Abstract
We have investigated the role of Sonic hedgehog (Shh) in the development of facial structures by depriving chicken embryos of the most anterior sources of this morphogen, including the prechordal plate and the anterior ventral endoderm of the foregut, before the onset of neural crest cell (NCC) migration to the first branchial arch (BA1). The entire forehead, including the foregut endoderm, was removed at 5- to 10-somite stage (ss), which led to the absence of the lower jaw when the operation was performed before 7-ss. If the embryos were deprived of their forehead at 8- to 10-ss, they were later on endowed with a lower beak. In embryos that were operated on early, the NCCs migrated normally to BA1 but were subjected to massive apoptosis a few hours later. Cell death did not occur when forehead excision was performed at a later stage. In this case, onward expression of Shh in the ventral foregut endoderm extended caudally over the excision limit, and we hypothesized that absence of Shh production by the endoderm in embryos that were operated on early could be responsible for the NCC apoptosis and the failure of BA1 development. We thus provided exogenous Shh to the embryos that were operated on before 7-ss. In this case, the development of the lower jaw was rescued. Therefore, Shh derived from the ventral foregut endoderm ensures the survival of NCCs at a critical stage of BA1 development.
Keywords: branchial arch, craniofacial skeleton, chicken/quail chimeras, prechordal plate
Formation of the facial skeleton in vertebrates results from complex developmental processes that require the migration of cranial neural crest cells (NCCs) and a series of epithelio-mesenchymal interactions. The NCCs arising from the posterior diencephalon and anterior mesencephalon give rise to the frontonasal skeleton, whereas those exiting from the posterior mesencephalon and from rhombomeres 1 and 2 colonize the first branchial arch (BA1) to form the skeleton of the maxilla and mandible (1–3). This rostral domain of the neural crest was designated the FSNC (facial skeletogenic neural crest) (4). The posterior rhombomeres yield NCCs, which participate in the formation of the medial and posterior parts of the hyoid cartilage (1–3).
Excision of the complete FSNC in 5- to 6-somite stage (ss) chicken embryos, before the onset of cell migration, results in a striking phenotype in which the facial processes and skeleton do not develop and the fore- and midbrain morphology is disturbed (4). If removal of cephalic NCCs is performed earlier (at 3-ss), the anteriormost cephalic vesicle does not develop, and the two eye anlagen fuse on the midline, giving rise to cyclopia (5). This phenotype resembles the most severe forms of human holoprosencephaly (HPE), a syndrome that includes a variety of malformations, such as a complete absence of the lower jaw, failure of forebrain hemisphere division, cyclopia, proboscis, and facial clefting.
Mutations of the Sonic hedgehog (Shh) gene, encoding for the morphogen Shh, have been found in cases of HPE in humans (6, 7). Moreover, disruption of Shh in the mouse showed that this gene is required for many morphogenetic processes, including establishment of limb polarity, dorsoventral patterning of the nervous system, and development of the foregut and of the axial and cranial skeleton (8–12).
Recent studies have contributed to the understanding of the role of Shh in facial development. Neutralizing Shh action in the chicken cranial mesenchyme at 7- to 10-ss by implanting Ab-secreting hybridoma cells induces the death of the FSNC (facial skeletogenic neural crest) cells 24 h after treatment and loss of BA1 derivatives (13). Blocking hedgehog signaling specifically in mouse NCCs results in similar cell death kinetics followed by severe head skeleton abnormalities (14). Both of these studies suggest that Shh is important for survival of the FSNC cells migrating to BA1. The absence of Shh in mutant mice results in strong abnormalities in gene expression in BA1 during development. In these mice, expression of Sox9, Twist, and Fgf8 is absent (15, 16). Similarly, in zebrafish, loss of Shh or of smoothened (Smo) results in the absence of craniofacial cartilages (17). However, none of these studies have identified the source of Shh that is critical for facial skeleton development. Possible candidates are the prechordal plate (PcP), the foregut endoderm, the floor plate (FP), the notochord (No), and the facial ectoderm.
It has been recently demonstrated that Shh produced by the forebrain neuroectoderm and the facial ectoderm is essential for upper face and nasofrontal bud development, whereas suppression of these sources of Shh has no effect on BA1 derivatives (18, 19), which suggests that the source of Shh that is critical for BA1 development might be the pharyngeal endoderm. In a previous study from our group, grafts of anterior foregut endoderm in the migration pathway of the NCCs to BA1 induced the duplication of the lower beak skeleton (20). Conversely, excision of the corresponding endodermal region in the early chicken embryo (5- to 6-ss) prevented the development of definite cartilage elements belonging to BA1 (20). Other experiments showed that NCCs can only differentiate into cartilage when cocultured or cografted with anterior foregut endoderm and maxillary ectoderm (21–23). Moreover, defects in the development of the visceral skeleton were observed in zebrafish embryos of casanova (cas) and bonnie and clyde (bon) mutants in which endoderm does not develop (24).
