Abstract
NO inhibits cytotoxic T lymphocyte killing of target cells, although the precise mechanism is unknown. We hypothesized that NO decreases exocytosis of cytotoxic granules from activated lymphocytes. We now show that NO inhibits lymphokine-activated killer cell killing of K562 target cells. Exogenous and endogenous NO decreases the release of granzyme B, granzyme A, and perforin: all contents of cytotoxic granules. NO inhibits the signal transduction cascade initiated by cross-linking of the T cell receptor that leads to granule exocytosis. In particular, we found that NO decreases the expression of Ras, a critical signaling component within the exocytic pathway. Ectopic expression of Ras prevents NO inhibition of exocytosis. Our data suggest that Ras mediates NO inhibition of lymphocyte cytotoxicity and emphasize that alterations in the cellular redox state may regulate the exocytic signaling pathway.
Keywords: granzyme, inflammation, lymphocyte, mitogen-activated protein kinases, Ras
NO plays a complex set of roles in the immune system (1–4). NO is generated from l-arginine by one of three isoforms of NO synthase (NOS): neuronal NOS (NOS1), endothelial NOS (NOS3), and inducible NOS (iNOS or NOS2) (5, 6). NO can act as an innate immune effector, inhibiting the replication of diverse pathogens such as Mycobacterium tuberculosis, Leishmania, and coxsackievirus (7–12). However, NO and the reactive nitrogen intermediates produced by oxidation of NO can be harmful to the host. For example, NO plays a role in LPS-induced hypotension, LPS-induced lung damage, autoimmune vasculitis, autoimmune encephalomyelitis, autoimmune nephritis, and acute allograft rejection (13–21). Furthermore, NO can suppress inflammation and decrease cell injury. For example, NO inhibits vascular inflammation in part by decreasing endothelial exocytosis of factors that would otherwise promote leukocyte adherence to the vessel wall (22). NO also modulates the immune response, inhibiting T lymphocyte proliferation and differentiation, B lymphocyte proliferation and antibody production, and immune cell production of cytokines (12).
NO may also be able to modulate inflammation in part by regulating immune cell killing of target cells. Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells kill infected cells or tumor cells by several distinct pathways, including activation of the Fas/Fas ligand pathway and exocytosis of cytolytic granules. These cytolytic granules contain perforin, serine proteases (including granzymes), and other effector molecules that promote death of target cells. When a T cell or NK cell recognizes its target, an immunological synapse is formed, a cluster of signaling, adhesion, and cytoskeletal proteins that includes the T cell receptor or a NK cell receptor, tyrosine kinases such as Lck and ZAP-70, and adaptor proteins such as LAT (linker of T cell activation) and Grb2 (23–25). This cluster of signaling molecules activates downstream pathways, including Ras, Raf, MAPK/ERK kinase (MEK), and ERK. The microtubule organizing center directs the cytotoxic granules toward the synapse. Intracellular calcium levels rise, triggering exocytosis of cytolytic granules.
Prior studies suggest that NO may regulate cytotoxic cells. Some studies suggest that NO blocks activation of NK and CTL cells; other studies suggest NO has no effect (26–30). NO can also regulate lymphokine-activated killer (LAK) cell killing of target cells, but the precise effect of NO is controversial. Various studies have suggested that NO increases (31–37), decreases (26, 38), or has no effect (27, 28) on LAK cell cytotoxicity. Possible explanations for discrepancies in these studies are the different pharmacological and genetic models used to alter NO production and the different ex vivo and in vivo models used. Finally, the molecular targets of NO are not well defined.
We hypothesized that NO regulates LAK cell cytotoxicity by inhibiting the exocytosis of cytolytic granules. We find that NO inhibits LAK cell exocytosis in part by its effects on Ras, a critical component of the exocytic signaling cascade. NO inhibition of cytolytic granule exocytosis is a mechanism by which NO might decrease inflammation in autoimmune diseases or transplant rejection.
