Abstract
Activation of naïve T cells generally requires T cell receptor-mediated contact with MHC-bound peptides on viable antigen-presenting cells such as dendritic cells (DC). Here evidence is presented that dissociated cell membrane fragments from a DC line can be used as an effective substitute for viable DC. Ultracentrifuged material derived from sonicates of IFN-γ-matured DC is enriched in small membrane vesicles that closely resemble exosomes. When complexed with MHC class I-restricted specific peptide, vesicles from DC sonicates generate strong responses by purified naïve CD8+ cells in vitro in the absence of normal antigen-presenting cells and can also efficiently prime T cells for tumor rejection in vivo. Both in terms of total yields from DC and relative immunogenicity, membrane vesicles from DC sonicates are much more effective than classic exosomes and may be a valuable tool for tumor immunotherapy.
Keywords: immunotherapy, T cell priming, tumors
T cell activation requires T cell receptor (TCR) recognition of peptide/MHC ligands plus costimulation resulting from the interaction of various molecules on T cells, e.g., CD28, with complementary molecules on dendritic cells (DC), e.g., B7-1 (CD80) and B7-2 (CD86) (1–3). Contact with these ligands drives T cells to proliferate and differentiate into effector cells.
In addition to responding to pathogens and other foreign antigens, T cells have specificity for a spectrum of self-antigens, including tumor-associated antigens (4–8). Although self-reactivity of T cells is generally suppressed by stringent tolerance mechanisms (2, 9–12), T cell responses to tumor-associated antigens can be induced by injection of antigen- or peptide-loaded DC (5, 6, 13, 14). DC-based immunotherapy can be highly effective for tumor rejection in certain situations, but there are intrinsic drawbacks with this approach. In addition to problematic long-term storage, DC generated ex vivo home poorly after s.c. injection, although the few cells that reach the draining lymph nodes (LN) generate effective T cell responses (15–17). DC injection i.v. leads to efficient homing to the spleen, but T cell responses in the spleen may fail to eliminate s.c. tumors (15–17).
The problem of suboptimal homing of DC can be avoided by the use of exosomes secreted by DC (18, 19). DC-derived exosomes can be stored for prolonged periods in vitro and generate efficient antitumor responses after s.c. injection in vivo. Exosomes are secreted from viable cells, but total yields of exosomes are quite low, which limits their clinical use. Exosome yields are especially restricted for mature DC, and for this reason exosomes are generally prepared from immature DC. Because immature DC express only low levels of costimulatory molecules, the immunogenicity of exosomes from these cells is indirect and requires uptake and presentation of antigen by mature host DC.
In considering alternatives to injecting exosomes or intact DC, it is notable that the direct immunogenicity of peptide-loaded mature DC in vitro is resistant to cell fixation (20, 21). Hence, the immunogenicity of these cells presumably reflects their dense expression of MHC/peptide plus high levels of costimulatory/adhesion molecules. If so, one might expect that the direct immunogenicity of mature DC could be mimicked by plasma membrane fragments from these cells. In line with this prediction, using a DC line, DC2.4 (22), we show here that ultracentrifuged vesicles derived from sonicates of mature DC are strongly immunogenic for naïve T cells both in vitro and in vivo. Such vesicles are directly immunogenic in the absence of antigen-presenting cells (APC), at least in vitro, and are obtainable in much larger quantities than exosomes.
Results
Preparation of Membrane Vesicles.
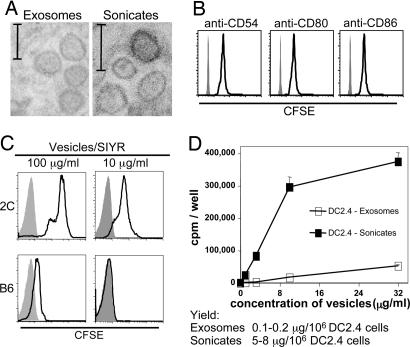
To prepare membrane vesicles from DC2.4 cells, cells were disrupted with a Dounce homogenizer. After removal of nuclei by light centrifugation, supernatants were sonicated and then centrifuged at 10,000 × g. Thereafter, the supernatants were subjected to ultracentrifugation (100,000 × g) for 1 h. Electronmicroscopic examination of the pelleted material showed a heterogenous mixture of membrane fragments and small organelles. Based on examining multiple sections throughout the pellet, approximately one-third of the material had the morphology of small (50- to 100-nm) round membrane vesicles (Fig. 1A Right). Of the remaining material, the lighter (upper) portion of the pellet also contained ribosomes and small irregular membrane fragments whereas the heavier (lower) portion consisted mostly of larger membrane fragments. Interestingly, the round membrane vesicles closely resembled classic exosomes released from intact DC2.4 (Fig. 1A Left). For the functional studies discussed below, pellets of ultracentrifuged membrane vesicles from DC2.4 sonicates and DC2.4 exosomes were resuspended in saline.
Fig. 1.
