Abstract
Recently, we have identified proinsulin (P-Ins)73-90 as an immunodominant T cell epitope of HLA-DRB1*0401 (DR4) subjects with β-islet cell autoimmunity and of HLA-DR4/CD4 double-transgenic mice immunized with human P-Ins. We have compared the fine specificities of one human CD4 T cell clone and two mouse T cell hybridoma clones recognizing this epitope, and, although these three clones all recognized the same core region (LALEGSLQK), there were major differences in how they interacted with the peptide (p)/HLA complex, reflecting the fact that human P-Ins is a foreign antigen in the mouse and an autoantigen in the type 1 diabetes patient. The human T cell clone was forkhead transcription factor 3 (Foxp3)-positive, a marker for regulatory T cell lineages, and secreted predominantly IL-5, IL-10, and low levels of IFNγ in response to P-Ins73-90. This finding is compatible with the previously detected regulatory cytokine pattern in subjects with β-cell autoimmunity. However, added N- or C-terminal amino acids drastically changed HLA and tetramer binding capacity as well as T cell reactivity and the cytokine phenotype of the P-Ins73-90-specific human CD4 T cell clone, suggesting a potential for this P-Ins epitope as a target for therapeutic intervention in HLA-DR4-positive humans with β-islet cell autoimmunity or recent-onset type 1 diabetes.
Keywords: Foxp3, HLA-DRB1*0401, type 1 diabetes
Proinsulin (P-Ins) is considered an important autoantigen in type 1 diabetes (T1D), because it is the only truly β-cell-specific target, and because autoreactivity to P-Ins is very common in T1D patients with the HLA-DRB1*0401 (DR4) DQ8 haplotype (1–6). The differences distinguishing autoreactive from foreign antigen reactive T cell responses to the same immunogenic epitope are still largely unknown (7). To understand the structural requirements for activation of self-reactive P-Ins73-90-specific T cells in T1D autoimmunity, we have isolated a Foxp3-positive CD4+ T cell clone from a DR4-homozygous T1D patient and compared the fine specificity of this T cell receptor to those of two murine T cell hybridoma clones derived from HLA-DR4 transgenic mice, in which this epitope is a foreign antigen (8). P-Ins73-90 is situated at the C terminus of the C peptide and also covers the enzymatic cleavage site of the insulin A chain (8). This site is proteolytically destroyed during the maturation of insulin before secretion and is, therefore, an indication that Proinsulin and not Insulin may be the actual autoantigenic target in T1D.
In human studies using purified CD4+ T cells from HLA-DR4-positive subjects with islet autoimmunity, P-Ins73-90 was also identified as an immunodominant epitope. This epitope was recognized by approximately two thirds of autoantibody-positive subjects, one third of recently diagnosed T1D patients, and a few control subjects (5, 9). The cytokines seen in response to P-Ins73-90 were predominantly IL-4 and IL-10 in subjects with β-cell autoimmunity (9). Moreover, in a consecutive follow up of three high-risk individuals, two of which developed T1D (within 1.5 and 2 years) this epitope was recognized among the first P-Ins epitopes by these two patients (5). A recent study of peptides eluted from a P-Ins-transfected B cell line homozygous for DR4 indicated that peptide P-Ins75-92 was naturally processed and presented (10). This study confirmed our previous data (5, 9, 11) and found that close to half of the DR4-positive T1D patients also responded to this peptide epitope. In nondiabetic subjects, P-Ins75-92 recognition was associated with production of the antiinflammatory cytokine IL-10, whereas, in T1D patients, production of IL-10 in response to P-Ins75-92 was associated with a delayed disease onset (10).
Self-reactive T cells with high-affinity T cell receptors (TCR) are usually eliminated in the thymus by negative selection, whereas low-affinity TCR may escape to the periphery (12, 13). An exception is the high-affinity CD25-positive “natural regulatory CD4 T cell,” which is already programmed for regulation in the thymus (14); these T cells are therefore capable of bypassing negative selection and can still be released to the periphery (12). The P-Ins73-90-specific regulatory CD4 T cells that we encounter in the T1D patients seem to be IL-10-producing T helper (Th)2/T regulatory (Treg) that function through their cytokine production (9–11). Either of these regulatory CD4 T cell lineages has been shown to express the Foxp3 marker (15), implying that acquired IL-10-dominant cytokine responses to P-Ins epitopes may potentially serve as a therapeutic target for delaying development of diabetes in humans.
