Abstract
Several neurodegenerative diseases are associated with the toxicity of misfolded proteins. This toxicity must arise from a combination of the amino acid sequences of the misfolded proteins and their interactions with other factors in their environment. A particularly compelling example of how profoundly these intramolecular and intermolecular factors can modulate the toxicity of a misfolded protein is provided by the polyglutamine (polyQ) diseases. All of these disorders are caused by glutamine expansions in proteins that are broadly expressed, yet the nature of the proteins that harbor the glutamine expansions and the particular pathologies they produce are very different. We find, using a yeast model, that amino acid sequences that modulate polyQ toxicity in cis can also do so in trans. Furthermore, the prion conformation of the yeast protein Rnq1 and the level of expression of a suite of other glutamine-rich proteins profoundly affect polyQ toxicity. They can convert polyQ expansion proteins from toxic to benign and vice versa. Our work presents a paradigm for how a complex, dynamic interplay between intramolecular features of polyQ proteins and intermolecular factors in the cellular environment might determine the unique pathobiologies of polyQ expansion proteins.
Keywords: huntingtin, Huntington's disease, protein misfolding, yeast
A variety of human diseases are characterized by the accumulation of aggregated, misfolded proteins (1, 2). In some cases, such as cystic fibrosis, disease is caused by the loss of a vital protein function due simply to this misfolding and aggregation. In other cases, however, protein misfolding creates novel, toxic properties. For example, in Alzheimer's disease, Parkinson's disease, and the polyglutamine (polyQ) disorders, misfolded proteins disrupt proper cellular function and cause cytotoxicity (3, 4). In all of these diseases the amino acid sequences of the particular misfolded protein and their interactions determined by its specific environment must govern toxicity. Yet, despite intense research, we know little about these intramolecular and intermolecular factors. Without identifying and understanding these factors at a molecular level it will be difficult to devise the most effective therapeutic strategies to treat protein misfolding diseases.
The polyQ diseases present a promising starting point to study this very general problem. In all of these diseases polyQ expansions constitute the molecular basis of disease (5). However, the disease pathologies (including the cell types most strongly affected) and the individual proteins that bear the disease-causing polyQ expansions are very distinct (5). Consequently, the amino acids flanking the polyQ region and the sets of cellular factors that specifically interact with each polyQ expansion protein are different. Combinations of these factors must account for their different pathobiologies. Recent studies have started to identify protein–protein and genetic interaction networks for polyQ proteins (6–8), but the current data do not yet provide a framework for understanding the distinct molecular pathologies caused by different polyQ expansion proteins.
We and others (9–12) have developed yeast models to study polyQ toxicity. The yeast models expressing a fragment of the human polyQ protein huntingtin (htt), htt exon I, recapitulate major features of proteins with polyQ expansions including their graded, polyQ length-dependent aggregation and toxicity (13). The yeast system offers the unique opportunity to dissect modulators of polyQ toxicity in a defined and uniform cellular environment. In addition, the numerous genetic tools available in yeast make it a powerful instrument to explore the intramolecular and intermolecular factors that govern polyQ toxicity. In our companion article (13) we investigated the effects of flanking amino acid sequences on polyQ toxicity. Here we explore the impact of protein–protein interactions on polyQ toxicity.
In our companion article, which provides background information crucial for the understanding of this article, we explored the effects of short amino acid sequences that have strong consequences on the toxicity of a polyQ-expanded htt exon I protein when present on the same polypeptide chain (13). A FLAG epitope can convert an otherwise benign polyQ protein into a toxic one. In contrast, proline-rich sequence can convert a toxic polyQ-expanded htt exon I protein into a benign one. Here we report an unexpected observation: the same amino acid sequences that modulate polyQ toxicity in cis can also do so in trans, i.e., when present in a separate polypeptide chain that contains a polyQ tract in the normal range.
We further identify a suite of glutamine-rich (Q-rich) proteins that interact with polyQ-expanded htt exon I and modulate its toxicity. We began with a protein that was previously established to affect toxicity of polyQ-expanded htt exon I in a conformation-specific manner (12). Rnq1, a protein rich in asparagines (N) and glutamines (Q), belongs to a small class of proteins known as the yeast prions, because it is capable of adopting an alternate, self-perpetuating conformational state. The toxicity of a FLAG-tagged polyQ-expanded htt exon I protein carrying a deletion of the proline-rich region depends on Rnq1 being in its prion conformation (12). We find that every combination of intramolecular and intermolecular factors that govern polyQ toxicity depended on the Rnq1 protein being in its prion conformation.