Results
Exogenous NO Inhibits LAK Cell Killing.
We first explored the effect of exogenous NO on the ability of LAK cells to kill target cells. We prepared LAK cells by isolating leukocytes from human donors and then stimulating them with IL-2 for 7 d. To confirm that these LAK cells can activate apoptosis in target cells, we cocultured the LAK cells with B cell lymphoma K562 cells and then measured poly(ADP-ribose) polymerase (PARP) cleavage by immunoblotting as a marker of apoptosis. Minimal PARP cleavage is detected in cells cocultured for 0 h together. However, LAK cells induce PARP cleavage after 2–6 h of coculture with K562 cells (Fig. 1A). The caspase inhibitors DEVD (Asp-Glu-Val-Asp) or IETD inhibit LAK cell-induced PARP cleavage.
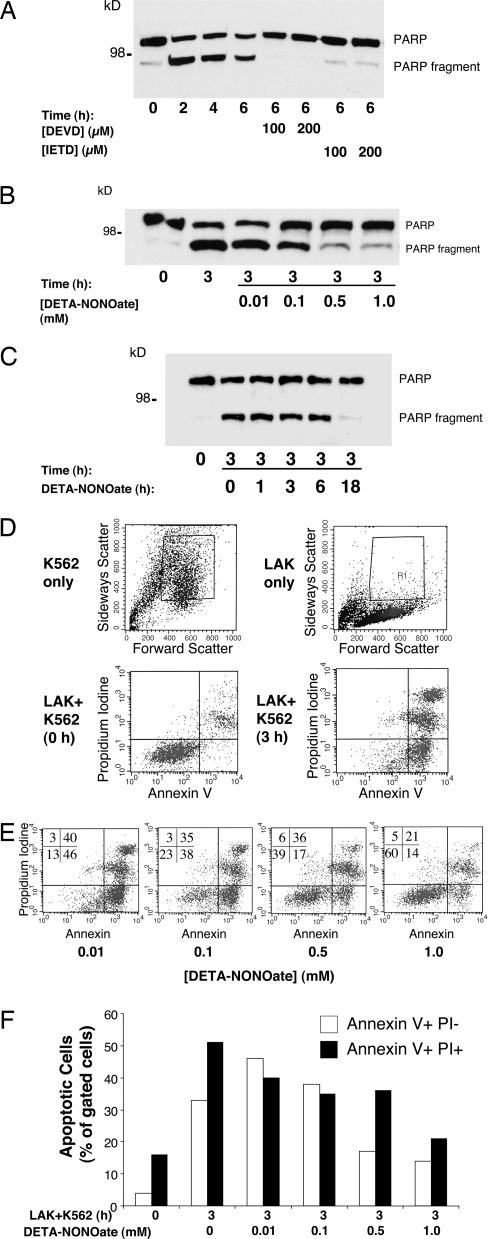
Fig. 1.
Exogenous NO inhibits LAK cell killing of K562 target cells. (A) LAK cells activate apoptosis in K562 target cells; measurement of PARP cleavage is shown. LAK cells were incubated with K562 cells for increasing periods of time, and cell lysates were analyzed by Western blotting with antibody to PARP. Some K562 cells were pretreated with inhibitors of apoptosis [DEVD (Asp-Glu-Val-Asp) or IETD]. The experiment was repeated more than five times with similar results. (B) NO inhibits LAK cell activation of apoptosis in K562 cells; dose–response is shown. LAK cells were pretreated with 0–1.0 mM DETA- NONOate for 18 h, and then LAK cell killing of K562 cells was assayed by monitoring PARP cleavage as above. (C) NO inhibits LAK cell activation of apoptosis in K562 cells; time course is shown. LAK cells were pretreated with 0.5 mM DETA-NONOate for 0–18 h, and then LAK cell killing of K562 cells was assayed by monitoring PARP cleavage as above. (D) LAK cells activate apoptosis in K562 cells as analyzed by FACS. FACS gates were set to include K562 cells (Upper Left) and exclude LAK cells (Upper Right). LAK cells were incubated with K562 cells for 0 h (Lower Left) or 3 h (Lower Right), and K562 cell apoptosis was analyzed by FACS for propidium iodide and annexin V staining. (E) NO donor decreases LAK cell killing of K562 cells as analyzed by FACS. LAK cells were pretreated with 0–1.0 mM DETA-NONOate for 18 h and incubated with K562 cells for 3 h, and K562 cell apoptosis was analyzed by FACS. Percentage of cells analyzed is shown for each quadrant. (F) Quantitation of NO inhibition of LAK cell killing of K562 cells by FACS analysis.
