune, and physiologic responses that are seen in asthma (7, 12), the importance of NO in the pathogenesis of these responses has not been defined, and the ability of NO to contribute to the pathogenesis of Th2 inflammation and remodeling has not been appropriately assessed. Our studies demonstrate that VEGF is a potent inducer of eNOS and iNOS and that this induction plays an important role in VEGF-induced inflammation, neovascularization, mucus metaplasia, DC hyperplasia, and AHR. These findings have a number of important implications regarding asthma pathogenesis. First, the demonstration that NO inhibition diminishes VEGF-induced inflammation is in accord with and provides a mechanistic explanation for prior studies that demonstrate that NOS inhibitors diminish aeroallergen-induced inflammation (37). Second, because viruses such as respiratory syncytial virus are potent stimulators of VEGF (38) and because the NO that is induced during these infections has anti-viral and anti-inflammatory properties (39, 40), the VEGF–NO pathway could contribute to the induction and control of virus-induced inflammation and remodeling in the normal and asthmatic airway. Lastly, NO is also a potent stimulator of VEGF elaboration (19, 41). Thus, one can easily envision a positive feedback/amplification loop in which stimuli such as viruses (38, 39, 42), endotoxin (43), or antigen (18) induce VEGF elaboration, the VEGF activates NOS to produce NO, and the NO in turn augments VEGF production. Such amplification loop could contribute to the intensity and chronicity of asthma and other VEGF-driven diseases. Collectively, these studies suggest that VEGF is an important stimulator of NO in asthma and that this activation plays an important role in the pathogenesis of the tissue and physiologic abnormalities in this disorder.
Mucosal immune responses are regulated by a complex system that involves anatomic barriers, macrophage regulation of T cell responses, and lung DC induction of CD4+ effector and regulatory T cells (44). Multiple lines of evidence suggest that VEGF is an important regulator of these responses. They include studies from our laboratory that demonstrated that VEGF abrogates pulmonary tolerance while increasing lung DC cell number and activation (7). To further define the mechanism of this VEGF response, we characterized the role(s) of NOS and NO in VEGF-induced immune responses. We expected VEGF-induced DC alterations would be mediated via an NOS-dependent mechanism(s). In accord with our expectations, VEGF-induced DC hyperplasia was diminished by all of the NOS-based interventions that were used. However, we were surprised to see that VEGF-induced DC activation was not similarly NOS-dependent. In fact, NO inhibition increased DC expression of CD54, CD80, CD86, and ICOSL. It is difficult to predict the consequences of these changes because the induction of respiratory tolerance requires costimulation with CD86 and ICOS–ICOSL interaction (44), whereas interventions that block ICOS–ICOSL interactions can diminish memory/effector T cell responses (45) and induce tolerance (46). It is clear, however, that VEGF stimulates lung DC hyperplasia and inhibits DC activation via simultaneously activated NO-dependent pathways.
One of the prominent paradoxes in pulmonary NO biology is the appreciation that, although NO is felt to be a bronchodilator and ENO is increased in asthma, asthmatic airway responses to agents such as methacholine are increased rather than depressed (19). Similarly, neither genetic deletion of iNOS in mice nor pharmacologic inhibition of NOS in asthmatics has provided protection against AHR (20, 22, 29, 47), and animals deficient in the NOSs that are expressed constitutively do not exhibit increases in airway tone or AHR (20). It has recently been appreciated that the effects of NO are mediated, at least in part, by downstream metabolites such as GSNO and that this endogenous bronchodilator is metabolized by GSNOR (29). A potential explanation for this NO–asthma paradox has recently been appreciated in studies that demonstrate that, although NO is increased after antigen challenge, so are the levels of GSNOR, resulting in GSNO depletion (29). Our studies demonstrate that, at baseline, GSNOR mRNA is not readily apparent in the murine lung but that l-NAME treatment or eNOS (but not iNOS) deletion induces GSNOR in the absence of additional stimulation. This finding suggests that basal GSNOR levels are inhibited by the low levels of constitutive eNOS (and possibly nNOS) activity. This inhibition would serve to control GSNO levels and maintain normal airway tone and can be thought of as another protective and homeostatic role of eNOS (see below). Our studies also demonstrate that VEGF induces AHR via NOS-dependent mechanisms and that VEGF is a potent stimulator of GSNOR gene expression. This finding allows for the exciting speculation that VEGF-induced AHR is mediated by GSNOR-induced depletion of GSNO. Lastly, our studies demonstrate that VEGF induction of GSNOR is ameliorated by treatment with l-NAME or the deletion of iNOS or eNOS. This finding suggests that VEGF stimulates GSNOR via its ability to induce eNOS and iNOS to produce high levels of NO and that GSNOR induction feeds back to control NO-mediated tissue responses. These observations suggest that VEGF is an important regulator of GSNOR at sites of Th2 inflammation such as the asthmatic airway and that this regulation contributes to asthmatic AHR.