In the work presented here, we have analyzed the role of Shh produced by the foregut endoderm in the patterning of BA1 and the development of its skeletal derivatives. At the early somitic stages, Shh is expressed dorsally to the foregut in the anterior No and FP. It is also expressed in the PcP and in the anterior ventral endoderm. However, this ventral endodermal expression of Shh remains limited to the transverse level of the prosencephalon up to the 6-ss. It extends caudally over the prosencephalic–mesencephalic boundary only from 7- to 8-ss onward. Owing to this time/space-regulated Shh expression in the ventral foregut, we were able to demonstrate the role of Shh produced by the foregut endoderm on BA1 development by examining chicken embryos deprived of the forehead region before or after 7-ss. Our results demonstrate that, among the different sources of Shh in the forehead, the foregut endoderm provides an early signal required for the development of BA1.
Results
Spatiotemporal Regulation of Shh Expression in the Cephalic Area of 5- to 25-ss Chicken Embryos.
To analyze Shh expression during early head development, chicken embryos at 5- to 25-ss were hybridized in whole mount and then cut in sagittal and parasagittal serial sections. These developmental stages include the delamination and migration of cephalic NCCs.
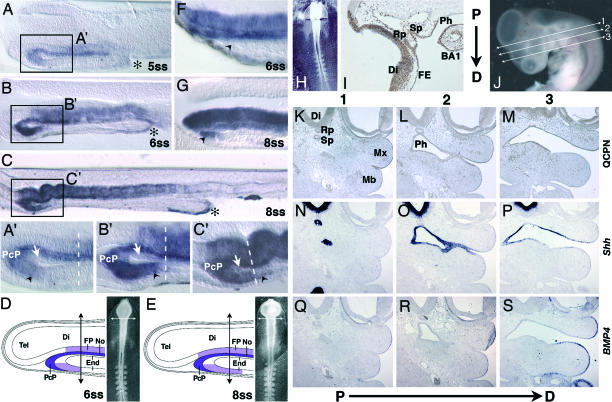
At 5-ss (Fig. 1A and A′), Shh transcripts are present in the No and associated FP corresponding to the level of the presumptive diencephalon and mesencephalon in continuity with the PcP located anteriorly. The Shh-positive PcP is abutted to the rostroventral endoderm, which also expresses Shh. At 6-ss, Shh transcripts in the ventral endoderm barely reach the level of the prosencephalic–mesencephalic boundary (Fig. 1 B and B′).
Fig. 1.
Shh expression in pharyhgeal endoderm. (A and A′) Sagittal section (50 μm) of a 5-ss chicken embryo showing Shh expression in midline cells, PcP, anterior ventral foregut endoderm (black arrowhead), and, more posteriorly, in the AIP (asterisk). (B and B′) At 6-ss, Shh transcripts are still present in the anterior ventral endoderm (black arrowhead) but remain absent in the dorsal foregut endoderm (white arrow). The PcP, the most anterior source of Shh at this stage, is in close contact with the rostral ventral endoderm. (C and C′) At 8-ss, a caudal extension of Shh expression in the ventral foregut endoderm has occurred and overpasses the prosencephalic–mesencephalic boundary (dashed line). (D and E) Schematic representations of Shh expression and the level of forehead excision (arrows) on chicken embryos at 6- and 8-ss. End, endoderm; Tel, telencephalon; Di, diencephalon; FP, floor plate; No, notochord. (F) Sagittal section of a chicken embryo immediately after forehead excision at 6-ss. Remaining ventral foregut endoderm (black arrowhead) is devoid of Shh transcripts. (G) At 8-ss, Shh-expressing foregut endoderm (black arrowhead) is present. (H–S) Forehead quail/chicken chimeras. (H) Chimera immediately after the graft of a quail forehead on a 6-ss chicken embryo. (I) A sagittal section (7 μm) of an E3.5 chimera grafted at 6-ss. QCPN immunostaining (brown) shows quail cells forming the ventral Di and the most anterior mesencephalon, the facial ectoderm (FE), Rathke’s pouch (Rp), Sessel’s pouch (Sp), dorsal and ventral pharyngeal endoderm (Ph) of BA1, and ectoderm of BA1. (J) E4.5 quail/chicken chimera with indications (lines 1–3) of proximodistal (P→D) serial sections. (K–S) Proximodistal serial sections were treated with QCPN (K–M) or were in situ hybridized for Shh (N–P) or BMP4 (Q–S). At E4.5, quail cells (K–M) show the same distribution observed in E3.5 embryos (I), forming the ectoderm of the maxillary bud (Mx) of Rathke’s pouch, the epithelium ventral to the diencephalon, and the endoderm of Sessel’s pouch and the anterior part of the pharynx. (N–P) Shh mRNAs are present in Di, Sp, and Ph. (Q–S) Bmp4 mRNAs are present in the ectoderm of both the Mx and mandible (Mb).