We next tested the effect of NO on LAK cell killing. We pretreated LAK cells with increasing concentrations of the NO donor DETA-NONOate [(Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate] for 18 h. After washing, the LAK cells were incubated with K562 cells for 3 h, and PARP cleavage was measured. DETA-NONOate treatment decreases PARP cleavage in a dose-dependent manner (Fig. 1B). To explore the length of time necessary for NO to inhibit cell killing, we pretreated LAK cells with DETA-NONOate for increasing periods of time and then incubated them with K562 cells for 3 h. Inhibition of PARP cleavage occurs between 6 and 18 h of exposure to NO (Fig. 1C).
We confirmed that DETA-NONOate inhibits LAK cell killing with another assay, FACS analysis of annexin V and propidium iodine staining. We set the gates of the FACS so that we analyzed only K562 cells and excluded LAK cells (Fig. 1D Upper). We then repeated our coculture of LAK and K562 cells and analyzed the cells by FACS for apoptosis. After 0 h of coculture, a low percentage of K562 cells display annexin V and propidium iodine staining. However, after 3 h of coculture, most K562 cells are positive for markers of apoptosis (Fig. 1D Lower). We then exposed LAK cells to the NO donor DETA-NONOate and measured their ability to kill K562 cells. DETA-NONOate decreases apoptosis of K562 cells as measured by annexin V and propidium iodine staining in a dose-dependent manner (Fig. 1 E and F).
These data show that exogenous NO inhibits LAK cell killing of target cells.
Endogenous NO Inhibits LAK Cell Killing of Target Cells.
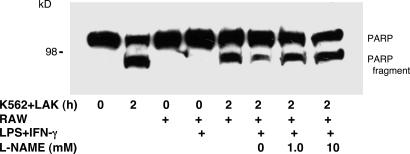
We next determined the effect of endogenously synthesized NO on LAK cell killing. We used RAW 264.7 macrophages as a source of NO. We treated RAW cells with LPS and IFN-γ for 18 h to activate expression of iNOS and then washed the cells. We then exposed LAK cells to soluble mediators, such as NO derived from these RAW macrophages, by plating LAK cells onto plastic inserts with permeable membranes and placing them adjacent to RAW cells for 6 h. The LAK cells were washed and cocultured with K562 cells for 2 h, and PARP cleavage was measured. There is no PARP cleavage in LAK and K562 cells cocultured for 0 h (Fig. 2). After 2 h of coculture, PARP cleavage is detected in cocultured cells (Fig. 2). Exposure of LAK cells to RAW cells decreases apoptosis, but only if the RAW cells are activated by LPS and IFN-γ. These data show that macrophages produce a soluble mediator that inhibits LAK cell killing. To show that this soluble mediator is NO, we pretreated RAW cells with the NOS inhibitor l-nitro-arginine methyl ester (l-NAME). l-NAME blocks the ability of activated RAW cells to inhibit LAK cell killing (Fig. 2).
Fig. 2.