We also describe a suite of aggregation-prone Q-rich proteins that can modulate polyQ toxicity. We show that deletions of genes encoding Q-rich proteins can eliminate or reduce the toxicity of an otherwise toxic polyQ htt exon I protein. Furthermore, the overexpression of other Q-rich proteins can convert nontoxic polyQ htt exon I proteins into toxic species. All these Q-rich proteins induce polyQ toxicity probably through direct interactions with the polyQ-expanded proteins. The polyQ toxicity induced by overexpression of Q-rich proteins strongly depended on the amino acids flanking the polyQ region of htt exon I: fragments that contain the proline-rich region produced no toxicity even in the presence of overexpressed Q-rich proteins. Importantly, in all these different scenarios polyQ toxicity can be observed only when Rnq1 is in its prion conformation. Our work presented here establishes that, within identical cellular environments, an interplay of intramolecular and intermolecular factors can determine whether the same polyQ expansion protein is benign or lethal.
Results
25Q Is Recruited into 103Q Aggregates Regardless of the Sequences Flanking polyQ.
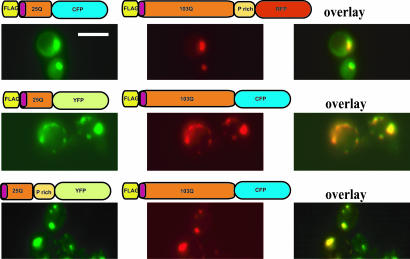
In our companion article, we have established that the amino acid sequences flanking the polyQ region in htt exon I have a profound effect on its toxicity (13). The ability of 103Q htt exon I protein to recruit other polyQ proteins (including the wild-type 25Q htt protein) into aggregates is postulated to play an important role in toxicity (14, 15). Therefore, we asked whether the FLAG epitope or the proline-rich region might alter the capacity of expanded polyQ proteins to recruit those with shorter polyQ regions into aggregates. To this end, we coexpressed various 103Q htt exon I proteins fused to cyan fluorescent protein (CFP) [or red fluorescent protein (RFP)] with various 25Q htt proteins fused to yellow fluorescent protein (YFP) (or CFP) and monitored their colocalization by fluorescence microscopy. Every pair of coexpressed 25Q and 103Q htt proteins tested colocalized in aggregates, regardless of their amino- or carboxyl-terminal modifications and their toxicity (Fig. 1 and data not shown). In contrast, coexpression of two 25Q htt constructs in various combinations never resulted in coaggregation (data not shown).
Fig. 1.
Recruitment of 25Q htt exon I proteins into inclusions formed by 103Q htt exon I is independent of the amino acid sequences flanking the polyQ stretch. Shown are three examples of fluorescent microscopic images of yeast cells coexpressing the indicated htt exon I proteins with 25Q or 103Q. Areas of colocalization are shown in yellow in merged images (overlay). (Scale bar: 5 μm.)
We took advantage of this general ability of 103Q proteins to recruit 25Q proteins into aggregates, to ask whether sequences that can mask or unmask the polyQ toxicity in cis (13) can also function in trans. Coexpression of two different 25Q proteins in any combination never caused toxicity (Fig. 2A and data not shown). Furthermore, 25Q proteins recruited into aggregates by 103Q htt proteins did not alter (i) the levels of the 103Q htt expressed, (ii) the morphology of the aggregates, or (iii) their resistance to solubilization by SDS (Fig. 2B and data not shown). They did, however, have dramatic effects on the toxicity of the 103Q proteins.
Fig. 2.
The proline-rich region and the FLAG tag can alter polyQ toxicity in trans. (A) Cells coexpressing the indicated htt exon I proteins or a vector control were spotted on plates that either induce (+Induction) or repress (−Induction) protein expression in three 5-fold dilutions. Toxicity is reflected by reduced growth of cells under inducing conditions. (B) A dot blot was performed with protein lysates from cells expressing the indicated combinations of different htt exon I proteins. The blot was probed with an anti-FLAG antibody. (C) Cells containing FLAGhtt103QΔProCFP and either a vector control or the indicated 25Q htt exon I proteins were spotted on plates that either induce (+Induction) or repress (−Induction) protein expression in three 5-fold dilutions. (D) Cells containing FLAGhtt25QΔProCFP and either a vector control or the indicated 103Q htt exon I proteins were spotted on plates that either induce (+Induction) or repress (−Induction) protein expression in three 5-fold dilutions.
