Abstract
The activity of cullin-containing ubiquitin protein ligase complexes is stimulated by linkage to cullin of the ubiquitin-like protein Nedd8 (“neddylation”). Neddylation is inhibited by the tight binding of cullins to CAND1 (cullin-associated and neddylation-dissociated 1) protein, and Nedd8 is removed from cullins by specific isopeptidase activity of the COP9/signalosome (CSN) complex. The mechanisms that regulate neddylation and deneddylation of cullins were unknown. We examined this problem for the case of SCFSkp2, a cullin1 (Cul1)-containing ubiquitin ligase complex that contains the S phase-associated protein Skp2 as the substrate-binding F-box protein subunit. SCFSkp2 targets for degradation the cyclin-dependent kinase (cdk) inhibitor p27 in the G1-to-S phase transition, a process that requires its phosphorylation and binding to cdk2-cyclin E. Because levels of Skp2, cyclin E, and the accessory protein Cks1 (cyclin kinase subunit 1) all rise at the end of G1 phase, it seemed possible that the neddylation of Cul1 in SCFSkp2 is regulated by the availability of the F-box protein and/or the substrate. We found that the supplementation of Skp2–Skp1 and substrate (along with further components necessary for substrate presentation to the ubiquitin ligase) to extracts of HeLa cells synergistically increased levels of neddylated Cul1. Skp2–Skp1 abrogates the inhibitory influence of CAND1 on the neddylation of Cul1 by promoting the dissociation of the cullin–CAND1 complex, whereas substrate, together with substrate-presenting components, prevents the action of CSN to deneddylate cullin. We propose a sequence of events in which the increased availability of Skp2 and substrate in the transition of cells to S phase promotes the neddylation and assembly of the SCFSkp2 ubiquitin ligase complex.
Keywords: cell cycle, Nedd8
Cullin-containing multiprotein complexes comprise the largest family of ubiquitin protein ligases. The best studied of these complexes is the SCF (Skp1-cullin1-F-box protein) class of ubiquitin ligases. They consist of cullin1 (Cul1) scaffold protein, the N-terminal domain of which binds the S phase-associated Skp1 adaptor protein while the C-terminal domain binds the ROC1 RING finger protein. Skp1 binds variable substrate-binding F-box proteins that may also interact directly with Cul1 (reviewed in refs. 1 and 2). We have been studying an SCF ubiquitin ligase that contains the F-box protein Skp2 (SCFSkp2) and that targets the cyclin-dependent kinase (cdk) inhibitor p27 for degradation in the transition of cells from G0/G1 to S phase of the cell cycle (3, 4). SCFSkp2 is assembled at the end of G1 by the rise in levels of its specific F-box protein, Skp2, and of its accessory protein, Cks1 (cyclin kinase subunit 1) (2, 4). As is the case with other cullin-based ubiquitin ligases, the assembly and activity of SCFSkp2 is regulated by the ligation of the small, ubiquitin-like protein Nedd8 to a specific lysine residue at the C-terminal domain of Cul1 (1). This process, also called “neddylation,” increases the ubiquitin ligase activity of SCF, in part by increasing its affinity for E2 (5). Neddylation also controls the assembly of cullin-based ubiquitin ligases, because nonneddylated cullins bind tightly to a protein called CAND1 (cullin-associated and neddylation-dissociated 1). Binding of CAND1 to Cul1 prevents its neddylation and also the binding of Skp1 to Cul1 and thus the assembly of the SCF complex (6–9). The crystal structure of the CAND1–Cul1 complex showed tight binding of CAND1 to both N-terminal and C-terminal domains of Cul1, thus accounting for both actions of CAND1 (10). Although it was initially reported that neddylation of Cul1 in complex with CAND1 caused the dissociation of the complex (6), subsequent experiments with more purified preparations did not detect neddylation or dissociation of Cul1 in complex with CAND1 (10). Because the initial experiments were carried out with Cul1–Cand1 complex immunoprecipitated from mammalian cells (6), it was suggested that some cellular factors may be required for the dissociation of the complex (10). Thus, an important problem is how the dissociation of Cul1 from CAND1 and its neddylation are initiated. The extent of the neddylation of cullins is also affected by the COP9/signalosome (CSN) complex, a large multiprotein complex that has a specific isopeptidase activity that deneddylates cullins (reviewed in ref. 11).
Although neddylation of cullins is important for the assembly and activity of cullin-based ubiquitin ligases, it is not clear how neddylation is regulated. It was previously suggested that the availability of a Skp1–F-box protein–substrate complex may trigger the neddylation of Cul1 (6, 11), but experimental evidence for this notion was very scanty. It was observed that the von Hippel–Lindau tumor suppressor gene product (pVHL), which is the substrate-binding subunit of a Cul2-containing ubiquitin ligase complex, stimulates the neddylation of Cul2 (12, 13). It was reported also that SCFβTrCP that contains the neddylated form of Cul1 preferentially binds to phosphorylated substrates of this ubiquitin ligase complex (see figure 1 in ref. 14). The molecular mechanisms responsible for the regulation of cullin neddylation were unknown and are the subject of this investigation.