Endogenous NO from activated macrophages inhibits LAK cell killing of K562 target cells. Apoptosis was measured by PARP cleavage. RAW cells were pretreated with LPS and IFN-γ for 18 h and then washed. LAK cells were plated onto plastic inserts with permeable membranes and cultured adjacent to RAW cells for 6 h. Some cultures were also treated with the NOS inhibitor l-nitro-arginine methyl ester (l-NAME) (0–10 mM). LAK cells were then washed and incubated with K562 for 2 h; the cells were harvested and analyzed for apoptosis by immunoblotting for PARP cleavage.
These data suggest that endogenous NO inhibits LAK cell killing of target cells.
NO Inhibits LAK Cell Exocytosis.
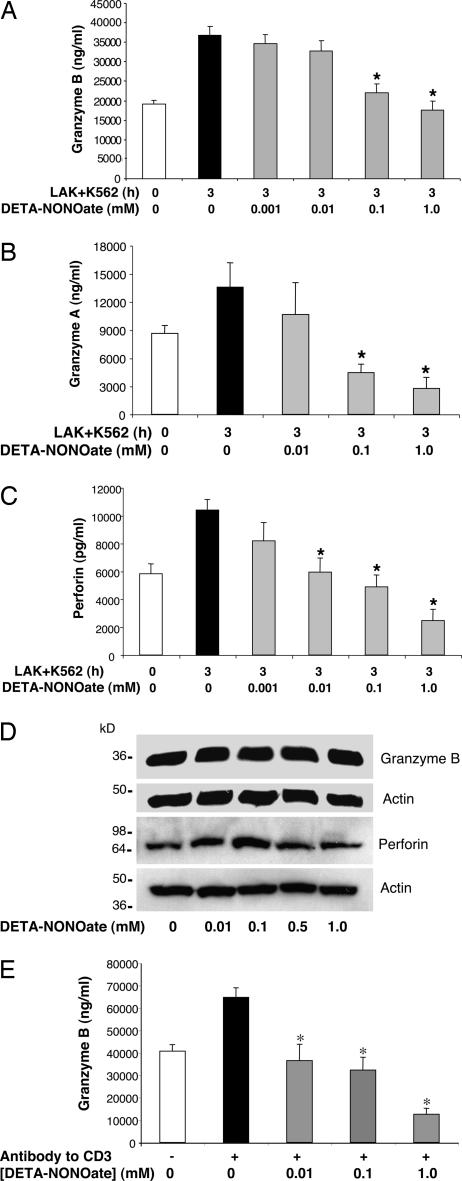
LAK cells activate target cell apoptosis by the exocytosis of granules containing compounds that mediate target cell death, including perforin and granzyme B. We next explored the effect of NO on LAK cell exocytosis of granzyme B. LAK cells were pretreated with DETA-NONOate for 18 h, washed, and incubated with K562 cells for 3 h, and the release of granzyme B into the cell media was measured by an ELISA. Exposure of LAK cells to K562 cells increases the release of granzyme B (Fig. 3A). DETA-NONOate decreases granzyme B release in a dose-dependent manner (Fig. 3A). DETA-NONOate also inhibits K562-triggered release of granzyme A and perforin, other components of LAK cell granules (Fig. 3 B and C). To confirm that NO does not affect the expression of granzyme B and perforin, we immunoblotted lysates of LAK cells exposed to DETA-NONOate. NO does not affect intracellular levels of granzyme B or perforin (Fig. 3D).
Fig. 3.