Results
Specificity and HLA-Restriction of the Human T Cell Clone 52c1
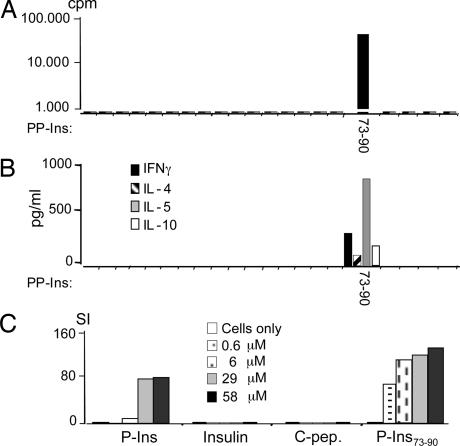
The human T cell clone 52c1 reacted exclusively to P-Ins73-90 (Fig. 1A) and produced significant levels of IL-5 (787 pg/ml), moderate IL-10 (72 pg/ml), and low IFNγ (298 pg/ml) levels after stimulation with the specific peptide (Fig. 1B). This clone also responded to the intact P-Ins antigen processed and presented by DR4 homozygous dendritic cells as well as the cognate peptide epitope P-Ins73-90, suggesting that this epitope was naturally processed and expressed (Fig. 1C). However, high P-Ins concentrations were required (6–58 μM), suggesting a modest avidity of the TCR (Fig. 1C). Although the antigen-presenting cells (APC) in this experiment were in vitro-expanded human dendritic cells (16), the synthetic peptide in equimolar doses seemed to have stronger stimulatory capacity in vitro than intact P-Ins antigen. Anti-HLA-DR mAbs completely abolished the T cell responses to peptide P-Ins73-90 (data are available in Figs. 6 and 7, which are published as supporting information on the PNAS web site). By using DR4- or DQ8-transfected Epstein–Barr virus-transformed B cell lines derived from a patient with Bare Lymphocyte Syndrome (BLS-1), as APC, the DR4 restriction of 52c1 was further affirmed (see Figs. 6 and 7).
Fig. 1.
Characterization of the human T cell clone. (A) Proliferation of the human P-Ins73-90-specific T cell clone, 52c1 to 21 overlapping preproinsulin (PP-Ins) peptides (16 mers; on x axis). (B) Cytokine secretion of the clone 52c1. In A and B, autologous BMNC were used as APC. (C) T cell clone was tested on different concentrations of P-Ins, insulin (Ins), C-peptide (C-pep), and P-Ins73-90 peptide by using allogeneic homozygous DR4 dendritic cells as APC.
Determination of the Core Epitope and DR4/TCR-Contacting Positions of P-Ins73-90.
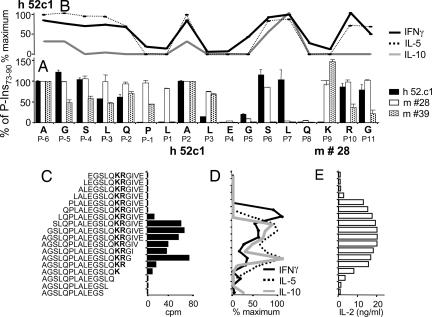
By testing a series of P-Ins73-90 peptide analogues with single alanine substitutions, we identified a set of altered peptides capable of inhibiting or altering proliferation (Fig. 2A) and cytokine secretion (Fig. 2B) by the human T cell clone as well as changing the IL-2 secretion levels of the mouse hybridomas (Fig. 2A). These alanine-substituted peptides blocked either the p/HLA interaction or the TCR activation. Alanine substitution of E83 in peptide position P4 inhibited profoundly the proliferation of all three T cell clones. Leucine80 in P1 was critical for the activation of the human clone and T cell hybridoma #39, whereas it had no apparent effect on hybridoma #28 (Fig. 2A). Alanine substitution of S85 in P6 had no effect on T cell activation of the human clone and hybridoma #28, whereas this residue was imperative for the activation of hybridoma #39 (Fig. 2A). The K88 residue in P9 was crucial for the responses of the human T cell clone, whereas reactivity of the two murine T cell clones was completely unaffected by substitution of this residue (Fig. 2A). Alanine substitutions in the primary TCR contacts P5 (G84) and P8 (Q87) completely abolished the proliferation of all three clones (Fig. 2A). P2 (A81) is in the position of a potential TCR-contact residue, whereas the alternative TCR contact P3 was of importance only for the human clone (Fig. 2A). Alanine substitution of the P7 residue (L86) seems to influence T cell reactivity of the two murine clones, whereas it has no influence on the reactivity of the human clone (Fig. 2A). The cytokine responses of the human T cell clone largely followed the pattern of the T cell proliferation (Fig. 2B).
Fig. 2.
Determination of DR4/TCR contacting positions of P-Ins73-90 and influence of the flanking amino acids on the activation and cytokine secretion of the human T cell clone. (A) Reactivity of the human T cell clone 52c1 (black bars) and two mouse hybridoma, #28 (open bars) and #39 (dotted bars) to a panel of alanine-substituted peptides of P-Ins73-90. The x axis displays the sequence of the peptide and assigned positions (P) concerning core HLA/TCR-binding residues. Data used for the human T cell clone express the cpm of each of the individual peptides in relation to P-Ins73-90 (100% value). For the murine T cell hybridoma, IL-2 production (pg/ml) has been similarly related to the IL-2 production of the P-Ins73-90 (100% value). Bars represent responses to a variant peptide in which that residue alone was substituted with alanine. (B) Cytokine reactivity of the human T cell clone to the panel of alanine-substituted peptides of P-Ins73-90. Maximum IFNγ response was 459 pg/ml, for IL-5 880 pg/ml, and for IL-10 13 pg/ml. (C–E) A panel of 18 N- or C-terminally truncated peptides was used to identify a minimal core epitope of P-Ins73-90 by the human T cell clone (C) and by the mouse hybridoma #39 (E). Cytokine secretion profile of the human T cell clone after stimulation with truncated peptides of P-Ins73-90 (D). In this experiment, maximum response for IFNγ was 1,154 pg/ml; for IL-5, response was 2,817 pg/ml; and for IL-10, response was 72 pg/ml.