Comparison of exosomes and sonicates from DC2.4 cells. (A) EM images of exosomes and sonicates prepared from IFN-γ-stimulated DC2.4 cells. (Scale bars: 100 nm.) Representative sections from the midsection of the ultracentrifuged pellets are shown. (B) Binding of sonicates from CFSE-labeled DC2.4 cells to synthetic beads coated with mAbs specific for CD54, CD80, or CD86. Shaded areas represent fluorescence activity of beads coated with isotype control mAb. Note that CFSE is used solely as a marker and not to measure proliferation. (C) Binding of sonicates from CFSE-labeled DC2.4 cells to 2C CD8+ cells and normal B6 CD8+ cells in the presence of vesicles plus 0.32 μM SIYR. (D) Proliferation of 2C CD8+ cells to 0.32 μM SIYR plus titrated amounts of exosomes and sonicates from DC2.4 cells. Data show [3H]thymidine incorporation (cpm) by 5 × 104 2C cells per well at 72 h (mean of triplicate culture ± SD) with addition of [3H]thymidine during the last 8 h of culture. Total yields (protein concentration) of exosomes and sonicates are shown at the bottom.
Expression of Costimulatory Molecules.
Surface expression of MHC class I and costimulatory molecules on intact DC2.4 cells was only modest but became conspicuous after overnight incubation with various Toll-like receptor agonists or IFN-γ (data not shown). IFN-γ treatment was particularly effective, and, unless stated otherwise, all DC2.4 cells used to prepare membrane vesicles from cell sonicates were preincubated overnight with IFN-γ. As shown by labeling of DC2.4 cells with carboxyfluorescein succinimidyl ester (CFSE) before sonication, the membrane vesicles from the cells expressed MHC class I (data not shown) as well as several costimulatory/adhesion molecules, including CD54, CD80, and CD86 (Fig. 1B).
Binding to T Cells.
As found previously for exosomes (23, 24), naïve CD8+ T cells were able to bind membrane vesicles from DC2.4 cells in vitro but only in the presence of specific peptide. Thus, naïve 2C TCR transgenic CD8+ cells, which have specificity for MHC class I Kb plus SIYRYYGL (SIYR) peptide, showed strong binding of CFSE-labeled DC2.4 (Kb) sonicated vesicles in the presence of SIYR peptide (vesicles/SIYR) (Fig. 1C). By contrast, uptake of vesicles/SIYR by normal polyclonal B6 CD8+ cells was negligible.
Immunogenicity of Membrane Vesicles Versus Exosomes.
In previous studies, purified naïve 2C CD8+ cells responded well in the absence of APC to peptide-pulsed exosomes released from transfected Drosophila cells and also from normal DC (23). These data applied to 2C responses to MHC class I Ld and the strong QL9 peptide. DC2.4 exosomes and SIYR peptide also elicited proliferation from purified 2C CD8+ cells, albeit at a low level (Fig. 1D). This response correlates with Kb/SIYR being a weaker ligand for the 2C TCR than Ld/QL9 (25). Significantly, membrane vesicles prepared from DC2.4 sonicates were strongly stimulatory for 2C CD8+ cells (Fig. 1D).
The key finding in the above experiment is that, in the presence of specific peptide, sonicates from DC2.4 cells were strongly stimulatory for naïve 2C CD8+ cells in vitro in the absence of APC. Sonicates were clearly superior to exosomes in two respects. First, in terms of protein concentration, sonicates were more potent than exosomes by a factor of 10- to 30-fold. Second, total yields of immunogenic material per 106 cells were ≈50-fold higher for sonicates than for exosomes (Fig. 1D, below graph). All of the experiments discussed below refer to vesicles prepared from ultracentrifuged DC2.4 sonicates. For simplicity these preparations are referred to as “vesicles.”
Features of in Vitro Responses to Vesicles.
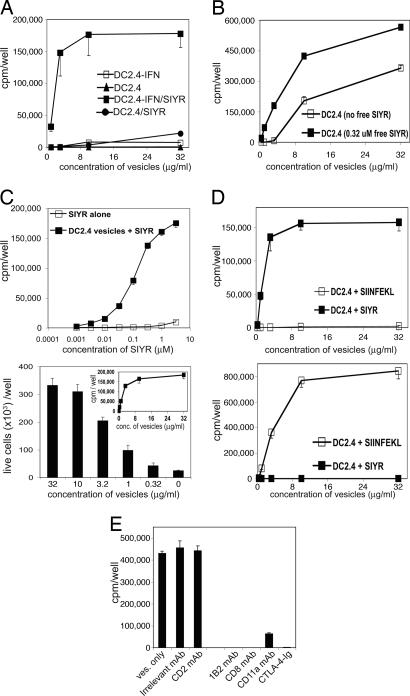
Vesicles prepared from unstimulated DC2.4 were poorly immunogenic for 2C cells, as seen by low proliferative responses to SIYR peptide (Fig. 2A). In contrast, vesicles prepared from IFN-γ-induced DC2.4 cells were highly immunogenic for 2C cells, correlating with the higher level of costimulatory molecules on these cells.
Fig. 2.