Exogenous NO inhibits LAK cell exocytosis. (A) NO inhibits granzyme B release from LAK cells stimulated by K562 cells. LAK cells were pretreated with DETA-NONOate for 18 h and added to K562 cells for 3 h, and the amount of granzyme B released into the media was measured by an ELISA (n = 4 ± SD; ∗, P < 0.01 vs. 3 h without DETA-NONOate). (B) NO inhibits granzyme A release from LAK cells stimulated by K562 cells. LAK cells and K562 cells were prepared as above, and the amount of granzyme A released into the media was measured by an ELISA (n = 3 ± SD; ∗, P < 0.01 vs. 3 h without DETA-NONOate). (C) NO inhibits perforin release from LAK cells stimulated by K562 cells. LAK cells and K562 cells were prepared as above, and the release of perforin into the media was assessed by an ELISA (n = 3 ± SD; ∗, P < 0.01 vs. 3 h without DETA-NONOate). (D) NO does not affect the LAK cell content of granzyme B and perforin. LAK cells were treated with DETA-NONOate for 18 h, and cell lysates were immunoblotted with antibody to granzyme B or perforin. (E) NO inhibits LAK cell exocytosis triggered by antibody to CD3. LAK cells were pretreated with NO donors or left untreated and then stimulated with antibody to CD3, and exocytosis was monitored with an ELISA for granzyme B (n = 3 ± SD; ∗, P < 0.01 vs. CD3 with 0 mM DETA-NONOate).
Exocytosis of cytotoxic granules for LAK cells can also be induced by antibody to CD3. Accordingly, we pretreated LAK cells with DETA-NONOate and then added antibody to CD3. DETA-NONOate also inhibits granzyme B release induced by antibody to CD3 (Fig. 3E). Taken together, these data suggest that NO inhibits exocytosis of LAK cells.
NO Inhibits Intracellular Signaling at the Level of Ras.
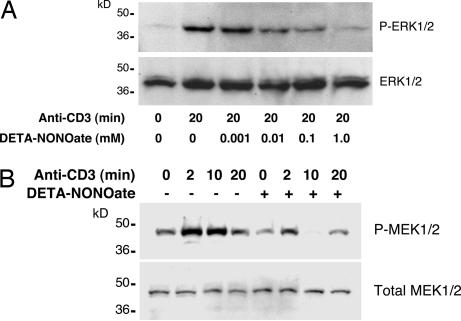
Stimulation of the T cell antigen receptor leads to the formation of an immunological synapse, activating tyrosine kinases and adaptor proteins, which in turn triggers downstream pathways, including Ras, Raf, MEK, and ERK, ultimately culminating in exocytosis of lytic granules. To see whether ERK1/2 is a target of NO, we pretreated LAK cells with DETA-NONOate and then activated LAK cell exocytosis with antibody to CD3 for 20 min. NO inhibits ERK1/2 phosphorylation in a dose-dependent manner (Fig. 4A). NO also inhibits phosphorylation of MEK1/2, a kinase that is upstream of ERK (Fig. 4B). These data suggest that NO inhibits exocytosis by regulating a pathway upstream of MEK and ERK.
Fig. 4.
Exogenous NO inhibits the MAPK pathway in LAK cells. (A) NO inhibits CD3 activation of ERK1/2 in a dose-dependent manner. LAK cells were pretreated with control or DETA-NONOate for 18 h and then stimulated with antibody to CD3, and cell lysates were immunoblotted for ERK1/2 and phospho-ERK1/2. (B) NO inhibits CD3 activation of MEK over time. LAK cells were pretreated with control or DETA-NONOate for 18 h and then stimulated with antibody to CD3, and cell lysates were immunoblotted for MEK and phospho-MEK.