Overall, these results suggest that all three T cell clones recognize the peptide core epitope (LALEGSLQK) of P-Ins73-90 by the same structural requirements in primary p/MHC and TCR contacts, although with slightly different constraints and varying secondary positions. The bolded “K” indicates that this amino acid belongs to the enzymatic cleavage site and not to the c-peptide region, as do the remaining amino acids of this epitope.
Influence of Flanking Amino Acids on Activation of the Clones.
One characteristic feature of murine P-Ins73-90-specific T cells was the requirement of flanking N- and C-terminal amino acids, which protrude from the peptide-binding groove of the DR4 molecule (8). For the human T cell clone, N- and C-terminal-truncation studies revealed complete abolition of proliferation either when L77 in P3 was removed or K88 in P9 was lost (Fig. 2C). Deletion of the N-terminal amino acid S76 in P4 caused a shift in cytokine secretion from a Th2/Treg to a Th0 phenotype by completely abolishing IL-10 secretion, decreasing IL-5 secretion (2.1 times), and increasing IFNγ secretion (2.2 times) (Fig. 2D). Furthermore, removal of L77 in P3 eliminated IL-5 secretion and provoked a complete switch to a Th1-type response (Fig. 2D). In contrast, removal of the C-terminal amino acid R89 in P10 completely eliminated the IFNγ production (Fig. 2D). Taken together, the quality of the T cell response to P-Ins73-90 of this human T cell clone also depended markedly on flanking peptide regions. Reactivity of the mouse T cell hybridoma disappeared when K in P9 was gone or P79 in P1 was removed (Fig. 2E). It should be emphasized that the C-terminal truncations influenced the responses of the human vs. the murine T cell clones in a very different manner (Fig. 2 C and E).
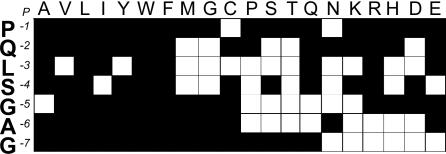
In addition, the contribution of the N-terminal overhang (consisting of seven neutral amino acids) to the activation of the mouse T cell hybridomas was analyzed by using 140 P-Ins73-90 peptides substituted in each position from 1 to 7 with 19 naturally occurring amino acids (Fig. 3). The nature of the permissive residues in the majority of positions was diverse. Position P3 showed the highest restriction and allowed for only seven substitutions (neutral: A, I, W, F, and Q; basic: R; acidic: E), whereas position P1 was the most promiscuous (17 substitutions, except C and N). The human T cell clone and the two mouse hybridomas differed significantly in their requirements for position P1. Substitution of P79 to A completely abolished proliferation as well as IL-5 and IL-10 secretion of the human clone, whereas it did not alter reactivity of the mouse hybridoma, appreciably (Fig. 2 A and B).
Fig. 3.
Influence of systematic amino acid substitutions in the N-terminal overhang residues 73–79 of P-Ins73-90 on the activation of the mouse hybridoma #39. Each position of the N-terminal overhang P-Ins73–79 was substituted by all 19 naturally occurring amino acids. Open blocks indicate that substitution of this particular amino acid residue had a significant influence on the activation (response <40–50% of the P-Ins73-90 peptide), whereas filled blocks stand for no effect (responses >60% of the P-Ins73-90 peptide).
This finding indicates that, although the three T cell clones recognized the P-Ins73-90/DR4 complex in the same binding register, LALEGSLQK, there were very different requirements for the TCR contact residues (Figs. 2A and 3). Although P3 is of importance for all three clones, P1 is essential only for the human T cell clone (Fig. 2A).
Dissociation Kinetics of P-Ins73-90 from DR4 and Influence of HLA-DM Editing.
We have shown recently that kinetic stability of the individual p/MHC complexes is closely associated with immunogenicity (17). Immunodominant peptides usually showed dissociation t1/2s >1 h, whereas nonimmunogenic peptides were <30 min (17). To investigate the dissociation kinetics of P-Ins73-90, or its shorter variant P-Ins79–90 (lacking the N-terminal overhang) with DR4, we analyzed p/HLA dissociation at either the conditions of the endosomal compartment (acidic, pH 5.5) or at the cell surface (neutral, pH 7), in the presence or absence of soluble HLA-DM (sDM), the protein-catalyzing dissociation of antigenic peptides in the endosomes (18) (Table 1). The dissociation t1/2 values for both peptides (in hours) at cell-surface conditions were much longer than in the more acidic conditions of the late endosomal compartment (Table 1). The longer P-Ins73-90 had t1/2s that were more than twice those of the shorter peptide under all conditions. Addition of sDM increased dissociation of both peptide P-Ins73-90/HLA-DR4 and peptide P-Ins79-90/HLA-DR4 complexes by a factor of five at endosomal pH, whereas it had marginal impact at cell-surface conditions.