At 8-ss (Fig. 1 C and C′), the PcP remains the most anterior Shh-expressing structure. At this stage, it forms a continuous pattern of expression with the anterior ventral endoderm, so that it is difficult to distinguish the borderline between these two tissues. From this stage on (8- to 10-ss), Shh expression progresses backward inside the anterior ventral foregut, whereas the dorsal foregut endoderm remains deprived of Shh transcripts. Shh expression present in the anterior intestinal portal (AIP) as early as 5- to 6-ss (Fig. 1 A and B) does not spread into the growing ventral endoderm as AIP moves backward, so that a large part of the ventral foregut endoderm remains free of Shh transcripts during that period of development (Fig. 1C). At 12-ss, the future oral ectoderm, in contact with the Shh-producing endoderm, starts to express Shh (data not shown). Up to 16-ss, Shh expression in the ventral head region is restricted to the oral membrane ectoderm and to the anterior part of the foregut endoderm (Fig. 4, which is published as supporting information on the PNAS web site).
Chicken embryos hybridized at stage 15 (Hamburger and Hamilton numbering; 25-ss) show Shh expression throughout the pharyngeal and gut endoderm, especially in BA1 endoderm. Shh is also expressed in the frontonasal prominence ectoderm at this stage (Fig. 4).
The Origin of BA1 Endoderm.
To explore the fate of the ventral endoderm expressing Shh at the early somitic stages, and to see whether it could include the stripe of endoderm able to induce the development of an extra lower jaw in our previous experiments (20), we substituted the entire forehead region of chicken embryos at 5- to 6-ss [embryonic day (E) 1.5] (Fig. 1H) with its counterpart from stage-matched quail embryos (n = 5). Quail/chicken forehead chimeras were killed 2 or 3 days after the operation (E3.5, n = 3; E4.5, n = 2) and analyzed in serial sagittal sections treated with the QCPN mAb, which identifies quail cells. This experiment revealed that not only were the telencephalon, diencephalons, and the most anterior part of the mesencephalon, together with the upper facial ectoderm, of the quail type (as expected), but the major part of the pharyngeal endoderm of BA1 was also formed by quail cells and thus was derived from the graft, whereas the major part of BA1 ectoderm was of host type (Fig. 1I).
To reconstitute more precisely the respective origin of the lateral pharyngeal endoderm and ectoderm, we analyzed two chimeras at E4.5. Consecutive coronal serial sections (see Fig. 1J) were either immunolabeled with the QCPN mAb (Fig. 1 K–M) or hybridized with Shh (Fig. 1 N–P) or Bmp4 (bone morphogenetic protein 4) (Fig. 1 Q–S) probes. These genes are expressed in the endoderm and distal ectoderm of BA1, respectively. The entire Shh-positive pharyngeal endoderm, Sessel’s pouch, the diencephalon, and Rathke’s pouch were found to be made up of quail cells (Fig. 1 K–M) (Fig. 5, which is published as supporting information on the PNAS web site). The Bmp4-positive ectoderm of the mandible was of the chicken host type and juxtaposed to the Shh-positive lateral pharyngeal endoderm made up of quail cells (Fig. 1 M, P, and S). Other quail/chicken forebrain chimeras (n = 2) were grown up to E10 to E11 and examined for head morphology. Although the upper beak was shorter than the lower beak, the facial skeleton was normally developed (Fig. 6, which is published as supporting information on the PNAS web site).
These results show that in the experiments published by Couly et al. (20) in 2002, zones I–III of the ventral endoderm corresponded to the endoderm of the Sessel’s pouch, of the stomodeum, and of the first branchial pouch.
The Rostral Foregut Endoderm Is Critical for Lower Jaw Development.