Peptide-specific immunogenicity of sonicated membrane vesicles from DC2.4 cells. (A) Effect of pretreating DC2.4 cells with IFN-γ. Membrane vesicles were prepared from untreated DC2.4 cells (DC2.4) or DC2.4 cells that had been incubated for 24 h with IFN-γ at 10 ng/ml (DC2.4-IFN). The data show proliferation of 2C cells cultured for 72 h with graded concentrations of vesicles plus 0.32 μM SIYR (DC2.4/SIYR and DC2.4-IFN/SIYR) or without SIYR. (B) Effect of adding SIYR to DC2.4 cells before versus before and after vesicle preparation. DC2.4 cells were preincubated for 72h with a combination of IFN-γ and 2.5 μM SIYR peptide and then sonicated to prepare membrane vesicles. The data show cpm of 2C CD8+ cells to vesicles with or without added free SIYR at 0.32 μM. (C) Influence of peptide concentration on proliferative response to vesicles. (Upper) Proliferation of 2C CD8+ cells cultured with graded concentrations of SIYR peptide with or without a fixed concentration of 10 μg/ml membrane vesicles. (Lower) Total numbers of live 2C cells after culture with the indicated concentration of vesicles plus a fixed concentration of 0.32 μM SIYR. (Inset) Proliferation (cpm) under the same conditions. (D) Peptide specificity of CD8+ cell responses to vesicles. The data show proliferation of 2C CD8+ cells (Upper) and OT-1 CD8+ cells (Lower) to vesicles plus either SIYR or SIINFEKL peptide (both at 0.32 μM). (E) Receptor/ligand interactions involved in 2C responses to vesicles plus SIYR. The data show proliferation of 2C CD8+ cells to vesicles (10 μg/ml) and SIYR (0.32 μM) in the presence of the indicated mAbs (5 μg/ml).
The above findings applied to peptide addition after vesicle preparation. Substantial, although lower, responses were elicited by IFN-γ-induced DC2.4 cells that were peptide-pulsed before sonication (Fig. 2B). The addition of extra peptide during culture considerably enhanced proliferation of 2C cells in response to the vesicles (Fig. 2B), implying significant elution of Kb-associated peptide during vesicle preparation. In general, adding peptide both before and after vesicle preparation was only slightly more effective than adding peptide just during T cell culture. Therefore, peptide was routinely added to vesicles only during the culture period with T cells.
In most experiments SIYR peptide was added to culture at 0.32 μM. This concentration of peptide was nonstimulatory in the absence of vesicles yet elicited nearly optimal responses in the presence of vesicles (Fig. 2C Upper). Higher concentrations of peptide (>1 μM) induced proliferation of 2C CD8+ cells in the absence of vesicles, presumably reflecting peptide presentation by the responding cells themselves. With respect to vesicle concentration, optimal proliferative responses of 2C CD8+ cells occurred with vesicles at 10 μg/ml for both [3H]thymidine incorporation and total yields of live cells (Fig. 2C Lower). However, proliferation was observed with concentrations of vesicles as low as 0.3 μg/ml.
Stimulation of CD8+ cells by vesicles was strongly peptide-specific. Thus, 2C cells responded well to vesicles/SIYR but not to vesicles/SIINFEKL peptide (Fig. 2D Upper). Conversely, OT-1 CD8+ cells responded to vesicles/SIINFEKL but not to vesicles/SIYR (Fig. 2D Lower).
Because DC2.4 cells express a variety of costimulatory/adhesion molecules, it was of interest to determine which of these molecules were important during vesicle stimulation of 2C cells. Proliferative responses to vesicles/SIYR were blocked or greatly reduced by CTLA-4–Ig and anti-CD11a mAb (Fig. 2E), indicating the importance of both CD28/B7 and lymphocyte function-associated antigen 1 (LFA-1)/CD54 interactions. Inhibition by anti-CD2 mAb was minimal, suggesting little or no contribution from CD2. As expected, proliferation was abolished by 1B2 anticlonotypic mAb and also by anti-CD8 mAb.
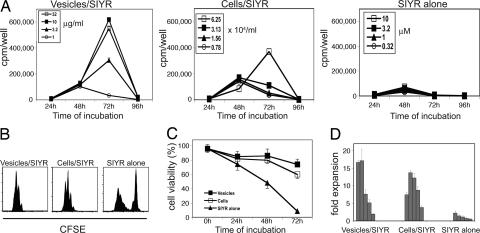
In the above experiments the responding T cells were highly purified and depleted of APC, implying that when loaded with specific peptide the vesicles were directly immunogenic for 2C cells. Hence, it was of interest to compare the response of 2C CD8+ cells to vesicles versus intact APC. This comparison is shown in Fig. 3. Here 2C CD8+ cells were cultured with titrated doses of vesicles/SIYR versus titrated numbers of intact IFN-γ-induced DC2.4 cells/SIYR. In both situations, 2C responses generally reached a peak on day 3 of culture. With intact DC2.4 cells/SIYR as APC, optimal proliferative responses required ≈6 × 104 cells per milliliter (1 × 104 per well). With vesicles/SIYR, comparable responses occurred with ≈3 μg/ml vesicles. These findings applied to [3H]thymidine incorporation, CFSE dilution, cell viability, and fold expansion of the responding T cells (Fig. 3 and data not shown). With SIYR peptide alone there was significant proliferation of a proportion of 2C cells, but most of the responding cells were nonviable by day 3 of culture (Fig. 3).
Fig. 3.