Table 1.
Peptide/HLA-DR4 complex stability
| Peptides | pH 5.5 |
pH 7.0 |
||
|---|---|---|---|---|
| No sDM | +sDM | No sDM | +sDM | |
| GAGSLQPLALEGSLQKRG | 5.1 ± 0.1 | 1.0 ± 0 | 127 ± 3.4 | 112 ± 7 |
| PLALEGSLQKRG | 2.8 ± 1.1 | 0.5 ± 0.1 | 52 ± 5.1 | 44 ± 0 |
P/HLA-DR4 complex stability was analyzed at pH 7 (cell-surface pH) or pH 5.5 (endosomal pH), in the absence or presence of soluble HLA-DM (sDM). The measurements represent the mean ± se of the dissociation (t1/2, in hours) computed from two or more independent dissociation curves.
Tetramer Analysis of the Human T Cell Clone 52c1.
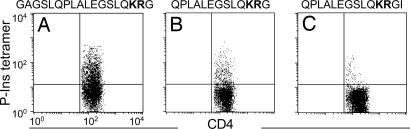
Binding of the human T cell clone to three different DR4 tetramers, including either the original peptide P-Ins73-90 or two shorter variants, P-Ins78-90 and P-Ins78-91, are depicted in Fig. 4. The specificity of tetramer binding to the human T cell clone, analyzed on day 7 of the restimulation cycles showed 30.8% binding to the original peptide P-Ins73-90, reduction of binding to a shorter variant P-Ins78-90 (10.55%), and an intriguing reduction of binding to tetramer with P-Ins78-91 (1.89%), which was extended by only 1 aa (the naturally occurring I91) at P12 at the C terminus.
Fig. 4.
Binding of DR4 tetramers, including either P-Ins73-90 (A) or two shorter variants of the P-Ins73-90 peptide, P-Ins78–90 (B) and P-Ins78–91 (C) to the human T cell clone.
Foxp3 Expression.
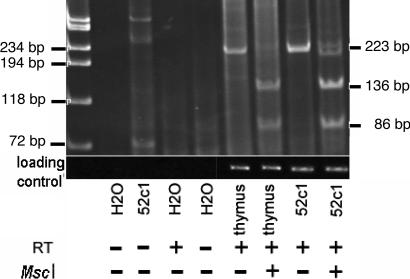
Fig. 5 demonstrates the presence of Foxp3 mRNA in the 52c1 clone. The correct identity of the 223-bp RT-PCR product was demonstrated by restriction digestion and confirmed by sequencing. Human thymus was used as a positive control.
Fig. 5.
Demonstration of Foxp3 mRNA expression from the 52c1 clone by RT-PCR. A band of the expected 223-bp size was obtained, of intensity comparable with that of a human thymus sample. Upon digestion with MscI, the sequence produced the expected restriction fragments. The band was absent when reverse transcriptase (RT) was omitted. Cyclophilin was used as loading control. These RT-PCR results were obtained in three independent experiments.
Discussion
The majority of DR4 subjects with islet autoimmunity (antibody-positive subjects and T1D patients) show autoreactivity to P-Ins73-90 (refs. 5, 9, 10, and 19 and J.A.O. and G.S., unpublished observation).
Two T cell hybridomas recognizing human P-Ins and P-Ins73-90 were previously generated in HLA-DR4 transgenic mice (8), and, to compare autoreactive with foreign antigen-specific responses to the same epitope, we generated a human proinsulin-specific CD4+ T cell clone from a recently diagnosed DR4 patient recognizing P-Ins73-90 and the intact P-Ins antigen (but not matured insulin). This clone was exclusively restricted by HLA-DR4 (see Figs. 6 and 7) and was found to express Foxp3 (Fig. 5), a selective molecular marker of the Treg cell lineages (15, 20). The cytokine profile of this clone had the signature of a Th2/Treg type of a CD4+ T cell, which is compatible with what has also been reported in T1D patients and HLA-DR4-positive humans with β-islet cell autoimmunity (5, 9, 21). A similar drift from early T cell proliferation and IFNγ responses over a stage with continued antigen-specific T cell proliferation with a shift to predominant IL-10 production (but without T cell proliferation) has also been reported in hemophiliac patients during the evolution of hepatitis C virus (HCV) infection (22). These hemophiliac patients, who, aside from their coagulation defect are healthy, did, over time, develop chronic HCV disease with loss of viral control.
