Abstract
The type II secretion pathway of Pseudomonas aeruginosa is involved in the extracellular release of various toxins and hydrolytic enzymes such as exotoxin A and elastase. This pathway requires the function of a macromolecular complex called the Xcp secreton. The Xcp secreton shares many features with the machinery involved in type IV pilus assembly. More specifically, it involves the function of five pilin-like proteins, the XcpT-X pseudopilins. We show that, upon overexpression, the XcpT pseudopilin can be assembled in a pilus, which we call a type II pseudopilus. Image analysis and filtering of electron micrographs indicated that these appendages are composed of individual fibrils assembled together in a bundle structure. Our observations thus revealed that XcpT has properties similar to those of type IV pilin subunits. Interestingly, the assembly of the type II pseudopilus is not exclusively dependent on the Xcp machinery but can be supported by other similar machineries, such as the Pil (type IV pilus) and Hxc (type II secretion) systems of P. aeruginosa. In addition, heterologous pseudopilins can be assembled by P. aeruginosa into a type II pseudopilus. Finally, we showed that assembly of the type II pseudopilus confers increased bacterial adhesive capabilities. These observations confirmed the ability of pseudopilins to form a pilus structure and raise questions with respect to their function in terms of secretion and adhesion, two crucial biological processes in the course of bacterial infections.
Pseudomonas aeruginosa is a gram-negative, opportunistic bacterial pathogen that is responsible for severe nosocomial infections and is also a key agent in the early deaths of patients suffering from cystic fibrosis (17). The organism is ubiquitous; it is found in many different ecological habitats and may infect a wide variety of hosts (32). These adaptive properties can be related to the large genome size of the bacterium (6.3 Mb) (37).
The pathogenicity of P. aeruginosa and its abilities to infect tissues and to colonize and establish itself on different surfaces is linked to the production of several toxins, hydrolytic enzymes, and adhesins. There are several secretory pathways that allow the extracellular release of P. aeruginosa enzymes and toxins (38). Whereas type I and type III pathways are thought to allow a one-step transport process of exoproteins across both inner and outer membranes of the cell envelope, the type II pathway drives exclusively translocation across the outer membrane (30). The translocation of the type II-dependent exoproteins across the inner membrane is achieved by either the Sec or Tat transport systems (40). For P. aeruginosa, two functional type II systems have been characterized. The Xcp system is required for secretion of exotoxin A, lipases, phospholipases C, alkaline phosphatase, or elastase (LasB) (12), and the Hxc system is required for secretion of the low-molecular-weight alkaline phosphatase LapA (1).
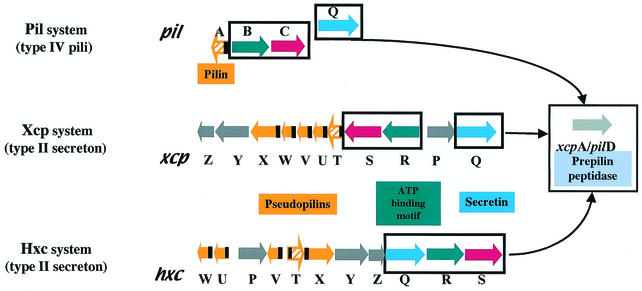
A number of adhesins are involved in P. aeruginosa attachment. In addition to alginate, an exopolysaccharide, extracellular appendages are crucial for biofilm formation (7). These include the flagella, the type IV pili (26), and the Cup adhesins (39). The type IV pili are responsible for twitching motility (41) and the major pilin subunit, PilA, is assembled into a pilus after processing of the PilA precursor by a prepilin peptidase, PilD (24). In P. aeruginosa, the function of this protein, also called XcpA, is used for both type IV pilus assembly and the type II secretion process (2, 25). In addition, type II secretion and type IV piliation machines consist of similar components, including the secretins PilQ, XcpQ, and HxcQ (4) and the “traffic ATPases” PilB, XcpR, and HxcR (28). Most interestingly, some components of the Xcp machinery share homologies with the pilin subunit and have been called pseudopilins (2, 25).
It has been a major issue to demonstrate that the pseudopilins may be assembled into a pilus-like structure. In this study we have confirmed, as has been shown for the Klebsiella oxytoca PulG pseudopilin (34), that the P. aeruginosa XcpT pseudopilin can be assembled into a pilus-like structure. Moreover, we further revealed interesting characteristics of the assembly and the structure of this cell surface appendage that we call a type II pseudopilus. This analysis showed that the structure is a bundled type of pilus with similarities to those described for enteropathogenic or enterotoxic Escherichia coli (10, 16) and Actinobacillus actinomycetemcomitans (21). We further addressed the relevance of this structure in terms of type II secretion and adhesion properties in P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. aeruginosa and E. coli strains were grown at 37°C in tryptic soy broth and Luria broth (LB), respectively. When required, media were supplemented with the following antibiotics at the indicated concentrations (in micrograms per milliliter): for E. coli, ampicillin, 50; kanamycin, 50; and streptomycin, 50; and for P. aeruginosa, carbenicillin, 300 to 500; and streptomycin, 2,000. The E. coli CC118λpir strain was used to propagate pKNG101 and derivative plasmids, while the TG1 strain was used for other plasmids. Plasmids were introduced into P. aeruginosa by triparental mating by use of the conjugative properties of pRK2013 or by electroporation (36). The P. aeruginosa strains used were PAO1 and its derivatives PAOΔHRQ (pilQ/hxcR), PAOΔXHR (xcpRS hxcR), PAOΔRSQ (xcpRS pilQ), PAOΔXHP (xcpRS hxcR pilQ), and D40ZQ (xcpP-Z); PAO222 and its derivative KS904 (xcpA); and PAK and its derivative PAKpilA/fliC (35). P. aeruginosa transconjugants were selected on Pseudomonas isolation agar supplemented with antibiotics.