Results
Levels of Neddylated Cullin1 in Extracts of HeLa Cells Are Increased by Skp2–Skp1 and p27 Substrate of SCFSkp2.
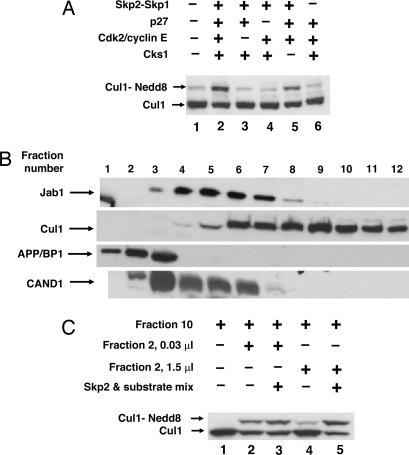
Initially, we examined the notion that increased availability of Skp2 and of phosphorylated p27 in transition to S phase stimulated the neddylation, and thus the activity, of the newly assembled SCFSkp2 ubiquitin ligase complex. As shown in Fig. 1A, the combined addition to HeLa cell extracts of Skp2–Skp1, its substrate p27, and further components necessary for the binding of phosphorylated p27 to the SCFSkp2 ubiquitin ligase (3, 4) markedly increased levels of neddylated Cul1 (lane 2). Because free Skp2 is not soluble, Skp2 was expressed and supplemented as a Skp2–Skp1 complex. Control experiments indicated that a similar amount of Skp1 had no significant effect (data not shown; see also below), suggesting that Skp2 is necessary. The substrate p27 was supplemented together with cdk2/cyclin E, which causes its phosphorylation on Thr-187 and is also necessary for its presentation to the ubiquitin ligase in a trimeric complex (15). The complete mixture also contained the accessory protein Cks1, which promotes the binding of phosphorylated p27 to Skp2 (4, 16). The omission of any of these components prevented the increase in the level of neddylated Cul1, with the exception of Cks1, which was only partially required (Fig. 1A). We have noted that the phosphorylation of p27 was also only partially required, because the addition of the protein kinase inhibitor staurosporine only partially decreased the stimulation of Cul1 neddylation by cdk2-cyclin E. This phenomenon may be related to the observation that neddylation of Cul1 in SCFSkp2 decreases the requirement for p27 phosphorylation for its ubiquitylation (unpublished data). For convenience of description, hereafter we refer to the complex of p27 with cdk2-cyclin E and Cks1 as “substrate” and to Skp2–Skp1 as “Skp2.” Although the stimulatory effects of Skp2 and substrate could be readily observed in crude extracts of HeLa cells, they had no significant influence on a purified neddylation system consisting of recombinant His-6-Cul1, Nedd8, the E1-like enzyme APP-BP1 (amyloid-β precursor protein binding protein 1)/Uba3, and the Nedd8-specific E2 Ubc12 (data not shown), which suggested the involvement of additional cellular factors in the control of cullin neddylation.
Fig. 1.
Skp2 and substrate synergistically increase levels of neddylated Cul1 in extracts and fractions from HeLa cells. (A) Increase in levels of neddylated Cul1 in extracts of HeLa cells by combined addition of Skp2–Skp1, p27 substrate, and other components required for the interaction of substrate with SCFSkp2. The neddylation of endogenous Cul1 in extracts of HeLa cells (3 μg of protein) was assayed as described in Materials and Methods, except that the concentration of APP-BP1-Uba3 was 0.01 nM. Skp2–Skp1 was added as indicated in the presence of either complete substrate mixture (lane 2) or substrate mixtures from which the indicated components were omitted (lanes 3–5). (B) Fractionation of HeLa cell extracts on a heparin-Sepharose column. Extracts of HeLa cells were fractionated as described in Materials and Methods. Samples of 3 μl of the indicated column fractions were subjected to immunoblotting with the indicated antibodies. (C) Reconstitution of the effects of Skp2 and substrate with fractions of extract separated on a heparin-Sepharose column. The effects of Skp2 and substrate on the neddylation of Cul1 were assayed as described in Materials and Methods, except that recombinant APP-BP1-Uba3 was not added. Samples (0.5 μl) of fraction 10 of the heparin column served as the source of Cul1. The indicated amounts of fraction 2 from the same heparin column were added as specified.