The reactivity pattern seen with the combined alanine-substituted peptide panel and the N- and C-terminally truncated peptide panels reported herein identified only one plausible binding register for the P-Ins73-90/HLA-DR4 complex, placing L80 in relative position P1, E83 in P4, S85 in P6, and K88 in P9 (Fig. 2 A–E). This finding suggested that the TCR contact residues would be found in P2 (A81), P5 (G84), and P8 (Q87), compatible with the T cell activation results obtained with all three clones (Fig. 2A). However, there was major variability in how these T cell clones were responding to the p/HLA complex. Although all three clones required the small glycine residue placed in the middle of the groove, the human clone had unique requirements for both P1 at the N terminus and P9 at the C terminus (Fig. 2 A and B), and the murine T cells were more flexible and depended predominantly on the C terminus of the p/HLA complex. However, whereas the human clone relied for activation on a combination of P8 (Q87) and P9 (K88), the murine T cells responded optimally to a combination of P7 (L86) and P8 (Q87) (Fig. 2A), implying that neighboring positions could also have an impact on the TCR contact (Fig. 2A). Although there seemed to be a somewhat closer relationship between the reactivity pattern of the two murine T cell clones, this was not reflected in their TCR sequences (results shown in Figs. 6 and 7).
The P-Ins73-90 epitope was originally found to benefit from an unusually long N-terminal addition of up to seven amino acids that was assumed to interact with the HLA molecule outside the binding groove (8). This finding is also in agreement with studies of immunogenic T cell epitopes published by others (23–25). With the goal to define particular amino acid sequences that would generally increase p/HLA-DR4 stability, we analyzed how substitution of each of these seven positions, one by one with each of the other 19 naturally occurring amino acids, would influence the reactivity of the murine T cell hybridoma clones (Fig. 3). Most of these seven positions allowed for a number of different amino acid substitutions (Fig. 3). However, P3, which was the most restricted residue, seemed to have a major effect on the cytokine phenotype, changing the responses from predominantly IL-5 and -10 to IFNγ for the human T cell clone (Fig. 2D).
From binding studies, we found that the long N-terminal overhang of the present P-Ins epitope exerts its effect through stabilizing p/HLA binding (Table 1). Previously, we have encountered a situation with a 5-aa N-terminal overhang, which did not influence p/HLA-DR4 complex stability but was capable of modulating the resulting cytokine phenotype through peptide–TCR interaction (26). P/HLA dissociation studies showed that the t1/2s of complexes with the longer peptide (P-Ins73-90) were approximately twice those of the shorter peptide (P-Ins79-90) under all conditions (Table 1). This same relationship was also found when sDM was present (Table 1). Although this P-Ins epitope is rather stable at cell-surface pH, the t1/2 values at lower pH may seem relatively low for an immunodominant epitope (Table 1). Considering that P-Ins is a self-protein (an autoantigen) in humans, which naturally would be expected to induce tolerance and not active immunity, the specific pH requirements of this T cell epitope may, therefore, have a unique role in regulating the immunogenic potential of P-Ins in HLA-DR4-positive humans.
The C-terminal truncation studies preserved T cell reactivity of the human T cell clone out to P10 (R89) (Fig. 2 C and D). Interestingly, addition of the next two native amino acids, P12 (I91) and P13 (V92), specifically inhibited all reactivity of the human T cell clone (Fig. 2 C–E). Notably, the inhibitory effect of the C-terminal addition of P11 observed with the human T cell clone was also detected in the P-Ins/DR4 tetramer studies shown in Fig. 4. Although tetramer binding was clearly enhanced by the longer N-terminal overhang (Fig. 4 A vs. B), the addition of P12 (I91) almost abolished tetramer binding (Fig. 4 B vs. C). Computer modeling studies suggest that the trimolecular complex of DR4, P-Ins73-90, and this human TCR may conform to a similar hairpin configuration at the C terminus (results not shown) as recently described by others for a major HIV epitope (27).
Proinsulin is considered a critical autoantigen in T1D patients with the HLA-DR4,DQ8 haplotype, but in vitro studies of human subjects with β-cell autoimmunity have generally shown low immunogenicity of P-Ins (2, 5, 9, 28, 29). In the present study a very high concentration (29 μM) of recombinant human P-Ins was required to activate the human T cell clone (Fig. 1D). In contrast, the mouse hybridomas were activated by P-Ins concentrations that were significantly lower (8). Moreover, only dendritic cells and not autologous blood mononuclear cells (BMNC) or DR4 lymphoblastoid cell lines (LCLs) were capable of processing the P-Ins73-90 epitope from intact P-Ins antigen to activate the human T cell clone, whereas murine spleen cells as well as HLA-DR4 positive human LCLs were capable of processing recombinant human P-Ins at sufficient levels to activate the murine T cell hybridomas (8). However, human P-Ins is a foreign antigen in the mouse but an autoantigen in humans, which may partially account for the differences in the T cell receptor repertoires of these two species. To escape thymic negative selection, a self-reactive TCR would be expected to be of low affinity and thus require a much stronger antigen challenge. Human and mouse P-Ins differ in 13 amino acids, and the P-Ins73-90 epitope differs in 6 amino acids (positions P4, P1, P5, P6, and P7). After initiation of β-cell destruction in inflamed islet cells, the accessible amount of free P-Ins is likely to be higher than in a healthy pancreas, and binding of P-Ins73-90 is more efficient at neutral cell surface conditions compared with the more acidic intracellular loading compartments (Table 1). Ongoing β-cell destruction may therefore favor the activation of pathogenic P-Ins-specific T cells and thereby further accelerate β-islet cell destruction.