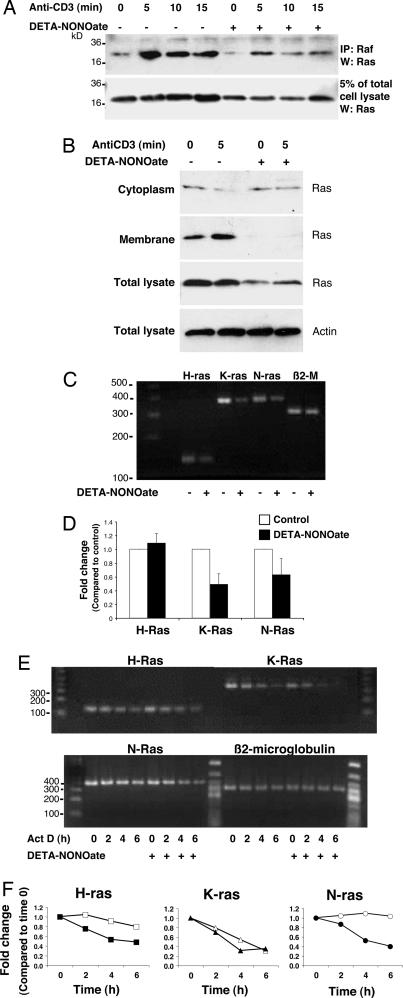
We next examined the effect of NO on Ras signaling. DETA-NONOate depresses Ras expression in LAK cells (Fig. 5A, lower blot). However, Ras can still be activated, as assessed by its ability to interact with Raf (Fig. 5A, upper blot). The NO donor not only decreases total Ras (Fig. 5B, third blot from top) but also appears to disrupt the membrane localization of Ras (Fig. 5B, second blot from top).
Fig. 5.
Exogenous NO inhibits Ras expression in LAK cells. (A) NO decreases Ras expression. LAK cells were pretreated with DETA-NONOate for 18 h and then stimulated with antibody to CD3. Cell lysates were precipitated with antibody to Raf, and precipitants were immunoblotted with antibody to Ras. Pretreatment with DETA-NONOate decreases Ras expression, but Ras can still interact with Raf. (B) NO inhibits Ras membrane localization. LAK cells were pretreated with DETA-NONOate for 18 h and then stimulated with antibody to CD3. Cell lysates were separated into cytoplasmic and membrane fractions and immunoblotted with antibody to Ras. Pretreatment with DETA-NONOate decreases Ras localization to membranes. (C) NO decreases steady-state Ras RNA levels. LAK cells were pretreated with DETA-NONOate for 18 h, and total RNA was analyzed by RT-PCR for Ras isoform mRNA or β2-microglobulin as a control. (D) NO decreases steady-state Ras RNA levels; quantification of the RT-PCR performed above is shown. (E) NO decreases stability of Ras RNA levels. LAK cells were pretreated with DETA-NONOate for 18 h, mRNA synthesis was inhibited by actinomycin D, and total RNA was analyzed by RT-PCR for Ras isoform mRNA. (F) Quantitation of NO’s effect on Ras RNA stability after actinomycin D treatment. The RT-PCR signal was measured by densitometry, normalized to the signal intensity at time 0, and then normalized to β2-microglobulin band intensity. NO pretreatment (filled symbols) decreases the mRNA stability of some isoforms of Ras compared with control (open symbols).
To further explore how NO decreases Ras protein, we analyzed the effect of DETA-NONOate on steady-state RNA levels of Ras. LAK cells were pretreated with control or DETA-NONOate, and Ras isoform RNA were measured by RT-PCR. DETA-NONOate decreases steady-state RNA levels of Ras isoforms (Fig. 5 C and D). Does NO alter the stability of Ras RNA? We pretreated LAK cells with DETA-NONOate for 16 h, added actinomycin D, and then harvested RNA at various times after the addition of actinomycin D and analyzed Ras isoform RNA by RT-PCR. DETA-NONOate decreases the stability of H-Ras and N-Ras isoform RNA over time (Fig. 5 E and F).
These data show that NO decreases Ras expression.
Overexpression of Ras Rescues LAK Cells from NO Inhibition.