There are three isoforms of NOS that are differentially distributed and regulated and play different roles in physiologic and pathologic responses (19, 32, 48, 49). Our studies demonstrate that VEGF induces eNOS and iNOS, but not nNOS, and that both eNOS and iNOS contribute to the pathogenesis of VEGF-induced vascular and extravascular pulmonary responses. These studies define the roles of NOS isoforms in the pathogenesis of pulmonary extravascular responses such as inflammation and mucin and Gob5 gene expression and, furthermore, characterize NOS isoform contribution to the regulation of GSNOR. eNOS is believed to be an important homeostatic regulator of microvascular permeability that maintains local tissue perfusion and contributes to crucial defense mechanism during inflammation (32). Our demonstration that eNOS inhibits DC activation and, at baseline, inhibits GSNOR mRNA accumulation are in accord with this concept because they would be expected to control pulmonary inflammation and airway tone. Our findings, however, also modify this conceptualization by demonstrating that eNOS, in addition to its protective effects, can contribute to the generation of pathologic extravascular responses in the lung and probably in other organs. Overall, these findings suggest that eNOS or iNOS isoform-specific interventions can be used to control the pathologic vascular and extravascular manifestations of VEGF. This treatment may be very important for diseases such as asthma where NO production may regulate tissue inflammation and viral replication (40) and diseases in which iNOS activation generates peroxynitrite and other reactive oxidant species (19).
Exaggerated VEGF production is believed to play an important role in the pathogenesis of a wide variety of diseases including tumor neovascularization, asthma, cystic fibrosis, viral infections, psoriasis, pulmonary edema, atherosclerosis, and retinopathies of the newborn and diabetic (1, 50, 51). The present studies demonstrate that VEGF is a potent stimulator of eNOS and iNOS and that these inductive events contribute to VEGF-induced pathologies. This finding suggests that the pathologic effects of VEGF in these disorders can be controlled by interventions that control NO production and that NOS isoform-specific interventions may maximize benefit while diminishing toxicity. This observation establishes the VEGF–NO pathway as a worthwhile site for future investigations designed to identify therapeutic agents that can be used in the treatment of these disorders.
Methods
Animals.
Transgenic mice (VEGF165) were generated and used in these studies, as described in ref. 7.
RNA Analysis.
RNA was isolated from frozen mice lungs by using TRIzol reagent (Life Technologies, Grand Island, NY), according to manufacturer’s instructions and subjected to RT-PCR analysis.
Airway Vasculature Staining and Airway Hyperresponsiveness.
These processes were performed as described in refs. 52 and 53, respectively.
T and Dendritic Cell Analysis.
Single cell suspensions from lungs of WT and transgenic mice receiving normal or dox water were prepared as described in ref. 54 and subjected to FACS anaylsis.
Supporting Information.
For more information, see Supporting Methods and Figs. 7–9, which are published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Kathleen Bertier for excellent administrative and secretarial assistance. This work was supported in part by National Heart, Lung, and Blood Institute (National Institutes of Health) Grants HL-74195 (to V.B.), HL-24136 and HL-59157 (to D.M.D.), and HL-64642, HL-61904, and HL-56389 (to J.A.E.) and the Angelworks Foundation (D.M.D.).