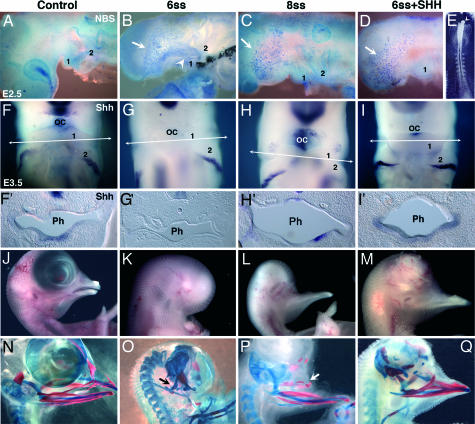
We have shown that, in embryos at 5- to 6-ss, Shh expression in the rostral ventral endoderm is restricted to the level corresponding to the prosencephalic region. In contrast, in embryos at 8-ss onward, Shh expression in the ventral endoderm extends largely beyond the prosencephalic–mesencephalic boundary (Fig. 1 A′–C′). To investigate the role of Shh derived from the foregut endoderm in the development of the facial skeleton, we removed the entire forehead in chicken embryos in ovo before or after 7-ss (Fig. 1 D and E). When the forehead was excised at 5- to 6-ss (n = 4), the entire rostral endoderm expressing Shh was removed, together with other sources of this morphogen: the PcP, No, and FP (Fig. 1 D and F). Forty-eight hours after the operation (E3.5), the embryos were completely deprived of Shh transcripts in both the anterior pharyngeal endoderm and the oral epithelium (Fig. 2G and G′), in contrast to control embryos (Fig. 2 F and F′). When the operation was performed in 8- to 10-ss embryos (8-ss, n = 2; 9-ss, n = 1; 10-ss, n = 2), a ventral endodermal source of Shh was maintained in the anterior pharyngeal endoderm caudally to the excision (Fig. 1 E and G), and, later on, at E3.5, Shh transcripts were found in the anterior pharyngeal endoderm of BA1 and oral epithelium (Fig. 2 H and H′) as in controls. These results suggest that Shh expression in these tissues depends on the presence of Shh in the anterior ventral foregut endoderm.
Fig. 2.
Role of Shh from poregot endoderm on NCC survival and facial skeletogenesis. (A–D) Cell death analysis by Nile blue sulfate (NBS) on chicken embryos in toto. (A) E2.5 control chicken embryo. (B–E) NBS staining was strong in BA1 (‘1’) of embryos whose foreheads were excised at 6-ss (white arrowhead) (B) but not at 8-ss (C) or after forehead excision at 6-ss and replacement by heparin beads (white arrowheads) soaked with Shh (100 μg/ml) (D and E). However, cell death was abundant in the remaining of the anterior head region (B–D, white arrows). (F–I) Whole-mount in situ hybridization for Shh at E3.5 showing the presence of Shh transcripts at the level of the oral cavity (OC) in a control embryo (F), an embryo excised at 8-ss (H), and a 6-ss excised embryo grafted with an Shh bead (I) compared with a nongrafted embryo (G), which does not show Shh expression at the level of the oral cavity. (F′–I′) Cross sections (50 μm) at the level of BA1 (arrows in F–I). Shh mRNAs are present in the pharyngeal (Ph) endoderm of a control embryo (F′), an embryo excised at 8-ss (H′), and an Shh-grafted embryo (I′). In contrast, an embryo excised at 6-ss (G′) is deprived of Shh mRNAs in BA1 pharyngeal endoderm. (J–Q) Morphology (J–M) and skeletal (N–Q) analyses of E11 to E12 embryos by using alcian blue staining for cartilage and alizarin red for bone. (K and O) Absence of upper and lower beak in an embryo excised at 6-ss. The proximal part of Meckel’s cartilage (quadratoarticular cartilage) alone was preserved (O, black arrow). (L and P) After forehead excision at 8-ss, a normal lower beak similar to control (J and N) developed, also with the presence of hypoplasic bone elements of the maxilla (white arrow). (M and Q) An E12 embryo that was excised at 6-ss and treated with an Shh bead; cartilages and bones corresponding to mandible and maxilla are present.
When the surgically altered embryos were grown up to E9–E11, we could see that the embryos that were operated on at 5- to 6-ss were devoid of the upper and lower beak. Only quadrate and articular cartilages had developed (n = 8 of 8) (Fig. 2 K and O). The embryos operated on at 8- to 10-ss were deprived of the upper beak but had a lower beak (n = 5 of 5) (Fig. 2 L and P) endowed of normal skeletal structures. The upper beak was devoid of nasal septum and premaxilla, normally derived from the frontonasal bud. It seemed, therefore, that Shh derived from the remaining part of foregut endoderm was critical for BA1 development to proceed.
Absence of Foregut Endoderm Does Not Disturb Cephalic NCC Migration into BA1 but Prevents NCC Survival.
To understand why chicken embryos deprived of the entire forehead region before 7-ss do not develop a jaw but the NCCs that normally colonize BA1 are untouched by the operation, we looked at NCC migration in BA1 at E2.5 by using HNK1 mAb. Embryos that were operated on before 7-ss (n = 4) showed numerous NCCs in BA1 (Fig. 7, which is published as supporting information on the PNAS web site). However, Nile blue sulfate staining showed massive cell death in BA1 (but not in BA2) in surgically altered embryos as compared with control embryos (Fig. 2 A and B). In contrast, embryos that were operated on after 7-ss developed normal BA1, and only a limited amount of cell death was seen (Fig. 2C). In E3.5 embryos excised before 7-ss, BA1s were of smaller size than their counterparts from control embryos and from embryos operated on after 7-ss (Fig. 2 F–H). In all embryos, including controls, BA2, BA3, and BA4 showed equivalent amounts of cell death, localized mainly in the proximal area of branchial arches (Fig. 8, which is published as supporting information on the PNAS web site). Thus, Shh from the foregut endoderm is not necessary for NCC migration in BA1 but is crucial for NCC survival. In contrast, excision of the foregut endoderm and withdrawal of this source of Shh does not affect the development of posterior arches and particularly of BA2, as demonstrated by the normal development of the hyoid bone in embryos operated on before 7-ss (Fig. 2O).