Construction of the P. aeruginosa mutants.
In this study, the mutants PAOΔHRQ (pilQ/hxcR), PAOΔRSQ (xcpRS pilQ), and PAOΔXHP (xcpRS hxcR pilQ) were constructed as previously described (1). The mutations were successively introduced to obtain strains containing more than one mutation. Briefly, 500-bp sections upstream and downstream the target genes were PCR amplified. The oligonucleotides were designed for amplifying fragments with overlapping 3′ and 5′ ends. Both fragments were ligated by performing an overlapping PCR. This was done by using the most-upstream and -downstream primers in a second run of PCR with a mix of the two fragments as the matrix. The resulting PCR product was cloned into the PCR2.1 plasmid (TA cloning kit; Invitrogen). A 1,000-bp BamHI-ApaI DNA fragment was then subcloned into the suicide pKNG101 vector. The resulting construct was transferred to P. aeruginosa by mobilization with pRK2013. The strains in which the chromosomal integration event occurred were selected on Pseudomonas isolation agar plates containing 2,000 μg of streptomycin per ml. Excision of the plasmid, resulting in the deletion of the chromosomal target gene, was performed after selection on LB plates containing 5% sucrose. Clones that became sucrose resistant and streptomycin sensitive were confirmed to contain the gene deletion by PCR analysis.
Protease plate assay.
Protease secretion by P. aeruginosa was tested after the organism was plated on tryptic soy agar plates containing 1.5% skim milk.
Shearing method.
The method to obtain the release of cell surface appendages was adapted from a previously described procedure (23). Overnight-grown bacteria were harvested from agar plates and suspended in LB supplemented with 10 mM MgCl2 to an optical density at 600 nm (OD600) of 5. The suspension was passed through the 19-gauge needle of a syringe and centrifuged to separate the bacterial pellet from the extracellular-appendage-enriched supernatant. The supernatant was recentrifuged to eliminate residual bacterial cells.
SDS-PAGE and immunoblot analysis.
Bacterial pellets or trichloroacetic acid-precipitated proteins from supernatants were suspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The amounts of proteins loaded from bacterial pellets or supernatants were equivalent to 0.1 or 0.5 OD600 unit of bacterial cell culture, respectively. Samples were heated for 7 min at 95°C and separated by electrophoresis on SDS-15% acrylamide gels. Immunoblotting was used for the detection of the XcpT and XcpZ proteins by using polyclonal antisera at a 1:5,000 dilution. Purified XcpT and XcpP proteins, tagged with a six-His tail, were used as the antigen source and injected into a rabbit to yield XcpT and XcpP antisera (Eurogentec).
TEM and immunogold labeling.
The negative-staining procedure was as follows. Bacteria harvested from agar plates were suspended in 15 μl of 10 mM Tris-0.15 M NaCl, pH 7.8. Suspensions were placed on copper grids and coated with Formvar and carbon, and bacterial cells were adsorbed for 1 min. The grids were incubated three times with drops of uranyl acetate (1%), air dried, and observed by using a Zeiss EM9 electron microscope. For immunogold labeling, harvested bacteria were suspended in 20 μl of phosphate-buffered saline (PBS). Adsorption on grids was performed as described above. Furthermore, grids were treated successively with PBS, 2% p-formaldehyde in PBS (fixation for 5 min), 5% bovine serum albumin (saturation for 15 min), PBS, 0.5% bovine serum albumin containing the primary XcpT antibody at a 1:100 dilution (2 h), PBS (three times), PBS containing the conjugated protein A-gold particles (10 nm diameter) for 1 h, PBS (three times), 1% glutaraldehyde (fixation for 5 min), PBS, and distilled water. Grids were negatively stained and observed by transmission electron microscopy (TEM) as described above.
Image analysis and filtering were performed with the MRC Cambridge image-processing system (8) and custom-written programs. Briefly, electron micrographs were examined to find in a type II pseudopilus straight regions of a constant width that showed reasonable structure as estimated by the contrast of diffraction spots in the two-dimensional Fourier transform image. The type II pseudopilus was isolated with a soft-edged cache and a filter constructed in the Fourier domain to enhance the visibility of the type II pseudopilus substructures and to attenuate noise by retaining information in or close to the obvious layer lines. The resulting Fourier filtered image was recached to hide artifactual echoes.
Adherence assay.
A biofilm formation assay was performed as described previously (39). A Falcon tube containing 1 ml of M63-derived minimal medium supplemented with 2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was inoculated with 108 bacterial cells. Incubation was performed at 30°C without agitation during a period of 4 h. The wells were rinsed with water, the bacterial film was stained with 1% crystal violet, and for quantification, the film was suspended in 400 μl of 95% ethanol, after which 600 μl of water was added and the OD600 was measured.
RESULTS
Extracellular exposure of the XcpT pseudopilin.