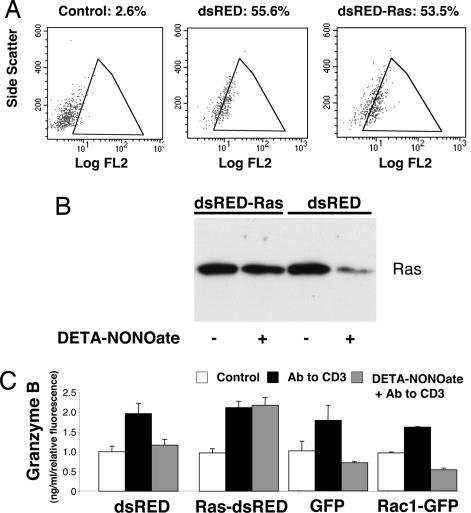
If NO inhibits exocytosis by targeting Ras, then overexpression of Ras should restore T cell exocytosis, despite treatment with NO. To test this hypothesis, we expressed Ras in LAK cells by transfecting cells with a vector that expresses the red fluorescent protein dsRED alone or a vector that expresses dsRED and N-Ras. Expression of dsRED was confirmed by FACS analysis (Fig. 6A). DETA-NONOate decreases Ras expression in cells transfected with dsRED alone. In contrast, DETA-NONOate does not decrease Ras expression in cells expressing ectopic Ras (Fig. 6B).
Fig. 6.
Overexpression of ras rescues LAK cell exocytosis from NO inhibition. (A) Transfection of LAK cells. LAK cells were transfected for 24 h with the plasmid dsRED, expressing a red fluorescent protein, or with the plasmid dsRED-Ras, expressing red fluorescent protein and N-Ras. Transfected LAK cells were analyzed by FACS for red fluorescent protein, using 488-nm excitation and 585-nm emission [fluorescence channel 2 (FL2)]. The percentage of FL2-positive cells is shown. (B) dsRED-Ras vector transfection maintains Ras levels after NO treatment of LAK cells. LAK cells were transfected with a control vector expressing dsRED or a vector expressing dsRED and Ras. Transfected cells were pretreated with DETA-NONOate or left untreated, and cell lysates were immunoblotted for Ras. (C) Ras transfection restores exocytosis in LAK cells inhibited with NO. LAK cells were transfected with vectors expressing dsRED or dsRED and Ras, or with vectors expressing GFP or Rac1 as controls. Transfected cells were pretreated with DETA-NONOate or left untreated and then stimulated with antibody to CD3. The release of granzyme B over 3 h was measured by an ELISA and normalized for transfection efficiency (n = 3 ± SD).
To evaluate the role of Ras as a target of NO, we treated the transfected cells with DETA-NONOate, activated the cells with antibody to CD3, and then measured exocytosis by granzyme B release. Overexpression of Ras has a minimal effect on exocytosis (Fig. 6C). As before, we found that DETA-NONOate inhibits exocytosis in cells transfected with dsRED alone. However, cells overexpressing Ras are protected from DETA-NONOate inhibition (Fig. 6C). (As negative controls, we also transfected cells with vectors expressing GFP or Rac1 and GFP, and we found that overexpression of Rac1 has no effect on NO inhibition of exocytosis, in contrast to Ras.). These data suggest that NO inhibits LAK cell exocytosis in part by decreasing Ras expression.
Discussion
Summary.
The major result of our study is that NO inhibits LAK cell cytotoxicity. Exposure of LAK cells to endogenous NO for 6 h suppresses LAK cell killing of target cells (Fig. 2). NO decreases Ras expression, a key component of the signaling pathway that leads to exocytosis of cytolytic granules (Fig. 5). Ectopic expression of Ras restores exocytosis to LAK cells (Fig. 6). Thus, NO inhibits LAK cell killing in part by targeting Ras.
How Does NO Decrease Ras Expression?