Comparison of the stimulatory activity of membrane vesicles versus intact DC2.4 cells. The data show proliferation of 2C CD8+ cells cultured with graded doses of vesicles plus 0.32 μM SIYR peptide (vesicles/SIYR), graded doses of intact irradiated IFN-γ-induced DC2.4 cells plus 0.32 μM SIYR (cells/SIYR), or graded amounts of SIYR without vesicles (SIYR alone). Proliferation was measured by [3H]-incorporation at 24–96 h (A), CFSE dilution of 2C cells at 72 h (B), 2C cell viability at 72 h (C), and fold expansion of 2C cells at 72 h (D) (relative to the number of cells initially cultured) in response to 3-fold dilutions of vesicles/SIYR (32 to 0.32 μg/ml), 2-fold dilutions of cells/SIYR (60 to 3.9 × 104 per milliliter), or 3-fold dilutions of SIYR alone (10 to 0.1 μM). 2C cells were cultured at 5 × 104 per well in 0.2-ml wells (A and D) or at 2 × 105 per well in 1-ml wells (B and C).
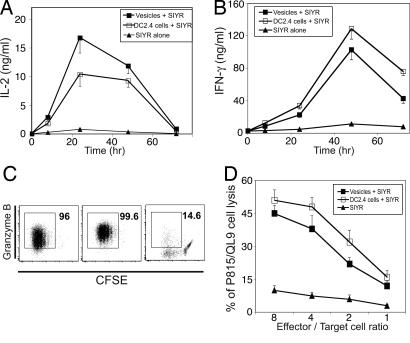
The above findings refer to T cell proliferation. Similar results were observed for differentiation of 2C cells into effector cells (Fig. 4). Thus, for vesicles/SIYR at 10 μg/ml and intact DC2.4 cells at 6.25 × 104 per milliliter, comparable 2C responses occurred with regard to IL-2 synthesis (Fig. 4A), IFN-γ synthesis (Fig. 4B), granzyme B synthesis (Fig. 4C), and lysis of peptide-loaded target cells (P815 cells expressing Ld plus QL9 peptide) (Fig. 4D).
Fig. 4.
Development of effector function of 2C CD8+ cells stimulated by membrane vesicles versus intact DC2.4 cells. Purified 2C CD8+ cells were stimulated with vesicles (10 μg/ml) plus 0.32 μM SIYR, intact irradiated IFN-γ-induced DC2.4 cells (6.25 × 104 per milliliter) plus 0.32 μM SIYR or with 0.32 μM SIYR alone. (A and B) 2C CD8+ cells were cultured at 5 × 104 per well, and culture supernatants were collected at 8–72 h to measure IL-2 (A) and IFN-γ (B) by ELISA. (C) Granzyme B synthesis was measured by culturing CFSE-labeled 2C CD8+ cells at 2 × 105 per well for 3 days with the above stimuli followed by fixing and permeabilizing the cells before staining for granzyme B. (D) Cells were cultured as for C, washed, and used as effector cells in a 51Cr-release assay with P815 (H-2d) cells pulsed for 1 h with 10 μM QL9 peptide.
Based on the above findings we conclude that vesicles plus peptide are strongly stimulatory for naïve CD8+ cells by all parameters measured. Qualitatively, responses to vesicles versus intact APC were indistinguishable.
In Vivo Responses.
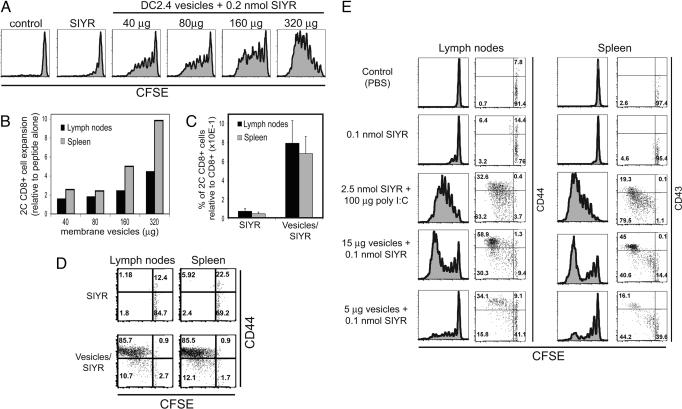
To examine responses in vivo, naïve CFSE-labeled 2C CD8+ cells were transferred i.v. into syngeneic B6 mice. One day later the recipients were injected i.v. with graded doses of vesicles plus a fixed amount of 0.2 nmol SIYR peptide or with SIYR alone. As shown in Fig. 5, a modest dose of vesicles (40 μg per mouse) plus peptide led to significant proliferation of TCR clonotype+ (1B2+) 2C cells as indicated by CFSE dilution and cell expansion measured on day 3 after priming (Fig. 5 A and B). With i.v. injection of vesicles, responses were more prominent in spleen than in LN, which presumably indicated that the vesicles lodged largely in the spleen. With a high dose of vesicles, virtually all of the injected 2C cells up-regulated CD44 and divided extensively in both spleen and LN, which contrasted with almost undetectable proliferation induced by peptide alone (Fig. 5 C and D). When 2C cells were injected i.v. and vesicles/SIYR were injected s.c., significant proliferative responses were apparent even with low doses of vesicles, i.e., 5 μg given in each rear footpad (Fig. 5E). By day 3 after s.c. vesicle injection, proliferation of 2C cells was apparent in spleen as well as the draining LN. In each site the proliferating cells up-regulated both CD44 (shown for LN) and CD43 (shown for spleen) (Fig. 5E).
Fig. 5.