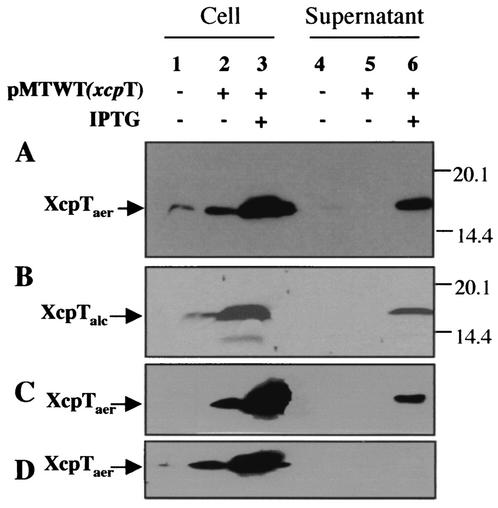
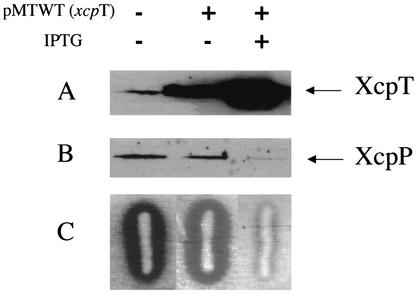
The pseudopilin-encoding xcpT gene was cloned as an 860-bp DNA fragment in the broad-host-range plasmid pMMB190, which contains the tac promoter, yielding pMTWT. P. aeruginosa strain PAO1 containing pMTWT was grown on agar plates supplemented, or not supplemented, with IPTG. A loop of cells was suspended in LB up to an OD600 of 5 and passed through the needle of a syringe to shear cell surface appendages. Bacterial cells and the supernatant were separated by centrifugation. Proteins contained in the supernatant were precipitated by adding 12% trichloroacetic acid, whereas cell pellets were directly suspended in SDS-PAGE buffer. Proteins were separated on polyacrylamide gels, followed by immunoblotting using antibodies directed against XcpT. As shown in Fig. 1A, XcpT was seen in the supernatant fraction of PAO1 strains containing pMTWT only when IPTG was added. The release of XcpT was not due to cell lysis since bacterial cells were viable and motile in all cases, as seen by observation under a light microscope (data not shown). In the absence of a shearing step, XcpT was also found in the supernatant fraction (data not shown). We concluded that upon XcpT overproduction, the protein is released from the bacterial cell or is assembled into a fragile structure at the cell surface.
FIG. 1.
XcpT overproduction and exposition at the bacterial cell surface. XcpT was released by the shearing of P. aeruginosa. (A) PAO1 strain bearing pMTWT (P. aeruginosa xcpT); (B) PAO1 strain bearing pMTA3 (P. alcaligenes xcpT); (C) P. aeruginosa xcpRS/hxcR mutant strain bearing pMTWT; (D) P. aeruginosa xcpRS/hxcR/pilQ mutant strain bearing pMTWT. Samples were analyzed by SDS-PAGE and immunoblotting. Lanes 1 to 3, bacterial pellet; lanes 4 to 6, extracellular-appendage-enriched supernatant. The position of XcpT has been indicated, with the use of “aer” or “alc” in subscript to indicate whether the pseudopilin is from P. aeruginosa or P. alcaligenes, respectively.
XcpT-containing pili are multifibrillar.
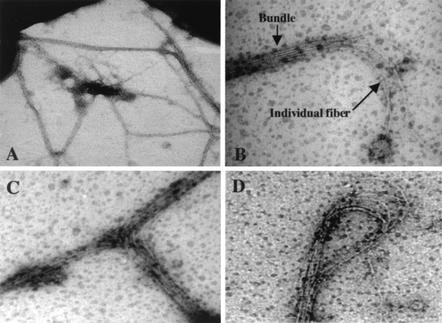
In order to distinguish whether the presence of XcpT in the supernatant is associated with extracellular release or the formation of cell surface XcpT-containing appendages, we examined the cells by high-resolution TEM. Interestingly, we observed several elongated structures sticking to the grids whereas others appeared to be attached to the cells (Fig. 2A). These thick and sticky structures could be seen only when we examined cells that overproduced XcpT. These structures appeared to be multifibrillar, consisting of bundle of six to seven filaments tightly packed together (Fig. 2B). Some structures formed a loop (Fig. 2D), split by raising individual filaments (Fig. 2B), or split by adopting a T form (Fig. 2C). We further checked the presence of XcpT within these bundled filaments by performing immunogold labeling using XcpT antibodies. Strikingly, the structures attached to the cell surfaces were fully labeled by gold particles, indicating that XcpT was found along the whole length of the bundled filament (Fig. 3). No XcpT labeling could be found at the cell surface of a PAO1 strain not overproducing XcpT (data not shown). The lengths of the structures measured up to 10 μm, and the thickness was between 20 and 100 nm, with each single filament having a diameter around 7 to 9 nm (data not shown). We concluded that, upon overproduction, XcpT is assembled at the cell surface into a multifibrillar structure, which we called the type II pseudopilus.
FIG. 2.
XcpT is assembled in multifibrillar structures. The results of negative staining and TEM analysis of a PAO1 strain that overproduces XcpT are shown. See the text for details.
FIG. 3.
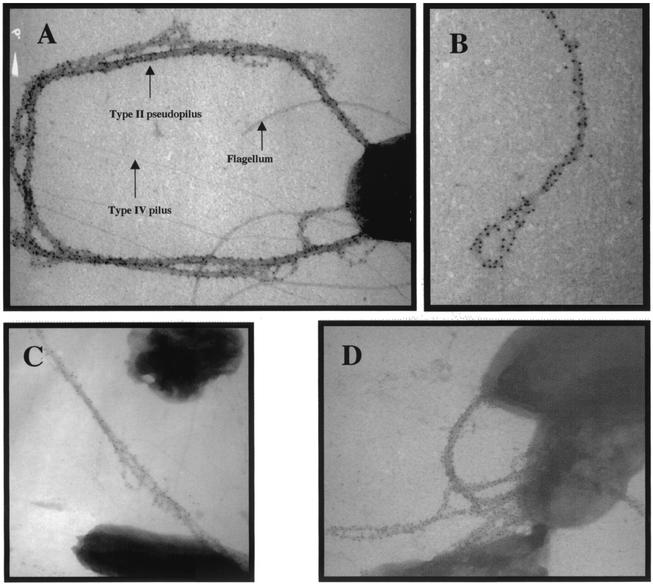
XcpT pseudopilin is a major component of the type II pseudopilus. Shown are the results of TEM analysis of PAO1/pMTWT after immunogold labeling with antibodies raised against XcpT. (A, C, and D) The type II pseudopili are seen attached to bacterial cells. (B) A type II pseudopilus with a loop structure is seen enlarged. In panel A, the positions of a type IV pilus, the flagellum, and a type II pseudopilus have been indicated.