The Proline-Rich Region in htt Exon I Can Antagonize polyQ Toxicity in Trans.
The proline-rich region that is normally located carboxyl-terminally to the polyQ region in the human htt protein has a strong protective effect when present on the same polypeptide chain as the polyQ expansion (in cis) (13). We asked whether the proline-rich region could antagonize the lethal effects of a toxic polyQ expansion in trans. When htt exon I proteins containing both 25Q and the proline-rich region were coexpressed with a usually toxic 103Q htt exon I protein, toxicity was greatly reduced (data not shown). Furthermore, toxicity was completely eliminated when this 25Q variant was constitutively expressed from a strong promoter from a multicopy plasmid, i.e., when it was present before induction of the toxic 103Q protein (Fig. 2C). Untagged, FLAG-tagged, and hemagglutin-epitope-tagged htt 25Q proteins containing the proline-rich region were equally effective at suppressing toxicity in trans (data not shown). The 25Q htt proteins were, however, unable to antagonize toxicity when they lacked the proline-rich region (Fig. 2C).
The Amino-Terminal FLAG Tag Can Induce polyQ Toxicity in Trans.
A FLAG-epitope tag confers toxicity to a 103Q htt exon I fragment when it is present on the same polypeptide chain as the polyQ expansion (in cis) (13). We asked whether the FLAG epitope could function in trans to transform a nontoxic 103Q htt protein into a toxic one. To this end, we expressed a nontoxic FLAG-tagged 25Q htt protein without the proline-rich region, with either of two different 103Q htt proteins. In one case the 103Q htt protein contained the proline-rich region, and in the other case it did not. The 25Q htt protein did not cause toxicity when coexpressed with a 103Q protein that lacked the proline-rich region (Fig. 2D). Surprisingly, it did produce toxicity when the 103Q protein contained the proline-rich region (Fig. 2D). That is, although the proline-rich region generally has the capacity to prevent toxicity both in cis and in trans, in this particular case it promoted toxicity.
In summary, the endogenous proline-rich region of htt and the exogenous FLAG tag can modulate polyQ toxicity both in cis and in trans. The FLAG tag has the ability to unmask toxicity, and the proline-rich region has a potent capacity to antagonize toxicity. Under certain circumstances, however, the proline-rich region could also promote toxic conformations in 103Q htt proteins.
The [RNQ+] Prion Is a Prerequisite for polyQ Toxicity.
Another factor that has a dramatic effect on the toxicity of 103Q htt exon I in yeast is the conformational status of a prion protein known as Rnq1 [rich in asparagines (N) and glutamines (Q)]. Rnq1 can exist in two different states, the prion state or the nonprion state. Only when Rnq1 is in the prion state does 103Q htt coaggregate with it and become toxic (12).
To test whether the amino acids flanking the polyQ region in htt exon I altered the ability of the Rnq1 prion to promote toxicity, we tested three different fusion proteins (one toxic and two nontoxic). Each was expressed in cells that (i) contained Rnq1 in its prion form, (ii) contained Rnq1 in its nonprion form, or (iii) did not express Rnq1, Δrnq1. [In separate experiments we found that the deletion of RNQ1 and its conformational states had no detectable effect on the expression of other proteins and on cell growth (T. Outeiro and S.L., unpublished observations).] The deletion of RNQ1 and its conformational state had no effect on the levels of accumulated 103Q htt protein (data not shown). They did, however, have strong effects on the aggregation and toxicity of the different 103Q htt proteins.
Each different 103Q htt protein aggregated efficiently in cells expressing Rnq1 in its prion conformation (Fig. 3A). Depending on the construct, some aggregates were also observed in cells that contained Rnq1 in its nonprion conformation and Δrnq1 cells, but in each case aggregation was much less than in cells that contained Rnq1 in its prion conformation (Fig. 3A). Moreover, in cells with Rnq1 in its prion conformation all three 103Q htt proteins colocalized with Rnq1 (Fig. 3B). However, the recruitment of the nontoxic 103Q htt proteins by Rnq1 did not convert them to a toxic state. Thus, the Rnq1 prion induces aggregation of each of the different 103Q htt proteins. It only induced toxicity, however, when the 103Q htt exon I protein contained the amino-terminal FLAG epitope and lacked the proline-rich region.
Fig. 3.