Recombinant Shh Can Replace the Foregut Endoderm in BA1.
Early excision of the forehead in chicken embryos at 5- to 6-ss prevents BA1 development. In contrast, excision at later stages (8- to 10-ss) (when the Shh-producing territory of the foregut endoderm has extended caudally) is followed by lower jaw development. We hypothesized that Shh from the ventral foregut endoderm was a required signal for this process. To test this hypothesis, we substituted the forebrain region with heparin beads soaked with 100 μg/ml recombinant mouse Shh in chicken embryos excised before 7-ss. The Shh beads were inserted in close contact with the most anterior region of the remaining ventral endoderm and ectoderm (Fig. 2E). Some embryos were treated with heparin beads soaked with PBS-BSA as a control. Nile blue sulfate staining of E2.5 treated embryos showed, as expected, that Shh beads could rescue NCCs from death in BA1 (n = 5) (Fig. 2D). Interestingly, Shh expression is restored in BA1 endoderm of these embryos (Fig. 2 I and I′). Moreover, in E10–E12 Shh-treated embryos, formation of a beak (n = 7 of 10) with the cartilages and bones derived from BA1 took place (Fig. 2 M and Q). In contrast, control embryos grafted with PBS-BSA-soaked beads (n = 3) showed massive cell death and developed only the proximal structures of BA1, such as the quadrate-articular process, as did nontreated embryos operated before 7-ss (not shown). These results show that the forehead exerts a critical effect on lower jaw development through Shh signaling originating from the foregut endoderm. Moreover, they suggest that the initial origin of Shh in the foregut comes from an induction by the PcP-produced Shh, which can be replaced by an exogenous source of this signaling morphogen. Finally, either the PcP or the Shh-soaked beads are able to induce and propagate the production of Shh in the ventral endoderm.
Shh Is Upstream of a Signaling Cascade in Jaw Development.
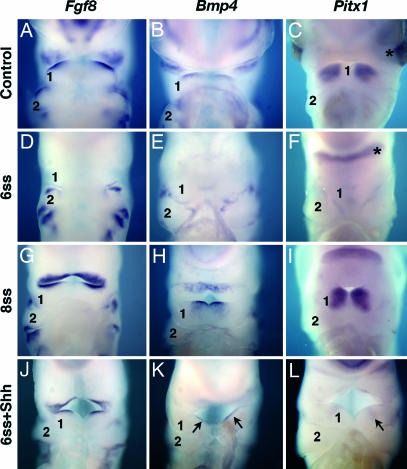
Because Shh beads were capable of restoring Shh expression in BA1 endoderm of embryos excised before 7-ss, we further investigated the changes occurring in gene expression in the absence of the initial ventral endodermal source of Shh. Thus, we analyzed the expression of a marker of BA1 development, the transcription factor Pitx1, and of Fgf8 and Bmp4, which are involved in the proximodistal patterning of BA1 (25). The observations were performed on forehead-deprived and control embryos at E3.5 and E4.5. Transcripts of Fgf8, Bmp4, and Pitx1 were down-regulated in BA1 ectoderm, oral epithelium, and mesenchyme in the embryos excised before 7-ss (Fig. 3D–F), whereas they were normally expressed in embryos excised after 7-ss (Fig. 3 G–I). In experimental embryos treated with Shh beads, the expression of Fgf8, Bmp4, and Pitx1 was restored in BA1 (Fig. 3 J–L). Pitx1 expression at E4.5 in the postoptic region of 6-ss excised embryos was not down-regulated, but, in the absence of eyes, the two lateral foci of expression associated to the eyes in controls were fused in a single transversal spot (Fig. 3F), showing that this expression was independent of endoderm-derived Shh. These results show that Shh derived specifically from the foregut endoderm has a critical role on NCC survival, thus allowing the patterning of BA1 and the formation of the beak.
Fig. 3.
Whole-mount in situ hybridization in E3.5 to E4.5 chicken control and surgically altered embryos. (A–I) Fgf8, Bmp4, and Pitx1 mRNAs are present in the oral epithelium and ectoderm of BA1 in control (A–C) and forehead-excised embryos after 8-ss (G–I) but not in embryos whose foreheads were excised at 6-ss (D–F). (J–L) Embryos whose foreheads were excised at 6-ss and replaced by a Shh bead show patterns of expression of those genes (black arrows) and a well developed BA1 (‘1’), in contrast to nontreated embryos, which exhibit a reduced sized BA1 and misexpression of these genes (D–F). An asterisk marks the expression of Pitx1 in the suborbital zone in F. In the absence of eyes, the two foci of expression join in a single transversal spot.