Image analysis of bundles.
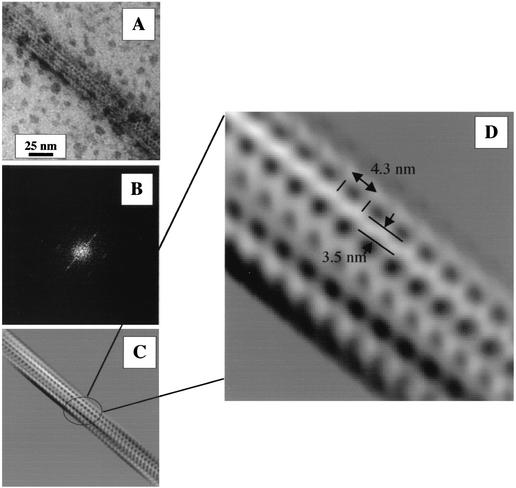
Image filtering of selected electron micrographs was performed in an attempt to clarify the possible structure and substructures of type II pseudopili (Fig. 4). The Fourier transformation (Fig. 4B) and real-domain filtering (Fig. 4C and D) were restricted to enhancing the visibility of the fibril substructure. The substructure was revealed to be a bundle of fibrils (Fig. 4C) which do not strictly run in parallel but have a twist. The fibrils of this twisted bundle appear to be ordered along their length and held together by projections, which are already visible in the original image (Fig. 4A) but much clearer after filtering (Fig. 4C and D). Along the axis of the fibrils, side projections alternate from right to left, suggesting a helical structure. The distance between two projections from the same side might represent the longitudinal repeat constituting the fibril. The length of the repeat is estimated to be 4.3 nm (Fig. 4D). The diameter of the electron-transparent fibril, excluding side projections, is evaluated to be 3.5 nm. Interestingly, assuming a cylindrical repetitive unit of the observed size (4.3-nm length and 1.7-nm radius) and a protein density of 1.2 g cm−3, we estimate a repeat unit mass of about 28 kDa. Since the relative molecular mass of the mature XcpT protein is 14 kDa, we may suggest that one repeat is made with two XcpT subunits.
FIG. 4.
Image treatment of electron micrographs of a negatively stained portion of the type II pseudopilus. (A) Original image; (B) Fourier transformation of the original image; (C) filtered image; (D) enlargement of the filtered image showing the type II pseudopilus substructure. The length of the observed longitudinal repeat (4.3 nm) and the approximate width of the electron-transparent fibril (3.5 nm) have been indicated.
Assembly of the XcpT pilus is not strictly dependent on the Xcp secreton.
The XcpT pilus was called a type II pseudopilus with reference to the type II secretion machinery in which XcpT is involved (12). We analyzed whether XcpT assembly was dependent on the function of other Xcp components. The plasmid containing xcpT (pMTWT) was introduced into strain D40ZQ (xcpP-Z), from which the whole xcp gene cluster is deleted (Fig. 5). Interestingly, the type II pseudopili were normally assembled by this strain, as was seen by immunogold labeling (data not shown). In contrast, when XcpT overproduction was induced in E. coli, no such structures could be seen (data not shown). This observation confirmed that the release of XcpT in the bacterial supernatant is not due to nonspecific leakage. We concluded that assembly of the type II pseudopilus requires machinery that is present in, and specific to, P. aeruginosa and that is either absent or not functional in E. coli. In P. aeruginosa, the pilins and pseudopilins are processed by the prepilin peptidase PilD/XcpA (2, 25), which is required for both type II secretion, including the recently discovered Hxc type II secretion, and type IV piliation (Fig. 5). Interestingly, the type II pseudopilus could not be formed when XcpT was overproduced in a mutant lacking the functional prepilin peptidase (data not shown). However, analysis of strains containing pMTWT and defective for either the pilus biogenesis system and the Hxc type II secretion system (pilQ/hxcR mutant), the pilus biogenesis system and the Xcp type II secretion system (pilQ/xcpRS mutant), or the two type II secretion systems (xcpRS/hxcR mutant) revealed the formation of type II pseudopili (Fig. 1C and data not shown). Thus, none of these pathways are individually essential for XcpT assembly. Only when we overexpressed XcpT in the xcpRS/hxcR/pilQ triple mutant were no extracellular type II pseudopilus appendages observed and no XcpT found in the sheared fraction (Fig. 1D). It should be noted that in terms of type II secretion and type IV piliation, none of the mutations tested here could be compensated for by the function of a gene from a distinct but similar system. For example, an hxcR mutant is defective for LapA alkaline phosphatase secretion, and this defect is not rescued by the functions of homologous genes in the pil and xcp systems, namely, pilB and xcpR (1). We concluded that XcpT assembly requires the function of the prepilin peptidase but might use, in a nonspecific manner, any of the PilD/XcpA-dependent systems, including the Xcp, Hxc, and Pil systems. These results are summarized in Table 1, and the different systems are described in the legend to Fig. 5.
FIG. 5.
Schematic representation of the pil, xcp, and hxc gene clusters. Genes are represented with arrows that reflect their transcription orientation. The genes encoding pilins and pseudopilins are represented in orange. The black boxes represent the regions encoding the conserved N-terminal ends of these proteins. The blue, red, and green genes encode components that are conserved between the three systems. The grey genes are found only in the xcp and hxc systems. The gene encoding the XcpA/PilD protein is unique and is found at the pil locus on the chromosome. However, it is represented apart since it is essential for the function of all three systems.
TABLE 1.