The RNQ1 prion status determines the aggregation of 103Q htt exon I proteins independent of the amino acids flanking the polyQ region. (A) Yeast cells that harbor Rnq1 in their prion state, in their nonprion state, or with a deletion of RNQ1 (Δrnq) were induced for the expression of the indicated htt exon I proteins and analyzed by fluorescence microscopy. (Scale bar: 15 μm.) (B) Yeast cells expressing Rnq-YFP (green) and the indicated htt exon I protein fused to CFP (red) were analyzed by fluorescence microscopy. Areas of colocalization are shown in yellow in overlaid images. (Scale bar: 10 μm.)
A Suite of Aggregation-Prone Proteins Alters polyQ Toxicity.
Next we examined the impact of other Q-rich proteins on polyQ-dependent aggregation and toxicity. We started with five proteins that were identified in a previous genetic screen (6). Deletions of any one of the genes encoding Rnq1, Def1, Ybr016w, Yir003w, and Ylr278c reduced the toxicity of a 103Q htt protein with the FLAG tag without the proline-rich region (6).
To determine whether these Q-rich proteins affected 103Q htt aggregation we expressed different 103Q htt proteins in each of the deletion mutants. The deletion of YIR003w or YLR278c reduced the aggregation of all htt103Q proteins (data not shown). In the DEF1 deletion strain, however, all three 103Q htt proteins continued to aggregate (data not shown). Among these proteins only Def1 is dispensable for aggregation but is required for toxicity of the 103Q protein with the FLAG tag and without the proline-rich region.
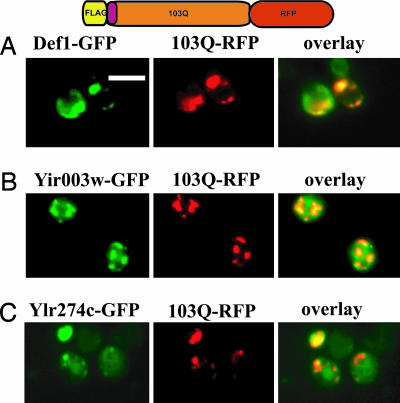
Furthermore, we asked whether these Q-rich proteins colocalized with 103Q proteins. For these experiments we used two different 103Q htt proteins: one toxic and one nontoxic fused to DsRed (RFP). These 103Q proteins were separately expressed in yeast strains that expressed carboxyl-terminal GFP fusions of Def1, Yir003w, or Ylr278c (16). In every case the Q-rich proteins colocalized with the 103Q proteins (Fig. 4C and data not shown).
Fig. 4.
Q-rich proteins that modulate polyQ toxicity colocalize with 103Q htt proteins. Cells expressing the GFP fusion proteins Def1 (A), Yir003w (B), and Ylr274c (C) (all green) together with the indicated 103Q htt exon I protein fused to DsRed (RFP; red) were analyzed by fluorescence microscopy. Areas of colocalization are shown in yellow in overlaid images. (Scale bar: 5 μm.)
Second, we examined the effects of five other Q-rich proteins on polyQ toxicity: Pan1, Ent2, Puf2, Cyc8, and Snf5. Each of the five proteins was fused to GFP and expressed in wild-type yeast cells. When moderately overexpressed alone, each Q-rich protein formed aggregates to some extent, but none was toxic (Fig. 5 A and B). However, each of these Q-rich proteins unmasked the toxicity of certain otherwise nontoxic 103Q htt proteins. Specifically, they caused 103Q proteins that carried either no amino-terminal tag at all or a hemagglutin tag to become toxic (Fig. 5C). But they did not convert 103Q proteins that contained the proline-rich region into toxic species (Fig. 5C), underscoring the protective function of the proline-rich region.
Fig. 5.
Overexpression of endogenous Q-rich proteins can induce polyQ toxicity. (A) Wild-type (W303) cells containing plasmids for the overexpression of PAN1, ENT2, CYC8, PUF2, or SNF5 fused to YFP under the control of a Cu2SO4-inducible promoter (CUP1) were spotted on plates that either moderately induce (100 μM additional Cu2SO4, +Induction) or weakly induce (no additional Cu2SO4, −Induction) expression. Shown are four 5-fold dilutions of cells per sample. (B) Fluorescence microscopy of cells expressing Pan1, Ent2, Cyc8, Puf2, or Snf5 fused to YFP under moderately inducing conditions (100 μM Cu2SO4). (C) Cells expressing the indicated htt exon I proteins together with a plasmid control or each of Pan1, Ent2, Cyc8, Puf2, or Snf5 fused to YFP were spotted on plates that either moderately induce (100 μM additional Cu2SO4, +Induction) or only weakly induce (no additional Cu2SO4, −Induction) expression. Four 5-fold dilutions for each sample were spotted. (D) Fluorescence microcopy of cells coexpressing Snf5 fused to YFP or Cyc8 fused to YFP (both red) together with the indicated htt exon I protein. Areas of colocalization are shown in yellow in overlaid images. (Scale bar: 5 μm.)