Discussion
We show in this report that Shh derived from the anterior ventral foregut endoderm is an early and necessary signal for jaw development. Surgical ablation of the entire forehead region in the chicken embryo up to 7-ss did not affect the migration of the cephalic NCCs into BA1 but prevented their survival and the formation of maxilla and mandible. Heparin beads soaked with mouse recombinant Shh that were deposited close to the pharyngeal endoderm after forehead excision rescued cell death in BA1 and allowed the development of a lower beak and maxilla with its skeletal elements. This treatment was able to induce expression of Shh in BA1 endoderm and expression of Fgf8, Bmp4, and Pitx1 in the ectoderm. Expression of these genes was down-regulated in the excised embryos.
The role of Shh on head development was first evidenced in Shh mutant mice that presented with holoprosencephaly, cyclopia, and absence of head skeleton (11). Facial malformations were also observed in humans with Shh mutations (6, 7). Although these observations demonstrated that cranial NCCs were not capable of forming cartilage and bones in the absence of Shh, it was impossible to determine which one among the different sources of Shh in the developing head (PcP, No, FP, or gut endoderm) had a role on facial development and by which mechanisms and at what time this action occurred.
In our work, we first focused on the chronological pattern of Shh expression in the foregut ventral endoderm. At 5-ss, Shh transcripts were detected in the anterior foregut ventral endoderm corresponding to the level of the presumptive forebrain in close contact with the PcP. At 6-ss, Shh transcripts were present in the ventral anterior endoderm and, more caudally, in the AIP but not between these two spots. Curiously, at these stages, the Shh expression domain progressed moderately in a caudal direction within the ventral endoderm and remained restricted to the anterior part of the foregut. It extended within the mesencephalic level from 8-ss onward. Thus, during cranial NCC delamination and migration (6- to 13-ss) (3), the ventral foregut endoderm comprises two regions: an anterior region that expresses Shh and a more posterior region that is negative for Shh (up to 16-ss) with the exception of the AIP, which is always Shh positive. These observations can be related to previous works that showed that different anteroposterior regions of the foregut endoderm present different capacities to support skeletal derivative development (20–22). Furthermore, replacement of the entire prosencephalic region (plus the anteriormost part of the mesencephalon, including the ectoderm, mesoderm, and endoderm) in the 6-ss chicken embryo by its quail counterpart showed that the BA1 endoderm expressing Shh at E3.5 originates from the quail-labeled region from the rostral foregut. This result shows that the anterior foregut endoderm expressing Shh gives rise to the endoderm of the first branchial pouch, including zone I–II and at least part of zone III as described in ref. 20.
The consequences of forehead excision experiments were dramatically different according to the stage at which they were performed. Up to 7-ss, when Shh is strictly limited to the forebrain region, no jaw developed. In contrast, when the operation was performed later (8- to 10-ss), Shh expression had already progressed caudally, and a jaw developed. When the initial anteroventral focus of Shh expression associated with the PcP is no longer in continuity with the foregut endoderm, the latter does not express this gene. This natural source of Shh can, however, be replaced by exogenous morphogen. Shh-soaked heparin beads placed in contact with the sectioned foregut were able to initiate Shh production by ventral foregut cells. This treatment is sufficient for insuring the survival of BA1-invading NCCs and the development of the jaw skeleton. This result strongly suggests that Shh induces the Shh gene to be transcribed, an observation already documented in various other systems (see refs. 9 and 26 and references therein). Moreover, the survival effect of Shh on NCCs has also been shown to take place during head development (13–15).
In the casanova mutant of zebrafish, which is deprived of the endoderm, the viscerocranial skeleton is completely absent, whereas the neurocranium is only slightly affected (24). In contrast, inhibition of Shh by neutralizing Ab in the neuroectoderm and facial ectoderm resulted in the hypoplasia of the nasal septum, but the lower beak developed normally (18). Taken together, these data and our results show that the development of the upper face is controlled by Shh derived from the neurectoderm and facial ectoderm, whereas lower face development is controlled by Shh derived from the ventral foregut endoderm.