Stimulation of 2C CD8+ cells by vesicles plus peptide in vivo. (A) CFSE-labeled 2C CD8+ cells were injected i.v. into syngeneic B6 recipients at 8 × 106 cells per mouse. One day later mice were injected i.v. with PBS (control), 0.2 nmol SIYR, or titrated doses of vesicles plus 0.2 nmol SIYR. Mice were killed 3 days later and analyzed for CFSE dilution of 1B2+ CD8+ cells in spleen and LN. Data for LN are shown. (B) Expansion of 1B2+ CD8+ cells in spleen and LN of mice injected with membrane vesicles relative to injection of peptide alone as in A. (C) CFSE-labeled 2C CD8+ cells (Ly5.2) were injected i.v. into B6.Ly5.1 recipients (4 × 106 per mouse). One day later mice were injected i.v. with 0.2 nmol SIYR or with 240 μg of vesicles plus 0.2 nmol SIYR. Mice were killed 3 days later. Numbers of donor Ly5.2+ CD8+ cells in LN and spleen relative to host Ly5.1+ CD8+ cells are shown. (D) CFSE versus CD44 expression for donor Ly5.2+ CD8+ 2C cells is shown for the same mice as in C. For C and D there were two mice per group. (E) CFSE-labeled Thy1.2+-marked 2C CD8+ cells were injected into Thy1.1+-marked B6 recipients at 4 × 106 cells per mouse. One day later mice were injected with PBS (Control), 0.1 nmol SIYR alone, 2.5 nmol SIYR plus 100 μg of poly I:C, or vesicles plus 0.1 nmol SIYR. SIYR plus poly I:C was injected i.p., whereas all other samples were injected into both footpads. Mice were killed 3 days later. CFSE profiles versus CD44 or CD43 expression of Thy1.2+ CD8+ cells are shown for inguinal LN or spleen, respectively. Data are representative of at least two independent experiments.
To examine effector function in vivo, we examined rejection of DP1 tumor cells, which are EL4 (H-2b) cells transfected with an SIYR peptide minigene (26). Preliminary experiments established that DP1 cells were rejected by normal syngeneic B6 mice but grew well in TAP−/− mice (data not shown); because TAP−/− mice are selectively depleted of CD8+ T cells (27), DP1 rejection is presumably controlled largely by CD8+ cells.
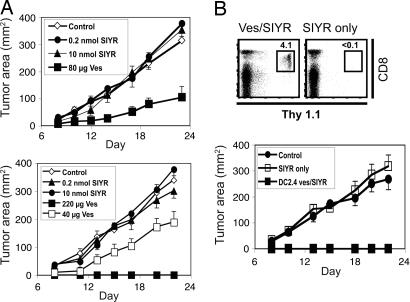
To measure tumor rejection, doses of 4 × 106 naïve 2C cells were transferred i.v. to B6.TAP−/− mice. After 1 day the host mice were injected i.v. with vesicles/SIYR or with SIYR alone; controls received PBS. At 3 days after 2C injection the hosts were injected s.c. with 2 × 106 DP1 tumor cells. In three separate experiments, two of which are shown in Fig. 6A, there was no reduction in tumor growth when 2C cells were injected alone or were coinjected with SIYR without vesicles, even with a high dose of 10 nmol peptide. With a modest dose of 80 μg of vesicles plus 0.2 nmol peptide per mouse, tumor growth was undetectable in 50% (four of eight) of the hosts (two of four in each of two experiments), and in the remaining hosts tumor growth was clearly reduced (Fig. 6A Upper). With a higher dose of vesicles (220 μg) tumor rejection was 100% (no growth in four of four mice) (Fig. 6A Lower).
Fig. 6.
Tumor rejection by 2C CD8+ cells. (A) A total of 4 × 106 purified 2C CD8+ cells were injected i.v. into TAP−/− mice. One day later mice were injected i.v. with PBS (Control), 0.2 nmol SIYR alone, 10 nmol SIYR alone, or vesicles plus 0.2 nmol SIYR. A total of 2 × 106 DP1 tumor cells were injected s.c. into these mice 3 days later, and tumor size was measured (n = 4 mice per group). For priming with vesicles the data refer to two of four mice; the other mice in this group did not develop tumors (Upper). Essentially identical results were seen in a second experiment (Lower). (B) Purified 2C CD8+ cells on a Thy1.1 background were stimulated in vitro with vesicles (10 μg/ml) plus 0.32 μM SIYR or 10 μM SIYR alone. After 3 days 4 × 106 in vitro activated cells were injected i.v. into B6 or TAP−/− mice. Fourteen days after transfer, survival of the donor (Thy1.1+) cells was measured in blood of B6 recipients (Upper); the data show the percentage of total white blood cells that were of donor Thy1.1+ origin. (Lower) TAP−/− recipients were then injected s.c. with 2 × 106 DP1 tumor cells 30 days after transfer. A total of 2 × 106 DP1 tumor cells were also injected s.c. into control TAP−/− mice. The data show tumor size (n = 4 mice per group).