The observed toxicity cannot simply be due to sequestration of the Q-rich proteins into aggregates because most are not essential in yeast (17). Moreover, each of the Q-rich proteins colocalized with each of the 103Q htt proteins in all combinations (Fig. 5D and data not shown) regardless of their toxicity.
We then asked whether the ability of the Q-rich proteins to unmask the toxicity of certain 103Q htt proteins depended on Rnq1 being in its prion conformation. To this end, we overexpressed the Q-rich proteins together with our complete set of 103Q htt proteins in yeast cells that harbor Rnq1 in nonprion conformation or in yeast cells with a deletion of the RNQ1 gene (Δrnq). In neither case did the overexpression of the Q-rich proteins produce toxicity (data not shown). Thus, the prion -conformation of Rnq1 is an absolute prerequisite for polyQ toxicity for all of the intermolecular and intramolecular factors tested in this study.
Discussion
PolyQ expansions are the common cause for several distinct neurodegenerative diseases. Each of these proteins causes disease when the polyQ region exceeds a threshold of ≈39 glutamines. These proteins are broadly expressed, yet different polyQ expansion proteins cause toxicity in different neurons, resulting in distinct pathobiologies. Despite intense research, the factors that determine the characteristic toxicities for each polyQ expansion protein in the different diseases are largely unknown. We hypothesized that the different amino acid sequences flanking the polyQ expansion proteins in combination with particular cellular environments determine polyQ toxicity. We tested this hypothesis in the yeast Saccharomyces cerevisiae because this organism uniquely allows the controlled manipulation of the sequences flanking a polyQ expansion and its proteomic environment, including the ability to control the conformational state of a particular htt-interacting protein, Rnq1.
In our companion article (13) we systematically characterized the effect of amino acid sequences flanking the expanded polyQ tract in htt exon I. We described flanking sequences that, when present on the same polypeptide chain as the polyQ tract, can convert a toxic protein to a nontoxic state and a nontoxic protein to a toxic state. In this study we demonstrate that the same flanking sequences can exert their toxic or protective functions in trans, i.e., when present on different polypeptide chains. We further show that the expression levels and the conformational state of other Q-rich proteins profoundly modulate polyQ toxicity. Like the flanking sequences, the Q-rich interacting proteins have the capacity to transform a nontoxic polyQ-expanded htt exon I protein to a toxic one and a toxic one to a nontoxic one.
How might short amino acid segments such as the FLAG-tag and the proline-rich region exert such profound effects on polyQ toxicity both in cis and in trans? Certainly, in cis they could simply have propagated some structural influence to the polyQ tract to which they were immediately adjacent (18, 19). But the fact that both the FLAG tag and the proline-rich region can function in trans suggests that toxicity might involve the recruitment of other proteins to misfolded htt fragments. Over the last decade the number of protein–protein interactions that have been shown to be governed by small segments of amino acids has increased greatly. To date, only a few candidate proteins that interact with negatively charged protein domains (such as the FLAG tag) are known [see our companion article (13)]. However, hundreds of proteins are now known to specifically interact with proline-rich regions. These include proteins with Src homology region 3 (SH3) domains, WW domains, and several other newly identified domains that bind proteins that contain short sequences rich in prolines (20). Counting SH3 domains alone, there are 25 in yeast and 332 in the human genome (21). Other candidates include molecular chaperones and protein remodeling factors such as prolyl isomerases. The number of possible interactions is daunting, but they are approachable through systematic experimentation in yeast, and such studies might be used to inform further analyses in neuronal cells.
It is also notable that the proline-rich region antagonized toxicity in trans only when it was present on an htt variant with a polyQ tract of the normal length (25Q). Because wild-type htt (25Q) can be sequestered into aggregates made of htt with a polyQ expansion (14, 15) our results suggest a direct mechanism by which wild-type alleles might ameliorate the toxicity of polyQ expansions: the polyQ expansion protein may recruit the rescuing activity of the proline-rich region to polyQ expansion proteins. Interventions aimed at altering the relative expression levels of the wild-type htt protein to polyQ-expanded htt might therefore be an attractive therapeutic strategy (14, 15, 22, 23).