The CD4 T cell clone reported herein, which has the phenotype of a down-regulatory Th2 cell, was derived by using clearly defined growth conditions for Th cells. It will be important to extend these studies to a larger number of T cell clones from different T1D patients, and these experiments are underway.
The fact that the present P-Ins73-90-specific T cell clone expresses Foxp3 and secretes T regulatory cytokines (similar to P-Ins73-90-specific CD4 T cells of T1D patients with the HLA-DR4,DQ8 haplotype) may be suggestive of a potential for beneficial effects in T1D patients. Recently, we have found that, in humans, there is an association between a significantly higher IL-10 response to this P-Ins epitope and the protective Ins-VNTR class III alleles (30). VNTR polymorphism upstream of the insulin promoter is implicated in the induction of self-tolerance to insulin by regulating the level of insulin transcription in the thymus, the long class III alleles resulting in 2- to 3-fold higher transcription than the short class I alleles (31). Changes in the length and amino acid composition of the therapeutic P-Ins73-90 epitope could, therefore, potentially silence the pathological islet-specific autoreactivity by inducing down-regulation by P-Ins-specific Treg cells, thus providing a credible target for diabetes prevention in DR4-positive humans.
Materials and Methods
Mouse T Cell Hybridoma.
Hybridomas #28 and #39 specific for P-Ins73-90 were isolated from transgenic mice expressing HLA-DRA1*0101, DRB1*0401, and human CD4 on a murine MHC class II null background, as already described (8, 32). The specificities of these hybridomas were analyzed in a Europium-based IL-2 assay (8).
Human T Cell Clone.
CD4+ T cells were enriched from BMNC of a DR4-homozygous T1D patient, as described in ref. 5. A T cell line was established by restimulation of CD4+ T cells with irradiated autologous BMNC as APC pulsed with P-Ins73-90 (50 μg/ml synthetic peptide for 3 h at 37°C). After 72 h, 15 units/ml IL-2 was added, and, after three rounds of restimulation (14 days each round), the analysis of the TCR Vβ repertoire of the line showed a major Vβ27 peak. Vβ27-positive cells were sorted by using Vβ27-specific monoclonal antibodies (Serotec, Oxford, U.K.) and MicroBeads (Miltenyi Biotec). This line was further expanded by repeated restimulation as above, and a long-term stable human P-Ins73-90-specific T cell clone, 52c1, was established. The monoclonality of the T cell clone was confirmed by TCR sequencing (see Figs. 6 and 7).
Monoclonal Antibodies, Cell Lines, and Dendritic Cells.
The following mAbs (20 μg/ml) were used in the proliferation-inhibition studies: W6/32 directed against HLA-class I molecules, L243 directed against HLA-DR, and Tü22 directed against HLA-DQ. Epstein–Barr virus-transformed B cell lines derived from a patient with BLS-1 were transfected with either DRB1*0401 or DQ8 (33). Allogeneic immature dendritic cells isolated from BMNC of a DRB1*0401-homozygous blood donor were also used as APC (16).
Antigens and Peptides.
Human P-Ins and insulin were kind gifts of Eli Lilly. Twenty-one overlapping peptides (16- to 17-aa long) that covered the preproinsulin (PP-Ins) sequence in steps of 6-aa were synthesized. In addition, three peptide sets were produced: (i) consisting of 15 peptides, each with an alanine substitution at each succeeding position, (ii) consisting of 18 truncated peptides at each single N- or C-terminal position, and (iii) consisting of 140 peptides with a 7-aa-long N-terminal overhang of the core epitope of P-Ins73-90, each position from 1–7 being substituted with 19 naturally occurring amino acids (Chiron, Mimotopes, Clayton, Australia).
Dissociation Kinetics and Cytokine Assay.
Dissociation rates of P-Ins73-90 and the shorter P-Ins 79-90 from DR4 in the presence or absence of soluble HLA-DM were analyzed by using fluorescein-labeled peptides together with soluble HLA-DR4 and soluble HLA-DM molecules, as described (17).
Cytokine measurements in the supernatants of stimulated T cell cultures were performed by using an antigen-capture ELISA (9). Detection limits were 34.2 pg/ml for IFNγ, 48.8 pg/ml for IL-5, and 4.9 pg/ml for IL-10.
Tetramer Staining of P-Ins73-90-Specific T Cells.