Abbreviations
- AHR
airway hyperresponsiveness
- DC
dendritic cell
- NOS
NO synthase
- iNOS
inducible NOS
- eNOS
endothelial NOS
- nNOS
neuronal NOS
- GSNO
S-nitrosoglutathione
- GSNOR
GSNO reductase
- Tg−
transgene-negative
- Tg+
VEGF transgenic
- l-NAME
NG-nitro-l-arginine methyl ester
- BAL
bronchoalveolar lavage
- dox
doxycycline.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tammela T., Enholm B., Alitalo K., Paavonen K. Cardiovasc. Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N., Gerber H. P., LeCouter J. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds L. P., Redmer D. A. Biol. Reprod. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- 4.Silha J. V., Krsek M., Sucharda P., Murphy L. J. Int. J. Obes. Relat. Metab. Disord. 2005 doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 5.Khurana R., Simons M., Martin J. F., Zachary I. C. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson-Berka J. L. Curr. Pharm. Des. 2004;10:3331–3348. doi: 10.2174/1381612043383142. [DOI] [PubMed] [Google Scholar]
- 7.Lee C. G., Link H., Baluk P., Homer R. J., Chapoval S., Bhandari V., Kang M. J., Cohn L., Kim Y. K., McDonald D. M., Elias J. A. Nat. Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He C. H., Waxman A. B., Lee C. G., Link H., Rabach M. E., Ma B., Chen Q., Zhu Z., Zhong M., Nakayama K., Nakayama K. I., Homer R., Elias J. A. J. Clin. Invest. 2005;115:1039–1048. doi: 10.1172/JCI23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias J. A., Lee C. G., Zheng T., Ma B., Homer R. J., Zhu Z. J. Clin. Invest. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charan N. B., Baile E. M., Pare P. D. Eur. Respir. J. 1997;10:1173–1180. doi: 10.1183/09031936.97.10051173. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino M., Takahashi M., Aoike N. J. Allergy Clin. Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y. C., Lee H. K. J. Allergy Clin. Immunol. 2001;107:1106. doi: 10.1067/mai.2001.115628. (lett.) [DOI] [PubMed] [Google Scholar]
- 13.Salvato G. Thorax. 2001;56:902–906. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vrugt B., Wilson S., Bron A., Holgate S. T., Djukanovic R., Aalbers R. Eur. Respir. J. 2000;15:1014–1021. doi: 10.1034/j.1399-3003.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino M., Nakamura Y., Hamid Q. A. J. Allergy Clin. Immunol. 2001;107:1034–1038. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 16.Kanazawa H., Hirata K., Yoshikawa J. Thorax. 2002;57:885–888. doi: 10.1136/thorax.57.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asai K., Kanazawa H., Otani K., Shiraishi S., Hirata K., Yoshikawa J. J. Allergy Clin. Immunol. 2002;110:571–575. doi: 10.1067/mai.2002.127797. [DOI] [PubMed] [Google Scholar]
- 18.Antony A. B., Tepper R. S., Mohammed K. A. J. Allergy Clin. Immunol. 2002;110:589–595. doi: 10.1067/mai.2002.127798. [DOI] [PubMed] [Google Scholar]
- 19.Ricciardolo F. L., Sterk P. J., Gaston B., Folkerts G. Physiol. Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 20.De Sanctis G. T., MacLean J. A., Hamada K., Mehta S., Scott J. A., Jiao A., Yandava C. N., Kobzik L., Wolyniec W. W., Fabian A. J., et al. J. Exp. Med. 1999;189:1621–1630. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid Q., Springall D. R., Riveros-Moreno V., Chanez P., Howarth P., Redington A., Bousquet J., Godard P., Holgate S., Polak J. M. Lancet. 1993;342:1510–1513. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 22.Hansel T. T., Kharitonov S. A., Donnelly L. E., Erin E. M., Currie M. G., Moore W. M., Manning P. T., Recker D. P., Barnes P. J. FASEB J. 2003;17:1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 23.Kroll J., Waltenberger J. Biochem. Biophys. Res. Commun. 1998;252:743–746. doi: 10.1006/bbrc.1998.9719. [DOI] [PubMed] [Google Scholar]
- 24.Bussolati B., Dunk C., Grohman M., Kontos C. D., Mason J., Ahmed A. Am. J. Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilani M. M., Mohammed K. A., Nasreen N., Tepper R. S., Antony V. B. Inflammation. 2004;28:245–251. doi: 10.1007/s10753-004-6047-y. [DOI] [PubMed] [Google Scholar]
- 26.Yague S., Alvarez Arroyo V., Castilla A., Gonzalez Pacheco F. R., Llamas P., Caramelo C. J. Nephrol. 2005;18:234–242. [PubMed] [Google Scholar]