Requirement for the Xcp, Hxc, or Pil system in the assembly of the type II pseudopilus
| Strain genotype | Activation of molecular machinea:
|
XcpT pseudopilus assemblyb | ||
|---|---|---|---|---|
| Xcp | Hxc | Pil | ||
| Wild-type | + | + | + | Yes |
| xcpP-Z | − | + | + | Yes |
| xcpA | − | − | − | No |
| hxcR pilQ | + | − | − | Yes |
| xcpRS pilQ | − | + | − | Yes |
| xcpRS hxcR | − | − | + | Yes |
| xcpRS hxcR pilQ | − | − | − | No |
XcpT was overproduced in different strains in which either one, two, or three of the Xcp, Hxc, and Pil systems are inactivated, as indicated by a − (a functional system is indicated by a +).
When the three systems were simultaneously defective, no type II pseudopilus could be assembled, whereas the functionality of only one of these systems was sufficient for assembly.
Heterologous assembly of the type II pseudopilus.
We tested whether pseudopilins from heterologous hosts could be assembled into a type II pseudopilus by P. aeruginosa. The Pseudomonas alcaligenes xcpT gene product was shown to cross-react with antibodies directed against P. aeruginosa XcpT. Western blot analysis (Fig. 1B) and immunogold labeling electron microscopy (data not shown) revealed that PAO1 containing plasmid pMTA3 (P. alcaligenes xcpT) is able to expose and assemble a type II pseudopilus containing the P. alcaligenes XcpT. The structure of the P. alcaligenes type II pseudopilus is identical in size and shape to the one formed by the P. aeruginosa pseudopilin XcpT (data not shown).
The type II pseudopilus interferes with type II secretion.
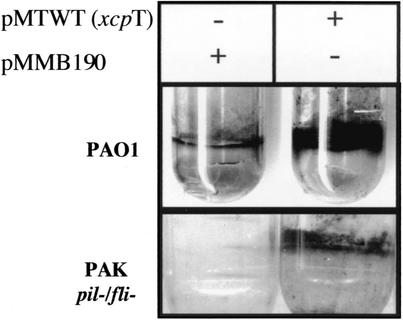
We analyzed whether the assembly of the elongated type II pseudopilus could alter the secretion of proteins that depend on Xcp for secretion, such as the major protease LasB. The proteolytic activity of P. aeruginosa LasB is routinely detected by the formation of a zone of hydrolysis on skim-milk plates (Fig. 6). Strikingly, the halo revealing LasB proteolytic activity was much reduced for PAO1 strains containing pMTWT (xcpT), compared to that of PAO1 containing pMMB190 (Fig. 6A). The integrity of the Xcp secreton was also investigated under conditions that allowed type II pseudopilus formation. Bacterial cells (PAO1/pMWT) grown on agar plates containing ITPG were harvested in LB. Samples equivalent to 0.1 OD600 unit were suspended in SDS-PAGE loading buffer, and proteins were separated on SDS-12% acrylamide gels. Proteins were transferred to nitrocellulose membranes and probed with antibodies directed against Xcp proteins. Strikingly, the level of XcpP protein was drastically reduced under conditions favoring type II pseudopilus formation, i.e., with expression of the xcpT gene upon IPTG addition (Fig. 6B). The levels of XcpZ and XcpY were also probed, but the amounts of these two proteins were not significantly affected (data not shown).
FIG. 6.
XcpT overproduction interferes with Xcp-dependent secretion. PAO1 strains containing pMTWT (xcpT gene cloned into pMMB190) where indicated or pMMB190 were grown on skim-milk plates in the presence of 2 mM IPTG where indicated. (A and B) A loop of cells was collected from plates and analyzed for XcpT and XcpP production by Western blotting with appropriate antibodies. (C) The zones of hydrolysis observed around the colonies are mostly due to the proteolytic activity of elastase (LasB).
The type II pseudopilus confers increased adherence capacity.
The type II pseudopilus has a structure similar to that of the bundled pili from enteropathogenic E. coli strains (10) or the Flp pili from A. actinomycetemcomitans (21), which are involved in attachment processes. We investigated whether the formation of the type II pseudopilus could confer increased adhesive properties to P. aeruginosa. An adhesion assay was performed as described in Materials and Methods (39). The thickness of the biofilm appeared significantly increased when the cells harbored the pMTWT plasmid compared to that of cells harboring the control plasmid pMMB190 (Fig. 7). Quantification experiments were performed in three independent assays as described in Materials and Methods. On average, for strains containing pMTWT, the level of attachment was significantly increased (2.5-fold) compared to that of the strain containing pMMB190. A similar experiment was performed with a nonadherent P. aeruginosa strain (PAKpilA/fliC). Introduction of pMTWT into this strain allowed formation of a clear film, whereas no zone of adhesion could be seen upon introduction of the pMMB190 cloning vector (Fig. 7). We concluded that type II pseudopilus formation linked to the presence of pMTWT increased the bacterial adherence capacity. Interestingly, the attachment level of a P. aeruginosa strain was also increased when the overexpressed xcpT gene originated from P. alcaligenes (pMTA3) (data not shown). This observation revealed the heterologous assembly of a P. alcaligenes type II pseudopilus in P. aeruginosa.
FIG. 7.
Attachment of P. aeruginosa PAO1 and PAKpilA/fliC strains that overproduce (with pMTWT, which contains the xcpT gene) or do not overproduce (with pMMB190, cloning vector) the XcpT pseudopilin. The formation of bacterial film on the walls of Falcon tubes is visualized after crystal violet staining.