Phycoerythrin-labeled HLA-DRB1*0401 tetramers (Beckman Coulter, San Diego) including either original P-Ins73-90 or two shorter forms of the same peptide, P-Ins78-90 and P-Ins78-91, were produced. On day 7 of the restimulation cycles, 5 × 105 cells of the human T cell clone 52c1 were incubated with 0.5 μg of each respective tetramer (5 h at 37°C) and anti-CD4 antibody (BD Pharmingen), and subsequently analyzed on a BD FACSCalibur flow cytometer (BD Biosciences). The same tetramer reagents loaded with an irrelevant peptide were used as controls for background staining.
Foxp3 Expression by the 52c1 Clone.
RNA from the human T cell clone 52c1 was reverse-transcribed by using random primers and Superscript II RNase H-Reverse Transcriptase (Invitrogen). cDNA (1.986 μg) was added to a reaction mix containing 0.2 μM sense primer for Foxp3 CATGATCAGCCTCACACCAC, 0.2 μM antisense primer CCACTTGCAGACACCATTTG, 0.2 mM dNTP mix (Invitrogen), 1.0 mM MgCl2 (Invitrogen), 1× Taq buffer (Invitrogen), and 0.06 units/μl of Taq polymerase. The PCR protocol was 35 cycles for 20 s at 94°C, 20 s at 55°C, and 20 s at 72°C. The final extension was at 72°C for 10 min. The 223-bp PCR product was electrophoresed with or without prior digestion with MscI, expected to cut the correct sequence to 136- and 86-bp fragments.
Supplementary Material
Acknowledgments
We acknowledge Dr. G. Seipke (Aventis, Frankfurt) and Eli Lilly Company (Bad Homburg, Germany) for supplying us with insulin, proinsulin, and XL He for peptide HLA modeling and F. Oswald for TCR alignment and homology analysis. This work was supported by German Research Council Center of Excellence Grants SFB 518 (to I.D-B., W.K., and B.O.B.) and GRK460 (studentship to S.R.); grants from the Eli Lilly Foundation International (to I.D-B.), the German Diabetic Child Foundation (to I.D-B.), and the German Diabetes Foundation (to I.D-B. and S.R.); National Institutes of Health Grant DK55364 (to G.S.); and grants from the Juvenile Diabetes Research Foundation (to J.A.O.) and The Greenwald Foundation (to M.C.).
Abbreviations
- APC
antigen-presenting cells
- BMNC
blood mononuclear cells
- Foxp3
forkhead transcription factor
- p
peptide
- P-Ins
proinsulin
- T1D
type 1 diabetes
- TCR
T cell receptor
- Th
T helper
- Treg
T regulatory.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Ziegler R., Alper C. A., Awdeh Z. L., Castano L., Brink S. J., Soeldner J. S., Jackson R. A., Eisenbarth G. S. Diabetes. 1991;40:709–714. doi: 10.2337/diab.40.6.709. [DOI] [PubMed] [Google Scholar]
- 2.Schloot N. C., Roep B. O., Wegmann D., Yu L., Chase H. P., Wang T., Eisenbarth G. S. Diabetologia. 1997;40:564–572. doi: 10.1007/s001250050716. [DOI] [PubMed] [Google Scholar]
- 3.Schenker M., Hummel M., Ferber K., Walter M., Keller E., Albert E. D., Janka H. U., Kastendiek C., Sorger M., Louwen F., Ziegler A. G. Diabetologia. 1999;42:671–677. doi: 10.1007/s001250051214. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacio E., Scirpoli M., Kredel K., Fuchtenbusch M., Ziegler A. G. J. Immunol. 1999;163:525–532. [PubMed] [Google Scholar]
- 5.Durinovic-Belló I., Boehm B.-O., Ziegler A.-G. J. Autoimmun. 2002;18:55–66. doi: 10.1006/jaut.2001.0566. [DOI] [PubMed] [Google Scholar]
- 6.Kent C. S., Chen Y., Bregoli L., Clemmings S. M., Kennyon N. S., Ricordi C., Herring B. J., Hafler D. A. Nature. 2005;435:224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 7.Hahn M., Nicholson M. J., Pyrdol J., Wucherpfennig K. W. Nat. Immunol. 2005;6:490–496. doi: 10.1038/ni1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Congia M., Patel S., Cope A. P., De-Virgiliis S., Sønderstrup G. Proc. Natl. Acad. Sci. USA. 1998;95:3833–3838. doi: 10.1073/pnas.95.7.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durinovic-Belló I., Schlosser M., Riedl M., Maisel N., Rosinger S., Kalbacher H., Deeg M., Ziegler M., Elliott J., Roep B., et al. Diabetologia. 2004;47:439–450. doi: 10.1007/s00125-003-1315-1. [DOI] [PubMed] [Google Scholar]