To identify the cellular factors that may be involved in the regulation of the neddylation of Cul1, we fractionated extracts of HeLa cells. In this fractionation, we tested the possible involvement of two factors that are known to have a negative influence on cullin neddylation: the CSN complex, which has deneddylation activity (11), and the protein CAND1, which binds to cullins and prevents their neddylation (6–9). Extracts of HeLa cells were subjected to chromatography on a heparin-Sepharose column, a procedure previously used for the purification of CSN (17). The position of known proteins in column fractions was followed by immunoblotting. As shown in Fig. 1B, the Jab1 subunit of the CSN complex eluted mainly in fractions 3–8. The Jab1-containing peak fractions coincided with enzyme activity that deneddylates Cul1 (data not shown). Cul1 eluted in a broad region of this column, spanning from fraction 5 to the end of the salt gradient (Fig. 1B), possibly because of its binding to different proteins in different complexes. APP-BP1, a subunit of the E1-like enzyme of Nedd8, eluted from the heparin column at low salt concentrations in fractions 1–3, whereas CAND1 eluted in a broader region in fractions 2–6 (Fig. 1B).
Because the strong deneddylating activity concentrated in CSN peak fractions interfered with the Cul1 neddylation assay, we tried to reconstitute the effects of F-box protein and substrate with fractions from both sides of the CSN peak. As the source of endogenous Cul1 for neddylation, we used fraction 10 from the heparin column. The neddylation of endogenous Cul1 was assayed in the presence of recombinant Nedd8 and Ubc12. When a small amount of fraction 2 of the heparin column (containing endogenous E1-like enzyme; Fig. 1B) was added to this system, significant neddylation of Cul1 could be seen (Fig. 1C, lane 2). However, the addition of a mixture of Skp2 and its substrate only slightly increased the neddylation of Cul1 under these conditions (Fig. 1C, lane 3). We observed, however, that a great increase in the amount of fraction 2 actually decreased the extent of the neddylation of Cul1 (Fig. 1C, lane 4) and that, under the latter conditions, the mixture of Skp2 and its substrate markedly stimulated the neddylation of Cul1 (Fig. 1C, lane 5). These observations suggested that fraction 2 of the heparin column contains a factor, or factors, that inhibits the neddylation of Cul1 and that Skp2 and substrate overcome the inhibitory influence of the factor(s). Because fraction 2 contains CAND1 (Fig. 1B), we next examined the possible involvement of CAND1 in this process.
The Inhibitory Influence of CAND1 on the Neddylation of Cul1 Is Overcome by Skp2 and Substrate.
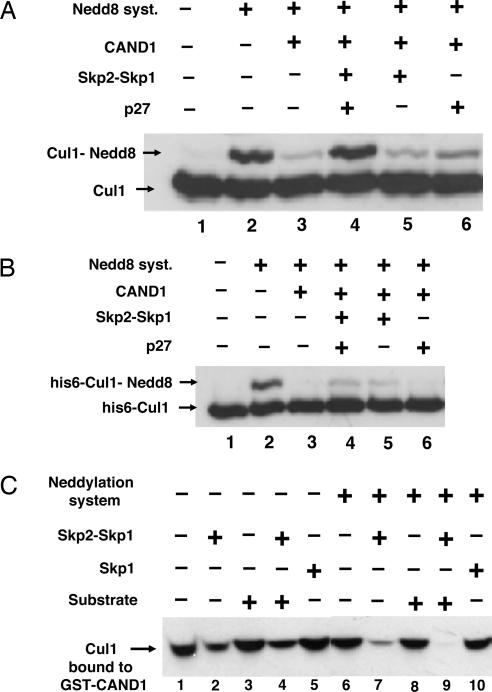
CAND1 is a protein that binds to the nonneddylated form of cullins and prevents their neddylation (6–10). We therefore examined whether Skp2, substrate, or both may overcome the inhibitory influence of CAND1 on the neddylation of Cul1. In the experiment shown in Fig. 2A, endogenous Cul1 (fractions 10–12 from the heparin column) was first incubated with recombinant purified CAND1 and then was subjected to the action of a neddylation system consisting of recombinant APP-BP1/UBA3, Ubc12, and Nedd8. As expected, CAND1 markedly inhibited the neddylation of Cul1 (Fig. 2A, lane 3). The supplementation of Skp2–Skp1 together with substrate completely overcame the inhibitory influence of CAND1 (lane 4). The stimulation of the neddylation of Cul1 under these conditions required the presence of both Skp2–Skp1 and p27 (lanes 5 and 6).
Fig. 2.