The above data refer to tumor rejection by T cells primed in vivo. Efficient tumor rejection also applied to T cells that were activated by vesicles/SIYR in vitro before transfer in vivo. Here 2C cells were cultured for 3 days in vitro with vesicles plus a low concentration of SIYR (0.32 μM) or with a high concentration of SIYR (10 μM) without vesicles. After transfer to B6 hosts (without peptide), the donor (Thy1.1+-marked) 2C cells activated by vesicles/peptide were clearly apparent in blood by FACS analysis at day 14 after transfer (Fig. 6B Upper). Furthermore, complete rejection of DP1 tumor cells was observed in TAP−/− mice when the tumor cells were injected s.c. at 30 days after T cell transfer (Fig. 6B Lower). By contrast, percentages of donor 2C cells activated by peptide alone in vitro were negligible in the blood of B6 recipients at day 14 after transfer. Additionally, these T cells failed to retard tumor growth in TAP−/− recipients.
Discussion
As discussed earlier, DC-derived exosomes are proving a very useful tool for tumor immunotherapy, but their use is limited by low yields, especially from mature DC. Here we show that this problem can be overcome simply by degrading DC into small fragments by sonication. Discarding nuclei and larger debris yielded material that, after ultracentrifugation, closely resembled small membrane vesicles. These vesicles had the size and morphology of exosomes and were strongly immunogenic for CD8+ cells when pulsed with peptide. In parallel studies, comparable immunogenic vesicles were obtained from a transfected Drosophila cell line (unpublished observations). Here separation of the sonicates on sucrose gradients, plus the ability to retard vesicle immunogenicity with a pre-sonication trypsin treatment, implied that the vesicles were derived largely from the plasma membrane.
As for exosomes, vesicle immunogenicity from sonicates was strictly peptide-dependent and peptide-specific, with peptide added to cells before sonication or, more effectively, to the vesicles at the time of T cell stimulation. Notably, the immunogenicity of the vesicles after peptide loading applied to purified naïve CD8+ cells in the absence of APC. Under these conditions, T cell stimulation required that, in addition to MHC/peptide, the vesicles coexpressed certain costimulatory/adhesion molecules, especially B7 and CD54. To be strongly immunogenic the vesicles had to be prepared from cells that had been pretreated to up-regulate costimulatory molecules, e.g., with IFN-γ. This finding parallels the stimulatory function of intact DC, where, to be immunogenic, these cells must express high levels of costimulatory molecules (1–3). As for exosomes (23, 24), T cell responses to peptide-loaded vesicles presumably reflect direct binding to T cells via a combination of TCR/MHC class I/peptide and LFA-1/CD54 interactions. Such binding, coupled with costimulation via CD28/B7 interactions, then signals proliferation and differentiation into effector cells. On this latter point, generation of effector cells seemed to be as efficient with vesicles as with intact DC, implying that the secretion of stimulatory cytokines such as TNF-α and IL-6 by APC (28, 29) is not essential.
As in vitro, peptide-loaded vesicles led to efficient proliferation of naïve CD8+ T cells in vivo followed by differentiation into effector cells capable of tumor cell elimination. The vesicles, given as a single injection, were immunogenic after either i.v. or s.c. injection, even for s.c. tumor rejection. This latter finding is interesting because, as mentioned earlier, rejection of s.c. tumors after immunization with intact DC is poor unless DC are given s.c. rather than i.v. (15–17). One possibility is that priming with vesicles is especially efficient at inducing up-regulation of appropriate homing molecules required for effector cell migration to s.c. sites. This idea remains to be examined.
How vesicles are presented to T cells in vivo is unclear. For exosomes derived from immature DC, antigen presentation involves uptake of exosomes by host APC followed by cross priming, i.e., degradation of native antigen into peptides, which are then ferried to the cell surface bound to MHC molecules (19). Such cross-presentation also applies to peptide-loaded exosomes (30, 31). Here the injected vesicles adhere to host cells and are presented to T cells on the surface of host DC: T cells recognize MHC/peptide on the bound exosomes and receive bystander costimulation from the adjacent costimulatory/adhesion molecules on the DC. This form of presentation initially depends on prior up-regulation of these molecules on host DC by injection of Toll-like receptor ligands such as CpG ODN. Significantly, this requirement did not apply to the vesicles used in the present study, presumably because the expression of costimulatory/adhesion molecules on the vesicles was sufficiently high to bypass the need for T cells to recognize these molecules on host DC. It is conceivable that, as in vitro, stimulation by the vesicles in vivo reflected direct uptake by the responding T cells. However, passive uptake and presentation by host cells is more likely. These possibilities remain to be investigated.
The potential of the vesicles described here for clinical tumor immunotherapy is still unknown, and it is clearly important to extend these studies to normal DC (autologous mature DC or B cell blasts) and to defined tumor antigens. Nevertheless, given the known success of exosomes for tumor immunotherapy, it is noteworthy that vesicles from mouse DC sonicates were much superior to exosomes. Thus, at least in vitro, vesicles were considerably more immunogenic than exosomes. The more important finding, however, was that total yields of material per 106 cells were ≈50-fold higher for vesicles than for exosomes. Vesicles from DC sonicates might also be useful as a vaccine for memory cell generation.
Materials and Methods
Mice.
C57BL/6 (B6), Thy1.1 (B6.PL), Ly5.1 (B6.SJL), and Tap1-deficient (TAP−/−) mice were from The Jackson Laboratory. 2C and OT-I transgenic mice were maintained in The Scripps Research Institute animal facility and used at 3–6 months of age.
Cell Lines and Media.