DISCUSSION
The type II secretion system, called the Xcp secreton in P. aeruginosa, is conserved in gram-negative bacteria (11). It is required for outer membrane protein translocation and involves 12 to 15 different components (12). A striking feature of these components is their relationship with the components involved in type IV pilus biogenesis (18). Type IV pili are retractile cell surface appendages involved in twitching motility (41). Remarkably, the structural subunit of the pilus, PilA, shares similarities with the pseudopilins, namely, XcpT, -U, -V, -W, and -X, in P. aeruginosa (2, 6, 25). The similarities between pilin and pseudopilins are essentially located at the N terminus of the proteins and include a short positively charged leader peptide that is cleaved by the leader peptidase and that precedes a highly conserved hydrophobic domain. Interestingly, the model for type IV pilus assembly proposes that the interactions between the different subunits of the pilus take place via these hydrophobic regions but that the globular C-terminal domain might append at the outside of the pilus axis (22). Could the pseudopilins be involved in the formation of a pilus-like structure (31)? We have not been able to identify Xcp-dependent structures present at the surface of a wild-type strain but absent in an xcp mutant (data not shown). However, it was shown recently by Sauvonnet and collaborators (34) that, under particular conditions, the K. oxytoca type II secretion system (Pul) is able to assemble a pilus-like structure. Indeed, E. coli cells overproducing the whole Pul machinery exposed a large structure at the cell surface, which was constituted mostly by the PulG pseudopilin.
Based on these observations, we developed conditions for which P. aeruginosa endogenously produced XcpT-containing pili. When analyzed by immuno-electron microscopy, these structures were fully labeled with gold particles. We concluded that the formation of such an appendage, which we called the type II pseudopilus, is not limited by the Xcp machinery itself but by the amount of pseudopilins that are produced. This observation might indicate that under laboratory growth conditions, the type II pseudopilus is not formed because the amount of XcpT produced is too low.
Unlike with the previous study of Sauvonnet and collaborators (34), we showed in this study that the formation of the type II pseudopilus could be obtained by the overproduction of the XcpT protein alone (the PulG homolog) and, furthermore, that it is not strictly dependent on the machinery to which XcpT belongs. Indeed, XcpT could be assembled into a pilus, even when it was overexpressed in an xcpP-Z mutant strain that lacks all known xcp genes except for xcpA. However, XcpT assembly is not seen in E. coli and requires the prepilin peptidase, XcpA/PilD in P. aeruginosa (24), which indicates that machinery such as Pil (type IV secretion) or Hxc (type II secretion) may substitute the function of the Xcp machinery for this process. This original observation was confirmed by the analysis of single, double, and triple mutations in the xcp, hxc, and pil gene clusters (Table 1). It is a possibility that XcpT has a preference for its cognate assembly pathway and might be rerouted to alternative pathways only in the absence of the Xcp system. This weak specificity is also confirmed by the observation that P. alcaligenes XcpT could be heterologously assembled in P. aeruginosa into a type II pseudopilus. In addition, it was, for example, shown that MS11 pilin from Neisseria gonorrhoeae or PpdD type IV pilin of E. coli can be assembled into pili in P. aeruginosa (33, 19).
We analyzed the relevance of the type II pseudopilus in terms of protein secretion. We suspect that, even though the assembly of elongated type II pseudopili reflects the obvious ability of the pseudopilins to form a pilus, such a structure should be much shorter under optimal secretion conditions. In support of this hypothesis, we observed that, under conditions in which the XcpT pseudopilin is overexpressed, the level of Xcp-dependent secretion is decreased, as was revealed by the low level of extracellular elastase (LasB). We could conclude that the type II pseudopilus might cross the outer membrane via the XcpQ secretin and thus disturb the release of elastase through this same channel at the same time. However, supplementary data showed that, under these same conditions, the stability of some Xcp components, and more particularly XcpP, are drastically affected. This observation might thus explain the dysfunction of the Xcp secreton. The instability of Pul secreton components upon overexpression of the XcpT homolog PulG was also observed (29). It is still a possibility that the continuous interaction of the pseudopilus with the XcpQ channel prevents the interaction of XcpQ with the XcpP component and results in its instability, as was previously reported (5, 15).
We analyzed more closely the structure of the type II pseudopilus and could see that it was not made of a single filament but was made of a bundle of fibrils. The type II pseudopilus thickness depends on the number of pili found in the bundle, which is between two and nine. Each pilus fibril had a diameter of about 7 nm. This is equivalent to the size proposed for the fibrils of N. gonorrhoeae type IV pili, which is about 6 nm (14), or P. aeruginosa pili, which is 5.2 nm (outer diameter) (22). The model proposed for assembly of N. gonorrhoeae or P. aeruginosa type IV pili takes into account five pilin monomers per helical turn (27, 22). The N-terminal hydrophobic α-helix faces the core of the pilus, whereas the C terminus is exposed to the aqueous environment. Interestingly, the electron-transparent fibril axis of the type II pseudopilus is estimated to be 3.5 nm, but that excludes the side projections (Fig. 4D). We may suggest that the side projections correspond to the C-terminal end of the structural subunits but that the fibril axis is made with the packing of the N termini.
It has recently been demonstrated that N. gonorrhoeae type IV pili cross the outer membrane via the pore formed by PilQ (42). P. aeruginosa XcpQ and PilQ belong to the same family of proteins, called secretins (4). These proteins form homomultimeric channels into the outer membrane. In the case of XcpQ, the channel cavity has a diameter of 9.5 nm (3), which is sufficient to allow passage of folded exoproteins (elastase is 6 nm long) or of a type II pseudopilus fiber (7 to 9 nm long) but not both. This hypothesis may also be supported by the fact that type II pseudopilus assembly interferes with the Xcp-dependent secretion of elastase. Finally, the formation of the type II pseudopilus as a bundle might come from the association of fibers issued from a distinct secretin channel.