Skp2 causes dissociation of Cul1 from CAND1 and, jointly with substrate, abrogates inhibition of neddylation. (A) Skp2 and substrate overcome inhibition by CAND1 of the neddylation of Cul1 in a partially purified preparation. Assay of the neddylation of Cul1 was carried out as described in Materials and Methods in the presence of 0.5 μl of fraction 9 of the heparin column as the source of Cul1. In lanes 3 and 5, a substrate mixture lacking p27 was added. Where indicated, CAND1 (70 nM) was added 5 min before the neddylation mixture. (B) Neddylation of purified recombinant Cul1 in the presence of CAND1 is only slightly stimulated by Skp2–Skp1. Experimental conditions were as in A, except that partially purified Cul1 was replaced by 35 nM recombinant purified His-6-Cul1-Roc1. (C) Skp2–Skp1, but not Skp1 or substrate, promotes dissociation of Cul1–Cand1 complex. Bacterially expressed GST–CAND1 (1.3 pmol) was preincubated with 4 μl of pooled fractions 10–12 from the heparin-Sepharose column for 30 min at 30°C in a buffer consisting of 50 mM Tris·HCl, pH 7.6/5 mM MgCl2/1 mM DTT/10% glycerol/10 mM phosphocreatine/0.1 μg/μl creatine phosphokinase/0.5 mM ATP/3 mg/ml BSA. Subsequently, Skp2–Skp1 (10 nM), Skp1 (15 nM), or complete substrate mixture (see Materials and Methods) was added as indicated, and a second incubation (at 20°C for 15 min) was carried out under conditions as described for the assay of neddylation of Cul1, except that the reaction volume was 40 μl and a neddylation system (consisting of APP-BP1-Uba3, Ubc12, and Nedd8) was added only in the indicated tubes. Next, 10 μl of glutathione-Sepharose beads (Amersham Pharmacia Biosciences) was added, and samples were rotated at 4°C for 90 min. The beads were washed three times with a buffer consisting of 50 mM Tris·HCl, pH 7.2/1 mM DTT/2 mg/ml BSA/100 mM NaCl/10% glycerol, eluted with SDS sample buffer, and subjected to immunoblotting for Cul1 for the estimation of Cul1 that remained bound to GST–CAND1.
The above experiments were carried out with endogenous partially purified Cul1. When we tried to replace it with recombinant His-6-Cul1, neddylation was effectively inhibited by CAND1, but there was only a very slight effect of Skp2 and substrate to overcome this inhibition (Fig. 2B). This finding suggested that some cellular factor(s), added with the partially purified preparation of Cul1, may still be required for the effectiveness of Skp2 and/or substrate.
Skp2 Promotes the Dissociation of Cul1 from CAND1, Whereas Substrate Prevents Deneddylation by CSN.
The observation that the combination of Skp2 and substrate overcomes the inhibition of the neddylation of Cul1 by CAND1 raised the question of whether these agents promote the dissociation of Cul1 from complex with CAND1. In the experiment shown in Fig. 2C, endogenous Cul1 (fractions 10–12 of the heparin column) was first incubated with GST–CAND1 to form a Cul1–Cand1 complex. Subsequently, the different effectors were added in the absence or presence of a neddylation system. After a further incubation, GST–CAND1 was isolated by glutathione beads, and the amount of Cul1 that remained associated with CAND1 was estimated by immunoblotting. Without neddylation, the addition of Skp2–Skp1 alone caused significant release of Cul1 from GST–CAND1 (Fig. 2C, lane 2). Substrate had no significant influence on Cul1 release when tested either by itself (lane 3) or in combination with Skp2–Skp1 (lane 4). In this case, too, Skp1 could not replace Skp2–Skp1 for Cul1 release (lane 5). Thus, we could not reproduce the results of Zheng et al. (7), who reported that Skp1 dissociates Cul1–Cand1 in the presence of ATP. In the presence of a neddylation system, Skp2–Skp1 caused more extensive release of Cul1 from complex with CAND1 (lane 7), whereas substrate alone had no influence (lane 8). However, the combination of substrate, Skp2–Skp1, and a neddylation system caused complete release of Cul1 from complex with GST–CAND1 (Fig. 2C, lane 9). These data suggested that Skp2–Skp1 promotes the dissociation of Cul1 from CAND1, whereas substrate increases the neddylation of Cul1 by a different mechanism.
Trying to distinguish between the effects of Skp2 and substrate and to identify further cellular factors that may be involved in the regulation of the neddylation of Cul1, we next purified endogenous Cul1 by binding to GST–CAND1, followed by affinity chromatography on glutathione-Sepharose. When purified Cul1–GST–CAND1 complex was incubated with neddylation mixture, no significant neddylation of Cul1 could be detected (Fig. 3A, lane 1), presumably because of the strong inhibitory action of CAND1. The addition of Skp2 and substrate stimulated the conversion of Cul1 to the neddylated form (lane 2). The omission of p27 did not decrease neddylation significantly (lane 3), whereas the omission of Skp2–Skp1 completely abolished the neddylation of Cul1 (lane 4). These data indicated that, with purified Cul1 in complex with CAND1, Skp2 alone, and not the substrate, stimulates Cul1 neddylation.
Fig. 3.