DC2.4, P815, and T cells were cultured in RPMI medium 1640 with 10% FCS, 2 mM l-glutamine, 1 mM Na-pyruvate, 10 mM Hepes, 5 × 10−5 M 2-mercaptoethanol, MEM nonessential amino acids, and antibiotics. DP1 cells were cultured in the same medium plus 0.5 μg/ml G-418.
Peptides.
SIYR (SIYRYYGL), QL9 (QLSPFPFDL), and SIINFEKL peptides were purchased from Sigma–Genosys, and purity was ≥95%.
Preparation of Membrane Vesicles.
Where indicated, DC2.4 cells were cultured with 10 ng/ml recombinant murine IFN-γ with or without 2.5 μM SIYR peptide for 24 h. Cells were then washed with PBS and resuspended in Dounce buffer with protease inhibitors. The cell suspension was incubated for 10 min at 4°C and transferred to Dounce homogenizer, and 30 strokes were delivered. Immediately thereafter, tonicity was restored to 0.15 M NaCl (final). The suspension was then centrifuged at 500 × g to remove the nuclear fraction (pellet). The supernatant was recovered, diluted with PBS, and sonicated. This suspension was centrifuged at 10,000 × g to remove mitochondria, any remaining nuclei fragments, and larger cell debris. The supernatant was recovered and centrifuged at 100,000 × g to pellet the vesicles. The pellet was resuspended in 1–2 ml of PBS. To remove possible aggregates thereafter, samples were spun at 7,500 × g for 5 min.
To prepare CFSE-labeled membrane vesicles, DC2.4 cells were cultured as described above, washed, and resuspended in warm PBS/0.1% BSA. Cells were then labeled with 10 μl of 5 mM Vybrant CFDA SE Cell Tracer kit per milliliter of cell suspension for 20 min at 37°C. Cells were washed as described for T cells and used for membrane vesicle preparation.
Exosomes were isolated from culture supernatant of DC2.4 cells used for preparation of membrane vesicles and were purified as described (23).
Cell Purification.
2C and OT-I CD8+ cells were purified from LN by using a negative selection kit (Miltenyi Biotec).
CFSE Labeling of T cells.
2C CD8+ cells were resuspended in 37°C PBS containing 0.1% BSA at 1–2 × 107 cells per milliliter and incubated with 1 μl of 5 mM CFSE per milliliter for 10 min at 37°C. Labeling was terminated by adding excess ice-cold PBS containing 10% FCS, and cells were then washed three times before use.
Antibodies and Flow Cytometry Analysis.
The following antibodies were used: phycoerythrin-conjugated anti-CD3 (145-2C11, Becton Dickinson), anti-CD8α (53-6.7, Becton Dickinson), anti-CD43 (1B11, Becton Dickinson), and anti-granzyme B (GB12, Caltag); allophycocyanin-conjugated anti-CD44 (IM7), anti-CD45.2 (104), and anti-CD90.2 (HIS51); Alexa Fluor 405-conjugated anti-CD4 (RM4-5, Caltag); biotin-conjugated anti-CD54 (YN1/1.7.4), anti-CD80 (16-10A1), anti-CD86 (Michel-17), anti-IL-2 (JES6-5H4), and anti-IFN-γ (XMG1.2); unconjugated anti-IL-2 (JES61A12) and anti-IFN-γ (RA-6A2). Antibodies were purchased from eBioscience unless otherwise stated. Cy5-conjugated 1B2 mAb was prepared by using a Cy5 labeling kit.
For intracellular staining of granzyme B, GolgiStop was added to cells for the last 5 h of incubation. Cells were then washed, stained for surface markers, fixed, permeabilized, washed, and analyzed. For staining of in vivo-activated cells, RBCs were lysed before surface marker staining. Streptavidin-coated beads were used to detect surface markers on membrane vesicles. Beads were coated with biotinylated anti-CD54, anti-CD80, anti-CD86, or isotype control mAbs. Coated beads were incubated with CFSE-labeled membrane vesicles, washed twice, and analyzed for bound material.
In Vitro Stimulation of 2C CD8+ T Cells.
In most experiments, 5 × 104 purified 2C CD8+ T cells were incubated with varying concentrations of membrane vesicles for 72 h. [3H]Thymidine at 1 μCi/ml (1 Ci = 37 GBq) was added to the cultures 8 h before harvest. When intact DC2.4 cells were used as stimulators, DC2.4 cells were incubated for 24 h with 10 ng/ml recombinant murine IFN-γ and 2.5 μM SIYR peptide, irradiated, washed, and added to wells with 2C CD8+ cells plus 0.32 μm free SIYR peptide.
Measurement of Proliferation of 2C CD8+ Cells in Vivo.
CFSE-labeled 2C CD8+ cells were injected i.v. The next day mice were injected with membrane vesicles given either i.v. or s.c. (both footpads). As a positive control, in some experiments SIYR and poly I:C were injected i.p. Mice were killed 3 days later. Donor cells were identified by anticlonotypic 1B2 mAb staining or by using Thy1.1/1.2 or Ly5.1/5.2 differences between donor and host.
ELISA and Cytotoxic T Cell Assays.
A total of 5 × 104 of 2C CD8+ T cells per well were incubated with membrane vesicles (10 μg/ml) plus 0.32 μM SIYR, intact DC2.4 cells (1.25 × 104 per well), plus 0.32 μM SIYR or with 0.32 μM SIYR alone. DC2.4 cells were treated as described for proliferation assays. Culture supernatants were collected and used for ELISA as described (32).