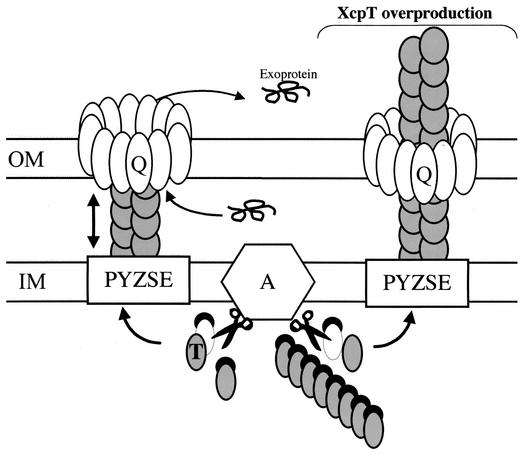
A model presenting the assembly of the type II pseudopilus is presented in Fig. 8. It is important to realize that the pseudopilus length required to cross the cell envelope is about 20 nm. According to our analysis of the type II pseudopilus, the length of a transperiplasmic structure might thus be given by five repeats of an asymmetric unit (the length of one unit is 4.3 nm). With each repeat having possibly two XcpT subunits per turn, one may expect the association of 10 XcpT molecules to be sufficient to span the periplasm. Assembly and disassembly of such a short structure might thus be possible without paying an energetically high cost. As previously mentioned, the existence of a transperiplasmic pseudopilus structure was recently reported for Xanthomonas campestris. The XcpT homolog XpsG was found in a periplasmic complex of 440 kDa (20). This complex is too large to contain only 10 XpsG subunits, but as was suggested by Hu et al., it might contain other pseudopilins, such as XpsH, which can be made by the autoassembly of several pseudopilus substructures or can be the result of XpsG aggregation (20). We are currently investigating whether additional pseudopilins can indeed be incorporated in the XcpT-containing type II pseudopilus and whether these additional pseudopilins can form pseudopili on their own.
FIG. 8.
Model for Xcp-dependent secretion and assembly of the type II pseudopilus. Under physiological conditions and upon removal of the N-terminal leader peptide (black crescent) by the prepilin peptidase XcpA (hexagon labeled “A”), pseudopilins, such as XcpT (in grey), are assembled into the Xcp secreton. The secreton is also composed of an inner membrane (IM) platform (white boxes labeled “PYZE”) and of the homomultimeric ring containing the outer membrane (OM) secretin XcpQ (Q). Under standard conditions (left part), the structure formed by the pseudopilins is proposed to be transperiplasmic. The positioning of the type II pseudopilus into the secretin channel might be part of a mechanism that prevents leakage from the periplasm through this large pore. The length of the type II pseudopilus might be controlled by the incorporation of minor pseudopilin subunits. The incorporation of the XcpX pseudopilin might stop type II pseudopilus elongation, as was previously proposed (6). Finally, the interaction of the Xcp machinery with exoproteins might somehow induce type II pseudopilus disassembly (two-headed arrow), give access to the channel, and allow the release of exoproteins into the extracellular medium. However, upon overproduction of the XcpT pseudopilin, an abnormally large type II pseudopilus that is exposed to the bacterial cell surface, probably through the XcpQ secretin, is formed (right part).
We have demonstrated that type II pseudopili aid in adherence by increasing the attachment capabilities of P. aeruginosa to plastic surfaces. The assembly of an extracellular, elongated, and bundled type II pseudopilus might be part of a distinct process. Under certain conditions, such as biofilm formation, type II secretion might be reduced (9). Under these conditions, formation of the type II pseudopilus might become advantageous. The formation of the pseudopilus obviously required an increased level of XcpT production, which was previously reported as the major P. aeruginosa pseudopilin (25). We will investigate whether increased expression of XcpT can be observed in biofilm-grown, compared to in planctonically grown, bacteria. The use of in-biofilm expression technology might be well suited for this study (13). It is indeed a possibility that the initiation of biofilm formation might be a natural switch to XcpT overproduction and to the formation of the mutifibrillar and adhesive structure herein called a type II pseudopilus.
Acknowledgments
We thank Corrine Reverbel for generating antibodies directed against XcpT and Jan Tommassen for helpful discussion.
Research in A.F.'s laboratory is supported by grants from the VLM (Vaincre La Mucoviscidose) and from the Programmes Internationaux de Coopération Scientifique (grant 848).
REFERENCES
- 1.Ball, G., E. Durand, A. Lazdunski, and A. Filloux. 2002. A novel type II secretion system in Pseudomonas aeruginosa. Mol. Microbiol. 43:475-485. [DOI] [PubMed] [Google Scholar]
- 2.Bally, M., A. Filloux, M. Akrim, G. Ball, A. Lazdunski, and J. Tommassen. 1992. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol. Microbiol. 6:1121-1131. [DOI] [PubMed] [Google Scholar]
- 3.Bitter, W., M. Koster, M. Latijnhouwers, H. de Cock, and J. Tommassen. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27:209-219. [DOI] [PubMed] [Google Scholar]
- 4.Bitter, W., and J. Tommassen. 1999. Ushers and other doorkeepers. Trends Microbiol. 7:4-6. [DOI] [PubMed] [Google Scholar]
- 5.Bleves, S., M. Gerard-Vincent, A. Lazdunski, and A. Filloux. 1999. Structure-function analysis of XcpP, a component involved in general secretory pathway-dependent protein secretion in Pseudomonas aeruginosa. J. Bacteriol. 181:4012-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleves, S., R. Voulhoux, G. Michel, A. Lazdunski, J. Tommassen, and A. Filloux. 1998. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX (GspK family). Mol. Microbiol. 27:31-40. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Crowther, R. A., R. Henderson, and J. M. Smith. 1996. MRC image processing programs. J. Struct. Biol. 116:9-16. [DOI] [PubMed] [Google Scholar]
- 9.Deziel, E., Y. Comeau, and R. Villemur. 2001. Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J. Bacteriol. 183:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg, M. S., H. Z. Zhang, and K. D. Stone. 1997. Biogenesis of the bundle-forming pilus of enteropathogenic Escherichia coli: reconstitution of fimbriae in recombinant E. coli and role of DsbA in pilin stability—a review. Gene 192:33-38. [DOI] [PubMed] [Google Scholar]