- 27.Lin Y. J., Markham N. E., Balasubramaniam V., Tang J. R., Maxey A., Kinsella J. P., Abman S. H. Pediatr. Res. 2005;58:22–29. doi: 10.1203/01.PDR.0000163378.94837.3E. [DOI] [PubMed] [Google Scholar]
- 28.Watkins D. N., Peroni D. J., Basclain K. A., Garlepp M. J., Thompson P. J. Am. J. Respir. Cell Mol. Biol. 1997;16:629–639. doi: 10.1165/ajrcmb.16.6.9191464. [DOI] [PubMed] [Google Scholar]
- 29.Que L. G., Liu L., Yan Y., Whitehead G. S., Gavett S. H., Schwartz D. A., Stamler J. S. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gratton J. P., Lin M. I., Yu J., Weiss E. D., Jiang Z. L., Fairchild T. A., Iwakiri Y., Groszmann R., Claffey K. P., Cheng Y. C., Sessa W. C. Cancer Cells. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 31.Bucci M., Gratton J. P., Rudic R. D., Acevedo L., Roviezzo F., Cirino G., Sessa W. C. Nat. Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 32.Cirino G., Fiorucci S., Sessa W. C. Trends Pharmacol. Sci. 2003;24:91–95. doi: 10.1016/S0165-6147(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 33.Mulrennan S. A., Redington A. E. Treat Respir. Med. 2004;3:79–88. doi: 10.2165/00151829-200403020-00002. [DOI] [PubMed] [Google Scholar]
- 34.Dinakar C. Curr. Allergy Asthma Rep. 2004;4:454–459. doi: 10.1007/s11882-004-0011-7. [DOI] [PubMed] [Google Scholar]
- 35.Kharitonov S. A., Barnes P. J. Proc. Am. Thorac. Soc.; 2004. pp. 191–199. [DOI] [PubMed] [Google Scholar]
- 36.Lemanske R. F., Jr Pediatrics. 2002;109:368–372. [PubMed] [Google Scholar]
- 37.Landgraf R. G., Russo M., Jancar S. Eur. J. Pharmacol. 2005;518:212–220. doi: 10.1016/j.ejphar.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 38.Lee C. G., Yoon H. J., Zhu Z., Link H., Wang Z., Gwaltney J. M., Landry M., Elias J. A. Am. J. Respir. Cell Mol. Biol. 2000;23:662–669. doi: 10.1165/ajrcmb.23.5.4188. [DOI] [PubMed] [Google Scholar]
- 39.Johnston S. L. Proc. Am. Thorac. Soc.; 2005. pp. 150–156. [DOI] [PubMed] [Google Scholar]
- 40.Proud D. Curr. Opin. Allergy Clin. Immunol. 2005;5:37–42. doi: 10.1097/00130832-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Milkiewicz M., Hudlicka O., Brown M. D., Silgram H. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H336–43. doi: 10.1152/ajpheart.01105.2004. [DOI] [PubMed] [Google Scholar]
- 42.Sigurs N. Am. J. Respir. Crit. Care Med. 2001;163:S2–6. doi: 10.1164/ajrccm.163.supplement_1.2011109. [DOI] [PubMed] [Google Scholar]
- 43.Ramanathan M., Giladi A., Leibovich S. J. Exp. Biol. Med. (Maywood) 2003;228:697–705. doi: 10.1177/153537020322800608. [DOI] [PubMed] [Google Scholar]
- 44.Macaubas C., DeKruyff R. H., Umetsu D. T. Curr. Drug Targets Inflammation Allergy. 2003;2:175–186. doi: 10.2174/1568010033484304. [DOI] [PubMed] [Google Scholar]
- 45.Greenwald R. J., Freeman G. J., Sharpe A. H. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 46.Nanji S. A., Hancock W. W., Anderson C. C., Adams A. B., Luo B., Schur C. D., Pawlick R. L., Wang L., Coyle A. J., Larsen C. P., Shapiro A. M. Am. J. Transplant. 2004;4:526–536. doi: 10.1111/j.1600-6143.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 47.Xiong Y., Karupiah G., Hogan S. P., Foster P. S., Ramsay A. J. J. Immunol. 1999;162:445–452. [PubMed] [Google Scholar]
- 48.Nathan C., Xie Q. W. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 49.Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C. O., Buerk D. G., Huang P. L., Jain R. K. Proc. Natl. Acad. Sci. USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celletti F. L., Waugh J. M., Amabile P. G., Brendolan A., Hilfiker P. R., Dake M. D. Nat. Med. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 51.Yang J. C., Haworth L., Sherry R. M., Hwu P., Schwartzentruber D. J., Topalian S. L., Steinberg S. M., Chen H. X., Rosenberg S. A. N. Engl. J. Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baluk P., Tammela T., Ator E., Lyubynska N., Achen M. G., Hicklin D. J., Jeltsch M., Petrova T. V., Pytowski B., Stacker S. A., et al. J. Clin. Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Z., Homer R. J., Wang Z., Chen Q., Geba G. P., Wang J., Zhang Y., Elias J. A. J. Clin. Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demedts I. K., Brusselle G. G., Vermaelen K. Y., Pauwels R. A. Am. J. Respir. Cell Mol. Biol. 2005;32:177–184. doi: 10.1165/rcmb.2004-0279OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.