- 10.Arif S., Tree T. I., Astill T. P., Tremble J. M., Bishop A. J., Dayan C. M., Roep B. O., Peakman M. J. Clin. Invest. 2004;113:451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durinovic-Belló I., Riedl M., Rosinger S., Maisel N., Kalbacher H., Deeg M., Schreckling H.-J., Schlosser M., Ziegler M., Kuehnl P., Boehm B.-O. Ann. N.Y. Acad. Sci. 2002;958:209–213. doi: 10.1111/j.1749-6632.2002.tb02971.x. [DOI] [PubMed] [Google Scholar]
- 12.Starr T. K., Jameson S. C., Hogquist K. A. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H., Chess L. J. Clin. Invest. 2004;114:1198–1208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng S., Wang J. H., Gray J. D., Soucier H., Horwitz D. A. J. Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot J. D., Rudensky A. Y. Nat. Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 16.van-Halteren A. G., van-Etten E., de-Jong E. C., Bouillon R., Roep B. O., Mathieu C. Diabetes. 2002;51:2119–2125. doi: 10.2337/diabetes.51.7.2119. [DOI] [PubMed] [Google Scholar]
- 17.Hall F. C., Rabinowitz J. D., Busch R., Visconti K. C., Belmares M., Patil N. S., Cope A. P., Patel S., McConnell H. M., Mellins E. D., Sønderstrup G. Eur. J. Immunol. 2002;32:662–670. doi: 10.1002/1521-4141(200203)32:3<662::AID-IMMU662>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Kropshofer H., Vogt A. B., Thery C., Armandola E. A., Li B. C., Moldenhauer G., Amigorena S., Hammerling G. J. EMBO J. 1998;17:2971–2981. doi: 10.1093/emboj/17.11.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sønderstrup G., McDevitt H. Immunol. Rev. 1998;164:129–138. doi: 10.1111/j.1600-065x.1998.tb01215.x. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot J. D., Gavin M. A., Rudensky A. Y. Nat. Immunol. 2003;4:330–336. [PubMed] [Google Scholar]
- 21.Durinovic-Belló I., Maisel N., Schlosser M., Kalbacher H., Deeg M., Eiermann T., Karges W., Boehm B.-O. Ann. N.Y. Acad. Sci. 2003;1005:288–294. doi: 10.1196/annals.1288.045. [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Layden T., Eckels D. Hum. Immunol. 2003;64:662–673. doi: 10.1016/s0198-8859(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 23.Moudgil K. D., Sercarz E. E., Grewal I. S. Immunol. Today. 1998;19:217–220. doi: 10.1016/s0167-5699(97)01233-4. [DOI] [PubMed] [Google Scholar]
- 24.Sant’Angelo D. B., Robinson E., Janeway C. A., Jr, Denzin L. K. Eur. J. Immunol. 2002;32:2510–2520. doi: 10.1002/1521-4141(200209)32:9<2510::AID-IMMU2510>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Arnold P. Y., La-Gruta N. L., Miller T., Vignali K. M., Adams P. S., Woodland D. L., Vignali D. A. J. Immunol. 2002;169:739–749. doi: 10.4049/jimmunol.169.2.739. [DOI] [PubMed] [Google Scholar]
- 26.Hall F. C., Visconti K. C., Ahmad R. C., Parry S. L., Miltenburg A. M., McConnell H. M., Mellins E. D., Sønderstrup G. Arthritis Rheum. 2003;48:2375–2385. doi: 10.1002/art.11132. [DOI] [PubMed] [Google Scholar]
- 27.Zavala-Ruiz Z., Strug I., Walker B. D., Norris P. J., Stern L. J. Proc. Natl. Acad. Sci. USA. 2004;101:13279–13284. doi: 10.1073/pnas.0403371101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semana G., Gausling R., Jackson R. A., Hafler D. A. J. Autoimmun. 1999;12:259–267. doi: 10.1006/jaut.1999.0282. [DOI] [PubMed] [Google Scholar]
- 29.Ellis T., Jodoin E., Ottendorfer E., Salisbury P., She J. X., Schatz D., Atkinson M. A. Diabetes. 1999;48:299–303. doi: 10.2337/diabetes.48.2.299. [DOI] [PubMed] [Google Scholar]
- 30.Durinovic-Belló I., Jelinek E., Eiermann T., Boehm B. O., Karges W., Marchand L., Polychronakos C. Diabetes. 2005;54(Suppl. 2):S18–S24. doi: 10.2337/diabetes.54.suppl_2.s18. [DOI] [PubMed] [Google Scholar]
- 31.Vafiadis P., Bennett S. T., Todd J. A., Nadeau J., Grabs R., Goodyer C. G., Wickramasinghe S., Colle E., Polychronakos C. Nat. Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 32.Fugger L., Michie S. A., Rulifson I., Lock C. B., McDevitt G. S. Proc. Natl. Acad. Sci. USA. 1994;91:6151–6155. doi: 10.1073/pnas.91.13.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovats S., Drover S., Marshall W. H., Freed D., Whiteley P. E., Nepom G. T., Blum J. S. J. Exp. Med. 1994;179:2017–2022. doi: 10.1084/jem.179.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.