To assay cytotoxic T lymphocyte activity, T cells were collected after 68 h of culture and used in a standard 51Cr-release assay. As a target, 51Cr-labeled P815 cells (1 × 104 per well) pulsed for 1 h with 10 μM QL9 peptide were used.
Tumor Rejection.
TAP−/− mice were injected i.v. with naïve 2C CD8+ cells (4 × 106 per mouse). The next day membrane vesicles were injected i.v. Three days after vesicle injection mice were injected s.c. with 2 × 106 DP1 cells. DP1 cells express a minigene encoding SIYR peptide (26).
Acknowledgments
This work was supported by U.S. Public Health Service Grants CA38355, AI21487, AI46710, and AG01743. This article is publication no. 18021-IMM from The Scripps Research Institute.
Abbreviations
- TCR
T cell receptor
- APC
antigen-presenting cell
- DC
dendritic cell
- LN
lymph node
- CFSE
carboxyfluorescein succinimidyl ester.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Guermonprez P., Valladeau J., Zitvogel L., Thery C., Amigorena S. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 2.Heath W. R., Carbone F. R. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 3.Alegre M. L., Frauwirth K. A., Thompson C. B. Nat. Rev. Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 4.Boon T., Coulie P. G., Van den Eynde B. J., Van der Bruggen P. Annu. Rev. Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 5.Davis I. D., Jefford M., Parente P., Cebon J. J. Leukocyte Biol. 2003;73:3–29. doi: 10.1189/jlb.0502261. [DOI] [PubMed] [Google Scholar]
- 6.Gilboa E., Nair S. K., Lyerly H. K. Cancer Immunol. Immunother. 1998;46:82–87. doi: 10.1007/s002620050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu W., Arraes L. C., Ferreira W. T., Andrieu J. M. Nat. Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg S. A., Yang J. C., Restifo N. P. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamradt T., Mitchison N. A. N. Engl. J. Med. 2001;344:655–664. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 11.Sprent J., Kishimoto H. Immunol. Rev. 2002;185:126–135. doi: 10.1034/j.1600-065x.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 12.Walker L. S., Abbas A. K. Nat. Rev. Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J., Palucka A. K. Nat. Rev. Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 14.Mende I., Engleman E. G. Ann. N.Y. Acad. Sci. 2005;1058:96–104. doi: 10.1196/annals.1359.018. [DOI] [PubMed] [Google Scholar]
- 15.Eggert A. A., Schreurs M. W., Boerman O. C., Oyen W. J., de Boer A. J., Punt C. J., Figdor C. G., Adema G. J. Cancer Res. 1999;59:3340–3345. [PubMed] [Google Scholar]
- 16.Mullins D. W., Sheasley S. L., Ream R. M., Bullock T. N., Fu Y. X., Engelhard V. H. J. Exp. Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada N., Tsujino M., Hagiwara Y., Tada A., Tamura Y., Mori K., Saito T., Nakagawa S., Mayumi T., Fujita T., et al. Br. J. Cancer. 2001;84:1564–1570. doi: 10.1054/bjoc.2001.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taieb J., Chaput N., Zitvogel L. Crit. Rev. Immunol. 2005;25:215–223. doi: 10.1615/critrevimmunol.v25.i3.30. [DOI] [PubMed] [Google Scholar]
- 19.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 20.Fujii S., Liu K., Smith C., Bonito A. J., Steinman R. M. J. Exp. Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii S., Shimizu K., Smith C., Bonifaz L., Steinman R. M. J. Exp. Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Z., Reznikoff G., Dranoff G., Rock K. L. J. Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 23.Hwang I., Shen X., Sprent J. Proc. Natl. Acad. Sci. USA. 2003;100:6670–6675. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segura E., Nicco C., Lombard B., Veron P., Raposo G., Batteux F., Amigorena S., Thery C. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 25.Lee P. U., Churchill H. R., Daniels M., Jameson S. C., Kranz D. M. J. Exp. Med. 2000;191:1355–1364. doi: 10.1084/jem.191.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho B. K., Palliser D., Guillen E., Wisniewski J., Young R. A., Chen J., Eisen H. N. Immunity. 2000;12:263–272. doi: 10.1016/s1074-7613(00)80179-x. [DOI] [PubMed] [Google Scholar]
- 27.Van Kaer L., Ashton-Rickardt P. G., Ploegh H. L., Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto H., Sprent J. J. Immunol. 1999;163:1817–1826. [PubMed] [Google Scholar]
- 29.Sepulveda H., Cerwenka A., Morgan T., Dutton R. W. J. Immunol. 1999;163:1133–1142. [PubMed] [Google Scholar]
- 30.Andre F., Chaput N., Schartz N. E., Flament C., Aubert N., Bernard J., Lemonnier F., Raposo G., Escudier B., Hsu D. H., et al. J. Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 31.Chaput N., Schartz N. E., Andre F., Taieb J., Novault S., Bonnaventure P., Aubert N., Bernard J., Lemonnier F., Merad M., et al. J. Immunol. 2004;172:2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 32.Ozaki M. E., Karlsson L., Peterson P. A., Webb S. R. J. Immunol. 1997;159:214–221. [PubMed] [Google Scholar]