Our approach using Shh beads as a source of Shh raises the question of the long-term action of this protein on the BA1 morphogenetic program. Shh beads are able to rescue cranial NCCs from death in BA1 and allow the formation of a lower beak. Later on, the presence of Shh turned out to be critical for insuring a normal expression pattern of genes controlling the proximodistal regionalization of BA1 ectoderm and its specification. Bmp4, Fgf8, and Pitx1 transcripts, which were absent in the BA1 ectoderm and mesenchyme in 6-ss excised embryos, were present in embryos that were operated on at later stages. Moreover, Shh beads rescued the expression pattern of these genes in embryos that were operated on early. These data suggest that Shh expressed in BA1 endoderm after grafting Shh beads has a role on the patterning of BA1 ectoderm. Thus, Shh from BA1 endoderm presents distinct roles, depending on the tissue considered: It acts as a survival signal for NCCs, as already shown in chicken and mouse (13, 14), and also for the endoderm itself (15), whereas it is an upstream signal responsible for inducing the expression of specific genes in BA1 ectoderm. Among these genes, Fgf8 was previously shown to be essential for proliferation and differentiation of BA1 NCCs (4). Our results are in agreement with the observation that, in Shh mutant mice, expression of Fgf8, Bmp4, Pax1, Twist, and Sox9 was lost in BA1 (15, 16). Moreover, it was recently shown that hedgehog signaling has a crucial role on the patterning of zebrafish stomodeum and, consequently, on jaw development (27). On the other hand, a contact between the ventral foregut endoderm expressing Shh and the oral ectoderm takes place as early as 6-ss and is followed by the expression of Shh in the ectoderm from 12-ss onward (28). Later on, this interaction will be responsible for the proximodistal regionalization of the oral epithelium (29).
In conclusion, the present work shows that Shh derived specifically from the ventral foregut endoderm is a critical and early signal for jaw development and morphogenesis. The expression of Shh in the foregut endoderm is induced by a more anterior source, which is likely to be the PcP. As soon as Shh expression is established in the ventral foregut endoderm (8-ss), a lower beak can develop even if the entire forehead region is removed, suggesting that, at this stage, upper and lower face morphogenesis are two independent processes. The ventral source of Shh is then responsible for at least two steps in BA1 development: the survival of cranial NCCs migrating in BA1 and the patterning of BA1 ectoderm and oral epithelium.
Materials and Methods
Microsurgery.
Forehead surgical excisions alone were performed in chicken embryos in ovo at 5- to 6-ss and 8- to 10-ss. The limit between the future prosencephalon and mesencephalon is not well defined at these stages, and the excised territory included part of the future midbrain. If compared with the experiments described in ref. 20, the foregut endoderm excised in the present work corresponds to zones I and II and part of zone III. Forehead grafts were performed from quail to chicken embryos in ovo at 5- to 7-ss according to techniques described in ref. 30. Surgically altered embryos were incubated again and killed at E2.5–E4.5 for whole-mount or section immunochemistry or in situ hybridization analyses and at E9–E12 for morphology and skeleton studies.
Shh Treatment of Excised Embryos.
Heparin acrylic beads (≈120 μm in diameter; Sigma, St. Louis, MO) were implanted in contact with the ventral endoderm and ectoderm of the 5- to 6-ss excised embryos. Heparin beads were soaked with 100 μg/ml mouse recombinant Shh (R & D Systems, Minneapolis, MN) and incubated for 1 h at 37°C and then overnight at 4°C before utilization. Beads treated with PBS were used as a control.
Embryo Processing.
In situ hybridization analyses on whole-mount embryos or sections with Fgf8 (31), Shh (9), Bmp4 (32), and Pitx1 (33) were performed as described in ref. 26. Whole-mount preparations and paraffin sections were treated for immunocytochemistry with either QCPN (for quail cells) or HNK1 mAb (for NCCs) as described in ref. 4. Whole-mount cell death detection was performed by Nile blue sulfate staining as described in ref. 34. When needed, cartilaginous structures subjected to immunocytochemistry with QCPN mAb on sections were counterstained with alcian blue. Whole-mount skeletons were visualized according to standard staining protocols by using alcian blue for cartilage and alizarin red for bone.
Supplementary Material
Acknowledgments
We thank Sophie Gournet and Michel Fromaget for illustrations. This work was supported by fellowships from the Fondation pour la Recherche Médicale and Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (to J.M.B.) and by the Centre National de la Recherche Scientifique and the Association pour la Recherche Contre le Cancer.