Substrate prevents the deneddylation of Cul1 by CSN. (A) The neddylation of affinity-purified Cul1 bound to GST–CAND1 is stimulated by Skp2 in the absence of substrate, and the requirement for substrate is restored by the fraction not adsorbed to GST–CAND1. Samples of 0.5 μl of affinity-purified Cul1 from HeLa cells bound to GST–CAND1 (see Materials and Methods) were subjected to the neddylation assay. Where indicated, 0.5 μl of the fraction not adsorbed to GST–CAND1, Skp2, or substrate mixture containing or lacking p27 was supplemented. (B) Experimental conditions were as in A except that the unadsorbed fraction was replaced by the indicated amounts of purified CSN (see Materials and Methods). (C) Substrate prevents the deneddylation of Cul1 (in complex with Skp2–Skp1) by CSN. The first incubation contained in a volume of 7 μl: 50 mM Tris·HCl (pH 7.6), 10% glycerol, 20 nM Skp2–Skp1, 0.5 μl of affinity-purified Cul1 in complex with GST–CAND1, and neddylation mixture as described above. After incubation at 20°C for 30 min, a mixture of 0.1 μg/μl hexokinase, 20 mM 2-deoxyglucose, and 20 mM DTT was added to stop the neddylation reaction. After 5 min, 0.05 μl of purified CSN was added in the presence (lane 4) or absence (lane 3) of complete substrate mixture. Substrate was added 5 min before CSN. After a second incubation (at 20°C for 30 min), samples were subjected to electrophoresis and immunoblotting with antibody to Cul1.
Because the effect of substrate was lost with purified Cul1 bound to GST–CAND1, we reasoned that substrate may counteract the effect of some negatively acting factor that was present in the crude preparation of Cul1 but was not adsorbed to GST–CAND1. When the unadsorbed fraction was added back to purified Cul1 bound to GST–CAND1, the combination of Skp2 and substrate still strongly stimulated the neddylation of Cul1 (Fig. 3A, lane 5), but, in this case, there was a strong requirement for substrate (lane 6). To identify the negatively acting factor, the unadsorbed fraction was subjected to gel filtration chromatography on a Superose-6 column. The factor eluted at a molecular mass of ≈500 kDa, in exact coincidence with the Jab1 subunit of CSN (data not shown). It should be noted that although fractions 10–12 of the heparin column, which served as the starting material for this preparation, were separated from most of the CSN peak (Fig. 2A), they still contained small amounts of CSN that was concentrated by further chromatography procedures. Because of the coincidence of the negatively acting factor with CSN and the known action of CSN to deneddylate cullins, we next examined whether the factor in the unadsorbed fraction could be replaced by CSN. As shown in Fig. 3B, the addition of small amounts of purified CSN restored the requirement for p27 substrate for the accumulation of the neddylated form of purified Cul1 (in complex with GST–CAND1) in the presence of Skp2 (lanes 5 and 7 vs. lane 3). In experiments of similar design, we found that in the presence of purified CSN, all components of the substrate-presenting complex, including cdk2-cyclin E and Cks1, were strongly required (data not shown). These observations suggested that the substrate complex may act by preventing the action of CSN to deneddylate Cul1. We tested this notion by the two-stage experiment shown in Fig. 3C. In the first incubation, purified Cul1 in complex with GST–CAND1 was neddylated in the presence of Skp2 (lane 2). Then, neddylation was stopped by the removal of ATP with hexokinase and deoxyglucose, and CSN was added in the absence (lane 3) or presence (lane 4) of substrate, followed by a second incubation. CSN caused deneddylation of Cul1 (lane 3), and this deneddylation was prevented by substrate (lane 4). These data indicated that substrate indeed acts by preventing the deneddylation of Cul1 by CSN.