Several studies have convincingly demonstrated that the interaction of polyQ-expanded htt proteins with other Q-rich proteins correlates with toxicity in mammalian cells (14, 24, 25). Because all of the previously studied Q-rich proteins are essential, their sequestration into polyQ aggregates, and the ensuing loss of their function could well have been the sole mechanism accounting for toxicity (26–30). Our experiments indicate that Q-rich proteins can make further contributions to toxicity. Individual deletions of five yeast genes encoding unrelated Q-rich proteins (Ybr016w, Ylr278c, Yir003w, Def1, and Rnq1) abolished the toxicity of a lethal polyQ-expanded htt variant (6). Not only are these proteins not essential, but deletions of the genes encoding them (except for Def1) have no detectable effect on growth under the many different conditions tested (17). Consequently, the toxicity of the interaction between these Q-rich proteins and polyQ-expanded htt fragments is not due to their own sequestration. It might be that they cause the sequestration of other essential proteins. Alternatively, the polyQ-expanded htt exon I might convert each of them to a novel toxic conformation. We note, however, that at least the Rnq1 protein is not itself toxic in either its soluble or its prion form. It seems more likely, therefore, that these Q-rich proteins directly interact with the polyQ-expanded htt to unmask its toxicity.
The moderate overexpression of five other Q-rich proteins, Pan1, Ent2, Cyc8, Puf2, and Snf5 (31), converted otherwise nontoxic htt variants into toxic species. These proteins always colocalized with polyQ-expanded htt exon I but produced a toxic interaction only when the htt protein lacked the proline-rich region. This observation underscores the importance of the proline-rich region in suppressing polyQ toxicity. Except for their high glutamine content, Pan1, Ent2, Cyc8, Puf2, and Snf5 are unrelated and are not toxic when overexpressed on their own. Notably, Pan1 is the only one of these proteins that is essential. Thus, htt is unlikely to create toxicity by sequestering these Q-rich proteins. Rather, Q-rich proteins seem to convert the benign htt exon I variants into toxic ones by physical interaction.
For every intermolecular and intramolecular factor we tested, polyQ-expanded htt exon I proteins formed aggregates and were toxic only when the protein Rnq1 was in its prion conformation. That is, although only certain types of aggregates formed by polyQ-expanded htt exon I proteins are toxic [see our companion article (13)], solubilization of the polyQ-expanded proteins always rescued toxicity in our model.
Our results therefore establish the prion conformation of Rnq1 as a general and essential prerequisite for polyQ aggregation and toxicity in yeast. Although the Rnq1 protein is not conserved from yeast to human, there are hundreds of Q-rich prion-like proteins in the human genome (O. King and S.L., unpublished observations). Prions are found in evolutionarily distant fungi and have been postulated to play broader roles in metazoan organisms, and at least one protein has been speculated to adopt a prion-like conformational change in neurons (32). Thus, we speculate that a similar phenomenon could occur in the polyQ diseases. Certain Q-rich proteins might exist in alternate conformations in different cell types, and in older versus younger neurons, but only a specific conformational state of these proteins might induce polyQ toxicity. Such phenomena might contribute to the cell type-specific and age-dependent polyQ toxicity in the different polyQ diseases and to the idiosyncratic nature of this toxicity, in which the same cell types exhibit different toxicities and stochastic variations in toxicities (33–35).
In summary, our findings begin to provide a framework for viewing the baffling variations in polyQ diseases and the poor correlation between the expression patterns of different polyQ expansion proteins and their distinct pathologies. PolyQ toxicity is likely determined by a combination of (i) toxic and protective effects of amino acid sequences flanking the polyQ region, which can function in cis and trans, (ii) a web of cell type-specific protein–protein interactions, and (iii) alternate conformational states of certain proteins in their cellular environment. Our work reveals unexpected layers of complexity in the forces that govern the toxicity of misfolded proteins. Although daunting, the factors we have uncovered may help guide future investigations in model organisms and human neurons.
Materials and Methods
Materials.
Chemicals were purchased from Sigma–Aldrich and Fluka. All yeast strains used in this study are in the W303 (MATα can1-100 ade2-1 his3-11, 15 trp1-1 ura3-1 leu23,112) genetic background or in BY4741 Mata haploid deletion strains (Research Genetics, Huntsville, AL). Yeast shuttle vectors for genomic integration and ectopic expression were used throughout this work as described (20). Plasmids were generated by using standard molecular biology techniques. The expression of htt fusion proteins was controlled by the inducible GAL1 or constitutive GPD1 promoters. The different htt exon I constructs are described in ref. 13. The plasmid expressing the RNQ fusion protein was described previously (21). Pan1, Ent2, Cyc8, Puf2, and Snf5 were expressed from single-copy plasmids under the control of the CUP1 promoter. For moderate induction 100 mM Cu2SO4 was added to the medium, and for weak induction no additional Cu2SO4 was added.