- 11.Filloux, A., M. Bally, G. Ball, M. Akrim, J. Tommassen, and A. Lazdunski. 1990. Protein secretion in gram-negative bacteria: transport across the outer membrane involves common mechanisms in different bacteria. EMBO J. 9:4323-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filloux, A., G. Michel, and M. Bally. 1998. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol. Rev. 22:177-198. [DOI] [PubMed] [Google Scholar]
- 13.Finelli, A., C. V. Gallant, K. Jarvi, and L. L. Burrows. 2003. Use of in-biofilm expression technology to identify genes involved in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:2700-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forest, K. T., and J. A. Tainer. 1997. Type-4 pilus-structure: outside to inside and top to bottom—a minireview. Gene 192:165-169. [DOI] [PubMed] [Google Scholar]
- 15.Gérard-Vincent, M., V. Robert, G. Ball, S. Bleves, G. P. F. Michel, A. Lazdunski, and A. Filloux. 2002. Identification of XcpP domains that confer functionality and specificity to the Pseudomonas aeruginosa type II secretion apparatus. Mol. Microbiol. 44:1651-1665. [DOI] [PubMed] [Google Scholar]
- 16.Giron, J. A., O. G. Gomez-Duarte, K. G. Jarvis, and J. B. Kaper. 1997. Longus pilus of enterotoxigenic Escherichia coli and its relatedness to other type-4 pili—a minireview. Gene 192:39-43. [DOI] [PubMed] [Google Scholar]
- 17.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobbs, M., and J. S. Mattick. 1993. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol. 10:233-243. [DOI] [PubMed] [Google Scholar]
- 19.Hoyne, P. A., R. Haas, T. F. Meyer, J. K. Davies, and T. C. Elleman. 1992. Production of Neisseria gonorrhoeae pili (fimbriae) in Pseudomonas aeruginosa. J. Bacteriol. 174:7321-7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, N. T., W. M. Leu, M. S. Lee, A. Chen, S. C. Cheng, Y. L. Song, and L. Y. Chen. 2002. XpsG, the major pseudopilin in Xanthomonas campestris pv. campestris, forms pilus-like structure between cytoplasmic and outer membranes. Biochem. J. 365:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kachlany, S. C., P. J. Planet, R. DeSalle, D. H. Fine, and D. H. Figurski. 2001. Genes for tight adherence of Actinobacillus actinomycetemcomitans: from plaque to plague to pond scum. Trends Microbiol. 9:429-437. [DOI] [PubMed] [Google Scholar]
- 22.Keizer, D. W., C. M. Slupsky, M. Kalisiak, A. P. Campbell, M. P. Crump, P. A. Sastry, B. Hazes, R. T. Irvin, and B. D. Sykes. 2001. Structure of a pilin monomer from Pseudomonas aeruginosa: implications for the assembly of pili. J. Biol. Chem. 276:24186-24193. [DOI] [PubMed] [Google Scholar]
- 23.Nunn, D., S. Bergman, and S. Lory. 1990. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J. Bacteriol. 172:2911-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunn, D. N., and S. Lory. 1991. Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc. Natl. Acad. Sci. USA 88:3281-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunn, D. N., and S. Lory. 1993. Cleavage, methylation, and localization of the Pseudomonas aeruginosa export proteins XcpT, -U, -V, and -W. J. Bacteriol. 175:4375-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 27.Parge, H. E., K. T. Forest, M. J. Hickey, D. A. Christensen, E. D. Getzoff, and J. A. Tainer. 1995. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature 378:32-38. [DOI] [PubMed] [Google Scholar]
- 28.Planet, P. J., S. C. Kachlany, R. DeSalle, and D. H. Figurski. 2001. Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc. Natl. Acad. Sci. USA 98:2503-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Possot, O. M., G. Vignon, N. Bomchil, F. Ebel, and A. P. Pugsley. 2000. Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. J. Bacteriol. 182:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pugsley, A. P. 1996. Multimers of the precursor of a type IV pilin-like component of the general secretory pathway are unrelated to pili. Mol. Microbiol. 20:1235-1245. [DOI] [PubMed] [Google Scholar]
- 32.Rahme, L. G., F. M. Ausubel, H. Cao, E. Drenkard, B. C. Goumnerov, G. W. Lau, S. Mahajan-Miklos, J. Plotnikova, M. W. Tan, J. Tsongalis, C. L. Walendziewicz, and R. G. Tompkins. 2000. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA 97:8815-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauvonnet, N., P. Gounon, and A. P. Pugsley. 2000. PpdD type IV pilin of Escherichia coli K-12 can be assembled into pili in Pseudomonas aeruginosa. J. Bacteriol. 182:848-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauvonnet, N., G. Vignon, A. P. Pugsley, and P. Gounon. 2000. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 19:2221-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson, D. A., R. Ramphal, and S. Lory. 1992. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect. Immun. 60:3771-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, A. W., and B. H. Iglewski. 1989. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 17:10509.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 38.Tommassen, J., A. Filloux, M. Bally, M. Murgier, and A. Lazdunski. 1992. Protein secretion in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 9:73-90. [DOI] [PubMed] [Google Scholar]
- 39.Vallet, I., J. W. Olson, S. Lory, A. Lazdunski, and A. Filloux. 2001. The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA 98:6911-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voulhoux, R., G. Ball, B. Ize, M. L. Vasil, A. Lazdunski, L. F. Wu, and A. Filloux. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20:6735-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wall, D., and D. Kaiser. 1999. Type IV pili and cell motility. Mol. Microbiol. 32:1-10. [DOI] [PubMed] [Google Scholar]
- 42.Wolfgang, M., J. P. van Putten, S. F. Hayes, D. Dorward, and M. Koomey. 2000. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 19:6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]