Discussion
In this study, we tried to gain insight into the mechanisms that regulate the state of the neddylation of the Cul1 component of SCFSkp2 and thus the activity of this ubiquitin ligase complex. It was suggested previously by other investigators that the availability of specific F-box proteins, along with their respective substrates, may control the neddylation and assembly of specific cullin-based ubiquitin ligase complexes (6, 11), but the underlying mechanisms remained unknown. In the case of the SCFSkp2 ubiquitin ligase that acts in the G1-to-S phase transition, it appeared reasonable to assume that such regulation may occur, because levels of Skp2, Cks1, cdk2-cyclin E, and phosphorylated p27 all rise markedly at this stage of the cell cycle (2, 4). In fact, we observed that the supplementation of Skp2 and substrate to cell extracts synergistically increased levels of neddylated Cul1 (Fig. 1A). Fractionation-reconstitution experiments indicated that these agents act by overcoming the effect of a factor (or factors) that inhibits the neddylation of Cul1 (Fig. 1D). Further work with purified components showed that Skp2–Skp1 abrogates the inhibitory influence of CAND1 on the neddylation of Cul1 (Figs. 2A and 3A), whereas substrate prevents the action of the CSN complex to deneddylate Cul1 (Fig. 3 B and C). Based on these findings, we propose the sequence of events shown in Fig. 4. The tight binding of CAND1 to Cul1 prevents neddylation and assembly of the SCF complex. The process is initiated by Skp2–Skp1, which induces the dissociation of the Cul1–Cand1 complex (Fig. 4, step1). We assume that this process is reversible (step 2), because in the absence of a neddylation system, the release of Cul1 is only partial (Fig. 2C). The dissociation of Cul1 from CAND1 allows its neddylation (Fig. 4, step 3), which converts the assembled SCF complex into a state that cannot associate with CAND1 and thus cannot be inhibited by it. The detailed mechanism by which Skp2–Skp1 initiates the dissociation of the Cul1–Cand1 complex remains to be elucidated. It is possible that an additional factor, which is tightly bound to Cul1–Cand1, is also involved, because endogenous Cul1 from HeLa cells that was purified by binding to GST–CAND1 was affected by Skp2–Skp1 much more than recombinant Cul1 (Figs. 2B and 3A). However, it is also possible that recombinant Cul1 is not a faithful copy of Cul1 because of misfolding and/or lack of modification. We observed that Skp1 cannot replace Skp2–Skp1 in the release of Cul1 from CAND1 (Fig. 2C). The crystal structure of the SCFSkp2 complex showed that Skp2 is bound to Cul1 not only by means of Skp1 but also by direct contact between Skp2 and Cul1 (18). The extended Skp1–Skp2–Cul1 interface may provide the tight interaction required for effective competition with CAND1 binding.
Fig. 4.
Proposed sequence of events in the neddylation and assembly of the SCFSkp2 complex. See the text (Discussion). The increase in the levels of Skp2–Skp1 in the transition of cells to S phase promotes the dissociation of Cul1 from CAND1 and thus allows its neddylation. The increase in the levels of substrate in the same stage of the cell cycle locks the SCFSkp2 complex in the neddylated and assembled state by preventing deneddylation. “Substrate” denotes phosphorylated p27 in ternary complex with cdk2-cyclin E and bound to Cks1.
Neddylated Cul1 is subject to deneddylation by CSN (Fig. 4, step 4), and substrate prevents the action of CSN (Fig. 3 B and C). We assume that substrate acts by binding to Skp2, which in turn is bound to neddylated Cul1 (Fig. 4), because the effect of p27 required the presence of cdk2/cyclin E, and Cks1, components that are known to be required for the binding of p27 to Skp2 (3, 4, 16). An obvious implication is that the prevention of deneddylation by bound substrate would facilitate its ubiquitylation by the SCF complex. Because Skp1–Skp2, along with substrate, binds to the N-terminal domain of Cul1 while neddylation takes place on K720 near the C terminus, the influence of substrate in preventing deneddylation was surprising. It is possible that binding of substrate to the N-terminal domain induces a conformational change in Cul1 that precludes the accessibility of neddylated K-720 to the active site of the CSN complex. It is obvious that although the present investigation provides an outline for the regulation of the neddylation and assembly of the SCFSkp2 complex, many of the molecular alterations involved remain to be elucidated. It also remains to be seen whether the neddylation and assembly of other SCF complexes and cullin-based ubiquitin ligases is subject to similar regulation by F-box protein and substrate.
Materials and Methods
Reagents.
Bacterially expressed and purified Nedd8 was generously provided by Cecile Pickart (The Johns Hopkins University, Baltimore, MD) and was also prepared as described in ref. 19. Bacterially expressed and purified CAND1 (10) was kindly provided by N. Zheng (University of Washington, Seattle, WA), and UBC12 was provided by J. Brownell (Millennium Pharmaceuticals, Cambridge, MA). Bacterially expressed and purified Δ109Skp2–Skp1 (20) was kindly provided by B. Schulman (St. Jude's Hospital, Memphis, TN). His-6-p27 and GST–CAND1 (expression plasmid kindly provided by Y. Xiong, University of North Carolina, Chapel Hill, NC) were expressed in bacteria and purified by chromatography on nickel-agarose and glutathione-Sepharose, respectively. Bacterially expressed Cks1 was purified as described in ref. 16. His-6-APP-BP1-Uba3, His-6-Cul1-ROC1, His-6-cyclin E-cdk2, and His-6-Skp1 were expressed in baculovirus-infected insect cells and purified as described in refs. 4 and 16. The antibodies used for immunoblotting were as follows: Cul1, Zymed catalog no. 71-8700; CAND1, Santa Cruz Biotechnology no. sc-10672; Jab1, Zymed no. 34-3000; APP-BP1, BD Biosciences 611864.
Fractionation of Extracts of HeLa Cells and Purification of CSN.