Yeast Methods.
Yeast media were prepared essentially as described (35) by using complete supplemental mixtures from Qbiogene. Transformation of yeast was performed by the lithium acetate method (36). Yeast integrating plasmids (pRS303 backbone) were linearized by restriction with BstXI before transformation. Induction of GAL1 promoter-controlled protein expression for fluorescence microcopy or dot blots was achieved by growing yeast cultures in selective media containing raffinose (2%) as the sole carbon source to mid-log phase followed by growth in galactose (2%) containing selective media for 8 h.
Growth Assays.
Yeast transformants were grown overnight in selective media with glucose (2%) as a sole carbon source. After the OD600 was determined cultures were diluted to equal concentrations. Cells were spotted in four 5-fold dilutions with the most concentrated spot containing ≈100,000 cells using a spotter (Frogger, V&P Scientific). Equal spotting was controlled by spotting the cells on yeast extract/peptone/dextrose (data not shown) and selective complete plates with glucose as a carbon source in parallel with galactose plates.
Dot Blots.
Dot blots of yeast protein extracts were performed exactly as were the filter retardation assays (10) except with poly(vinylidene difluoride) membranes instead of cellulose acetate membranes. Cells were harvested after 8 h of induction.
Fluorescence Microscopy.
Fluorescence microscopy was carried out by using a Zeiss Axioplan II microscope and openlab (ImproVision) software. Cells were imaged by using a ×100 objective after 8 h of induction. For colocalization studies, cyan, Texas red, and yellow filters were used to visualize CFP, DsRed, and YFP, respectively, and metamorph (Universal Imaging) software was used to merge the two fluorescent pictures.
Acknowledgments
We thank Michael Sherman (Boston University, Boston, MA) for the RFP fusion constructs and Karen Allendoerfer and members of the S.L. laboratory for critical discussion of the manuscript. M.L.D. was supported by a postdoctoral fellowship from the Huntington's Disease Society of America. P.J.M. was supported by National Institutes of Health Grant NS047237.
Abbreviations
- polyQ
polyglutamine
- htt
huntingtin
- Q-rich
glutamine-rich
- YFP
yellow fluorescent protein
- CFP
cyan fluorescent protein
- RFP
red fluorescent protein (DsRed).
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Soto C. FEBS Lett. 2001;498:204–207. doi: 10.1016/s0014-5793(01)02486-3. [DOI] [PubMed] [Google Scholar]
- 2.Dobson C. M. Semin. Cell Dev. Biol. 2004;15:3–16. doi: 10.1016/j.semcdb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Koo E. H., Lansbury P. T., Jr., Kelly J. W. Proc. Natl. Acad. Sci. USA. 1999;96:9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor J. P., Hardy J., Fischbeck K. H. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 5.Zoghbi H. Y., Orr H. T. Annu Rev. Neurosci. 2000;23:217–247. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]
- 6.Giorgini F., Guidetti P., Nguyen Q., Bennett S. C., Muchowski P. J. Nat. Genet. 2005;37:526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goehler H., Lalowski M., Stelzl U., Waelter S., Stroedicke M., Worm U., Droege A., Lindenberg K. S., Knoblich M., Haenig C., et al. Mol. Cell. 2004;15:853–865. doi: 10.1016/j.molcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Willingham S., Outeiro T. F., DeVit M. J., Lindquist S. L., Muchowski P. J. Science. 2003;302:1769–1772. doi: 10.1126/science.1090389. [DOI] [PubMed] [Google Scholar]