Abbreviations
- NCC
neural crest cell
- Shh
Sonic hedgehog
- PcP
prechordal plate
- BA1
first branchial arch
- En
embryonic day n
- ss
somite stage
- AIP
anterior intestinal portal.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Couly G., Grapin-Botton A., Coltey P., Le Douarin N. M. Development (Cambridge, U.K.) 1996;122:3393–3407. doi: 10.1242/dev.122.11.3393. [DOI] [PubMed] [Google Scholar]
- 2.Kontges G., Lumsden A. Development (Cambridge, U.K.) 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- 3.Le Douarin N. M., Kalcheim C. The Neural Crest. Cambridge, U.K.: Cambridge Univ. Press; 1999. [Google Scholar]
- 4.Creuzet S., Schuler B., Couly G., Le Douarin N. M. Proc. Natl. Acad. Sci. USA. 2004;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etchevers H. C., Couly G., Vincent C., Le Douarin N. M. Development (Cambridge, U.K.) 1999;126:3533–3543. doi: 10.1242/dev.126.16.3533. [DOI] [PubMed] [Google Scholar]
- 6.Belloni E., Muenke M., Roessler E., Traverso G., Siegel-Bartelt J., Frumkin A., Mitchell H. F., Donis-Keller H., Helms C., Hing A. V., et al. Nat. Genet. 1996;14:353–356. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- 7.Roessler E., Belloni E., Gaudenz K., Jay P., Berta P., Scherer S. W., Tsui L. C., Muenke M. Nat. Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 8.Echelard Y., Epstein D. J., St-Jacques B., Shen L., Mohler J., McMahon J. A., McMahon A. P. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 9.Riddle R. D., Johnson R. L., Laufer E., Tabin C. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 10.Roelink H., Augsburger A., Heemskerk J., Korzh V., Norlin S., Ruiz i Altaba A., Tanabe Y., Placzek M., Edlund T., Jessell T. M., et al. cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 11.Chiang C., Litingtung Y., Lee E., Young K. E., Corden J. L., Westphal H., Beachy P. A. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 12.Litingtung Y., Lei L., Westphal H., Chiang C. Nat. Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 13.Ahlgren S. C., Bronner-Fraser M. Curr. Biol. 1999;9:1304–1314. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- 14.Jeong J., Mao J., Tenzen T., Kottmann A. H., McMahon A. P. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore-Scott B. A., Manley N. R. Dev. Biol. 2005;278:323–335. doi: 10.1016/j.ydbio.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Washington Smoak I., Byrd N. A., Abu-Issa R., Goddeeris M. M., Anderson R., Morris J., Yamamura K., Klingensmith J., Meyers E. N. Dev. Biol. 2005;283:357–372. doi: 10.1016/j.ydbio.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Chen W., Burgess S., Hopkins N. Development (Cambridge, U.K.) 2001;128:2385–2396. doi: 10.1242/dev.128.12.2385. [DOI] [PubMed] [Google Scholar]
- 18.Marcucio R. S., Cordero D. R., Hu D., Helms J. A. Dev. Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Cordero D., Marcucio R., Hu D., Gaffield W., Tapadia M., Helms J. A. J. Clin. Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couly G., Creuzet S., Bennaceur S., Vincent C., Le Douarin N. M. Development (Cambridge, U.K.) 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- 21.Graveson A. C., Armstrong J. B. Differentiation. 1987;35:16–20. doi: 10.1111/j.1432-0436.1987.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 22.Seufert D. W., Hall B. K. Cell Differ. Dev. 1990;32:153–165. doi: 10.1016/0922-3371(90)90109-a. [DOI] [PubMed] [Google Scholar]
- 23.Bee J., Thorogood P. Dev. Biol. 1980;78:47–62. doi: 10.1016/0012-1606(80)90317-6. [DOI] [PubMed] [Google Scholar]
- 24.David N. B., Saint-Etienne L., Tsang M., Schilling T. F., Rosa F. M. Development (Cambridge, U.K.) 2002;129:4457–4468. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- 25.Wall N. A., Hogan B. L. Mech. Dev. 1995;53:383–392. doi: 10.1016/0925-4773(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 26.Charrier J. B., Lapointe F., Le Douarin N. M., Teillet M. A. Development (Cambridge, U.K.) 2002;129:4785–4796. doi: 10.1242/dev.129.20.4785. [DOI] [PubMed] [Google Scholar]
- 27.Eberhart J. K., Swartz M. E., Crump J. G., Kimmel C. B. Development (Cambridge, U.K.) 2006;133:1069–1077. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- 28.Withington S., Beddington R., Cooke J. Development (Cambridge, U.K.) 2001;128:309–320. doi: 10.1242/dev.128.3.309. [DOI] [PubMed] [Google Scholar]
- 29.Haworth K. E., Healy C., Morgan P., Sharpe P. T. Development (Cambridge, U.K.) 2004;131:4797–4806. doi: 10.1242/dev.01337. [DOI] [PubMed] [Google Scholar]
- 30.Martinovitch P. N., Pavlovic M. R. Nature. 1958;182:571–572. doi: 10.1038/182571b0. [DOI] [PubMed] [Google Scholar]
- 31.Crossley P. H., Minowada G., MacArthur C. A., Martin G. R. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 32.Francis P. H., Richardson M. K., Brickell P. M., Tickle C. Development (Cambridge, U.K.) 1994;120:209–218. doi: 10.1242/dev.120.1.209. [DOI] [PubMed] [Google Scholar]
- 33.Henrique D., Adam J., Myat A., Chitnis A., Lewis J., Ish-Horowicz D. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- 34.Jeffs P., Jaques K., Osmond M. Anat. Embryol. 1992;185:583–588. doi: 10.1007/BF00185617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.