Extracts from logarithmically growing HeLa cells were prepared as described in ref. 21 and were fractionated on a heparin-Sepharose column as described for the purification of CSN (17), with some modifications. All operations were performed at 0–4°C. HeLa cell extract (150 mg of protein) was dialyzed against 20 mM potassium phosphate containing 1 mM DTT (buffer A) and then was loaded on a HiPrep 16/10 Heparin FF column (Amersham Pharmacia Biosciences) equilibrated with buffer A. The column was washed with 90 ml of buffer A and eluted with a gradient of 0–600 mM NaCl (210 ml). Fourteen fractions of 15 ml were collected, concentrated by centrifuge ultrafiltration (Centriprep-30, Amicon), diluted 20-fold with 50 mM Tris·HCl, pH 7.2/1 mM DTT (buffer B), and concentrated again to a volume of ≈500 μl. Glycerol was added to a final concentration of 10% (vol/vol), and samples were stored at −70°C.
For further purification of CSN, fractions 5–7 of the heparin column (which contained the peak of CSN, judged by immunoblotting for its Jab1 subunit and deneddylating activity; see Results) were subjected to anion exchange chromatography on a MonoQ HR 5/5 column (Amersham Pharmacia Biosciences) with a gradient of 200–600 mM NaCl in buffer B. CSN eluted at 330–370 mM NaCl. The peak fractions were collected and subjected to gel filtration chromatography on a Superose-6 HR 10/30 column (Amersham Pharmacia Biosciences) equilibrated with buffer B that also contained 150 mM NaCl and 0.2 mg/ml soybean trypsin inhibitor. Fractions of 0.5 ml were collected, concentrated by ultrafiltration with a Centricon-30 (Amicon), diluted 20-fold in buffer B, and concentrated again to a volume of ≈100 μl. The central peak fractions of CSN (fractions 26–28) were collected, supplemented with glycerol to 10% (vol/vol), and stored at −70°C in small samples.
Affinity Purification of Cul1 from HeLa Cells by Formation of Complex with GST–CAND1.
Fractions 10–12 from the fractionation of HeLa cell extracts on a heparin-Sepharose column (see above) that contained Cul1 well separated from endogenous CAND1 (see Results) were pooled. Three hundred microliters of this material was mixed with ≈6 μg of GST–CAND1 in a buffer consisting of 50 mM Tris·HCl, pH 7.2/1 mM DTT/2 mg/ml BSA/100 mM NaCl/10% glycerol (buffer C) in a volume of 600 μl. We estimated that, under these conditions, the molar excess of CAND1 over Cul1 was at least 5-fold. The mixture was incubated at 30°C for 1 h and then mixed with 180 μl of glutathione-Sepharose beads (Amersham Pharmacia Biosciences) that had been equilibrated with buffer C. After rotation at 4°C for 90 min, the beads were separated from the unadsorbed fraction by centrifugation. The unadsorbed fraction was concentrated by ultrafiltration to a volume of 300 μl. The beads were washed three times with buffer C, and the Cul1–GST–CAND1 complex was eluted (at 4°C for 1 h) with 600 μl of a buffer similar to buffer C, except that the pH was 8.0 and it contained 10 mM glutathione. The eluate was concentrated by ultrafiltration, diluted 30-fold in buffer B, and concentrated again to a volume of 180 μl.
Assay of the Effects of Skp2 and Substrate on the Neddylation of Cul1.
Before the neddylation reaction, a “substrate mixture” was prepared for the formation of phosphorylated p27 in ternary complex with cdk2/cyclin E and in the presence of Cks1. The incubation of the substrate mixture for 10 neddylation reactions contained in a volume of 10 μl: 40 mM Tris·HCl (pH 7.6), 2 mg/ml ovalbumin, 1 mM DTT, 5 mM MgCl2, 1 mM ATP, 1.6 μM p27, 170 nM cdk2/cyclin E, and 4.4 μM Cks1. The substrate mixture was incubated at 30°C for 30 min. When the influence of the deletion of a component of the substrate mixture was tested, a similar incubation was carried out in the absence of the specified component. Unless otherwise specified, the assay for the neddylation of Cul1 contained in a volume of 10 μl: 50 mM Tris·HCl (pH 7.6), 5 mM MgCl2, 1 mM DTT, 3 mg/ml BSA, 1 mM ATP, 10% glycerol, 10 mM phosphocreatine, 0.1 μg/μl creatine phosphokinase, 0.5 mM ATP, 2.5 μM Nedd8, 100 nM Ubc12, 10 nM APP-BP1-Uba3, and a source of Cul1 as indicated in Figs. 1–3. Where indicated, 1 μl of substrate mixture and 10 nM Skp2–Skp1 were added. After incubation at 20°C for 30 min, samples were subjected to electrophoresis on an 8% polyacrylamide-SDS gel, transferred to nitrocellulose, and blotted with anti-Cul1 antibody. The extent of the neddylation of Cul1 was estimated by the formation of the slower migrating derivative of Cul1.