- 9.Krobitsch S., Lindquist S. Proc. Natl. Acad. Sci. USA. 2000;97:1589–1594. doi: 10.1073/pnas.97.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muchowski P. J., Schaffar G., Sittler A., Wanker E. E., Hayer-Hartl M. K., Hartl F. U. Proc. Natl. Acad. Sci. USA. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes R. E., Lo R. S., Davis C., Strand A. D., Neal C. L., Olson J. M., Fields S. Proc. Natl. Acad. Sci. USA. 2001;98:13201–13206. doi: 10.1073/pnas.191498198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meriin A. B., Zhang X., He X., Newnam G. P., Chernoff Y. O., Sherman M. Y. J. Cell Biol. 2002;157:997–1004. doi: 10.1083/jcb.200112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duennwald M. L., Jagadish S., Muchowski P. J., Lindquist S. Proc. Natl. Acad. Sci. USA. 2006;103:11045–11050. doi: 10.1073/pnas.0604547103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preisinger E., Jordan B. M., Kazantsev A., Housman D. Philos. Trans. R. Soc. London B. 1999;354:1029–1034. doi: 10.1098/rstb.1999.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazantsev A., Preisinger E., Dranovsky A., Goldgaber D., Housman D. Proc. Natl. Acad. Sci. USA. 1999;96:11404–11409. doi: 10.1073/pnas.96.20.11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O’Shea E. K. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 17.Giaever G., Chu A. M., Ni L., Connelly C., Riles L., Veronneau S., Dow S., Lucau-Danila A., Anderson K., Andre B., et al. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya A., Thakur A. K., Chellgren V. M., Thiagarajan G., Williams A. D., Chellgren B. W., Creamer T. P., Wetzel R. J. Mol. Biol. 2006;355:524–535. doi: 10.1016/j.jmb.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Ignatova Z., Gierasch L. M. J. Biol. Chem. 2006;281:12959–12967. doi: 10.1074/jbc.M511523200. [DOI] [PubMed] [Google Scholar]
- 20.Kay B. K., Williamson M. P., Sudol M. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- 21.Zarrinpar A., Bhattacharyya R. P., Lim W. A. Sci. STKE, 2003:RE8. doi: 10.1126/stke.2003.179.re8. [DOI] [PubMed] [Google Scholar]
- 22.Xia H., Mao Q., Paulson H. L., Davidson B. L. Nat. Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 23.Xia H., Mao Q., Eliason S. L., Harper S. Q., Martins I. H., Orr H. T., Paulson H. L., Yang L., Kotin R. M., Davidson B. L. Nat. Med. 2004;10:816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 24.Chai Y., Shao J., Miller V. M., Williams A., Paulson H. L. Proc. Natl. Acad. Sci. USA. 2002;99:9310–9315. doi: 10.1073/pnas.152101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson K. M., Li W., Ching K. A., Batalov S., Tsai C. C., Joazeiro C. A. Proc. Natl. Acad. Sci. USA. 2003;100:8892–8897. doi: 10.1073/pnas.1530212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunah A. W., Jeong H., Griffin A., Kim Y. M., Standaert D. G., Hersch S. M., Mouradian M. M., Young A. B., Tanese N., Krainc D. Science. 2002;296:2238–2243. doi: 10.1126/science.1072613. [DOI] [PubMed] [Google Scholar]
- 27.McCampbell A., Taylor J. P., Taye A. A., Robitschek J., Li M., Walcott J., Merry D., Chai Y., Paulson H., Sobue G., Fischbeck K. H. Hum. Mol. Genet. 2000;9:2197–2202. doi: 10.1093/hmg/9.14.2197. [DOI] [PubMed] [Google Scholar]
- 28.Nucifora F. C., Jr., Sasaki M., Peters M. F., Huang H., Cooper J. K., Yamada M., Takahashi H., Tsuji S., Troncoso J., Dawson V. L., et al. Science. 2001;291:2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 29.Schaffar G., Breuer P., Boteva R., Behrends C., Tzvetkov N., Strippel N., Sakahira H., Siegers K., Hayer-Hartl M., Hartl F. U. Mol. Cell. 2004;15:95–105. doi: 10.1016/j.molcel.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Zuccato C., Ciammola A., Rigamonti D., Leavitt B. R., Goffredo D., Conti L., MacDonald M. E., Friedlander R. M., Silani V., Hayden M. R., et al. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 31.Michelitsch M. D., Weissman J. S. Proc. Natl. Acad. Sci. USA. 2000;97:11910–11915. doi: 10.1073/pnas.97.22.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shorter J., Lindquist S. Nat. Rev. Genet. 2005;6:435–450. doi: 10.1038/nrg1616. [DOI] [PubMed] [Google Scholar]
- 33.Ross C. A., Poirier M. A. Nat. Med. 2004;10(Suppl.):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 34.Yamada M., Tsuji S., Takahashi H. Neuropathology. 2002;22:317–322. doi: 10.1046/j.1440-1789.2002.00457.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaiser C., Michaelis S., Mitchell A. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 36.Guthrie C., Fink G., editors. Methods in Enzymology: Guide to Yeast Genetics and Molecular Biology. Vol. 169. San Diego: Academic; 1991. [PubMed] [Google Scholar]