Acknowledgments
This work is dedicated to the memory of Dr. Cecile M. Pickart, an outstanding scientist, colleague, and friend. We thank all of the colleagues listed in the paper who generously provided reagents. This work was supported by grants from the Israel Science Foundation and the Israel Cancer Research Fund.
Abbreviations
- Cul1
cullin1
- SCF
Skp1-Cul1-F-box protein
- CAND1
cullin-associated and neddylation-dissociated
- CSN
COP9/signalosome
- cdk
cyclin-dependent kinase
- Cks1
cyclin kinase subunit 1
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Petroski M. D., Deshaies R. J. Nat. Rev. Mol. Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 2.Cardozo T., Pagano M. Nat. Rev. Mol. Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 3.Carrano A. C., Eytan E., Hershko A., Pagano M. Nat. Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 4.Ganoth D., Bornstein G., Ko T. K., Larsen B., Tyers M., Pagano M., Hershko A. Nat. Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami T., Chiba T., Suzuki T., Iwai K., Yamanaka K., Minato N., Suzuki H., Shimbara N., Hidaka Y., Osaka F., et al. EMBO J. 2001;20:4003–4012. doi: 10.1093/emboj/20.15.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Furukawa M., Matsumoto T., Xiong Y. Mol. Cell. 2002;10:1511–1518. doi: 10.1016/s1097-2765(02)00783-9. [DOI] [PubMed] [Google Scholar]
- 7.Zheng J., Yang X., Harrell J. M., Ryzhikov S., Shim E.-H., Lykke- Andersen K., Wei N., Sun H., Kobayashi R., Zhang H. Mol. Cell. 2002;10:1519–1526. doi: 10.1016/s1097-2765(02)00784-0. [DOI] [PubMed] [Google Scholar]
- 8.Hwang J.-W., Min K.-W., Tamura T.-A., Yoon J.-B. FEBS Lett. 2003;541:102–108. doi: 10.1016/s0014-5793(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 9.Oshikawa K., Matsumoto M., Yada M., Kamura T., Hatakeyama S., Nakayama K. I. Biochem. Biophys. Res. Commun. 2003;303:1209–1216. doi: 10.1016/s0006-291x(03)00501-1. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg S. J., Cascio T. C., Shumway S. D., Garbutt K. C., Liu J., Xiong Y., Zheng N. Cell. 2004;119:517–528. doi: 10.1016/j.cell.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Cope G. A., Deshaies R. J. Cell. 2003;114:663–671. doi: 10.1016/s0092-8674(03)00722-0. [DOI] [PubMed] [Google Scholar]
- 12.Liakopoulos D., Busgen T., Brychzy A., Jentsch S., Pause A. Proc. Natl. Acad. Sci. USA. 1999;96:5510–5515. doi: 10.1073/pnas.96.10.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada H., Yeh E. T. H., Kamitani T. J. Biol. Chem. 1999;274:36025–36029. doi: 10.1074/jbc.274.50.36025. [DOI] [PubMed] [Google Scholar]
- 14.Read M. A., Bronwell J. E., Gladysheva T. B., Hottelet M., Parent L. A., Coggins M. B., Pierce J. W., Podust V. N., Luo R.-S., Chau V., Palombella V. Mol. Cell. Biol. 2000;20:2326–2333. doi: 10.1128/mcb.20.7.2326-2333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montagnoli A., Fiore F., Eytan E., Carrano A. C., Draetta G. F., Hershko A., Pagano M. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitri D., Seeliger M. A., Ko T. K., Ganoth D., Breward S. E., Itzhaki L. S., Pagano M., Hershko A. J. Biol. Chem. 2002;277:42233–42240. doi: 10.1074/jbc.M205254200. [DOI] [PubMed] [Google Scholar]
- 17.Seeger M., Kraft R., Ferrel K., Bech-Otschir D., Dumdey R., Schade R., Gordon C., Naumann M., Dubiel W. FASEB J. 1998;12:469–478. [PubMed] [Google Scholar]
- 18.Zheng N., Schulman B. A., Song L., Miller J. J., Jeffrey P. D., Wang D., Chu C., Koepp D. M., Elledge S. J., Pagano M., et al. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 19.Whitby F. G., Xia G., Pickart C. M., Hill C. D. J. Biol. Chem. 1998;273:34983–34991. doi: 10.1074/jbc.273.52.34983. [DOI] [PubMed] [Google Scholar]
- 20.Schulman B. A., Carrano A. C., Jeffrey P. D., Bowen Z., Kinnucan E. R., Finnin M. S., Elledge S. J., Harper J. W., Pagano M., Pavletich N. P. Nature. 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- 21.Yudkovsky Y., Shteinberg M., Listovsky T., Brandeis M., Hershko A. Biochem. Biophys. Res. Commun. 2000;271:299–304. doi: 10.1006/bbrc.2000.2622. [DOI] [PubMed] [Google Scholar]