We found that NO decreases the expression of Ras, a key molecule in the signal transduction cascade from T cell receptor activation to cytolytic granule release. In particular, NO decreases steady-state protein levels and RNA levels of Ras by accelerating the degradation of some Ras isoform mRNA transcripts (Fig. 5). How does NO regulate Ras mRNA stability? NO can disrupt the stability of mRNA encoding other proteins, such as matrix metalloproteinase 9, by interfering with proteins that normally bind to AU-rich elements; AU elements are contained in the 3′ untranslated region of ras (39). NO decreases steady-state levels of K-Ras and N-Ras but not H-Ras (Fig. 5D). One mechanism by which NO alters N-Ras levels is by decreasing transcript stability (Fig. 5F). However, NO must decrease K-Ras levels by other mechanisms, because it does not affect K-Ras stability (Fig. 5F). Furthermore, even though NO decreases H-Ras mRNA stability (Fig. 5F), NO has no effect on overall H-Ras levels (Fig. 5D). These data suggest that NO regulates Ras isoform expression by a variety of mechanisms, one of which is at the level of mRNA stability. Because our experiments show that overexpressing Ras reverses NO inhibition of LAK cell killing, our experiments support the hypothesis that NO decreases LAK cell killing by suppressing Ras expression.
Our data do not show that NO affects Ras activity. In fact, Ras is still able to associate with Raf after DETA-NONOate treatment (Fig. 5A). Similarly, the decreased localization of Ras to the membrane may be due to the greatly diminished expression of Ras (Fig. 5B). Thus, our data do not show that NO inhibits Ras activity, only that NO regulates Ras expression.
Some prior studies have shown that NO can stimulate Ras activity in T lymphocytes. NO gas increases the GTPase activity of Ras by S-nitrosylation of a critical cysteine residue (40–43). Furthermore, endogenous NO synthesized by neuronal NOS in rat neurons in culture also activates Ras signaling (44). NO may directly activate Ras in part by promoting exchange of the guanine nucleotide GDP for GTP (45). In contrast, our data suggest that NO inhibits Ras signaling, not by modulating Ras activity but by decreasing Ras expression. Our studies differ from prior studies in several important aspects. We treated different cell types with different NO donors for longer periods of time. Considering our studies in the context of the work of others, these data suggest that short bursts of NO may activate Ras by posttranslational modification but that prolonged exposure to NO may block Ras signaling by regulating its expression.
Other Potential Targets of NO in Exocytic Signaling.
In addition to targeting Ras, NO may also affect other proteins that are part of the signaling pathway that activates granule exocytosis in leukocytes. T lymphocyte recognition of a target cell leads to the assembly at the inner cell membrane of a signaling complex that includes LAT, Grb2, phospholipase Cγ, GADS, SLP-76 (SH2-domain-containing leukocyte protein 76), VAV, and NCK (23–25). This complex activates small G proteins such as Rac and Ras, which in turn activate the MAPK cascade, leading to granule exocytosis. None of these proteins is known to be a target of NO (except for Ras, as discussed above). However, some of these proteins have cysteine residues that can be oxidized and thus are potential targets of NO. NO can also inhibit phosphatases by oxidizing the active-site cysteine residue, thereby indirectly increasing levels of tyrosine phosphorylation (46). S-nitrosylation of phosphatases such as SHP-2 that inhibit T cell signaling could potentially regulate granule secretion as well. Because the K562 target cells do not express Fas, cytolytic killing in our studies occurs through the granule pathway and not the Fas/Fas ligand pathway, which implies that NO inhibits cell killing through a Fas-independent pathway.
We have previously shown that NO inhibits exocytosis of endothelial granules by S-nitrosylation of N-ethylmaleimide-sensitive factor (NSF), an ATPase that is necessary for membrane fusion and SNARE (soluble NSF attachment protein receptor) recycling (22). However, a polypeptide that inhibits NSF had no effect on exocytosis of LAK cells compared with a control peptide (data not shown). Furthermore, exogenous NO rapidly inhibits endothelial exocytosis within 1 h of exposure, but exogenous NO had no effect on LAK cell exocytosis until >6 h of treatment. These data indirectly suggest that NO inhibits exocytosis of LAK cells by a mechanism other than directly modifying the exocytic machinery.
Physiological Implications.
Our data suggest a mechanism for how NO regulates inflammation in vivo: namely, by inhibition of lymphocyte exocytosis. This mechanism may explain some aspects of the divergent effects of NO in various animal models of inflammation. In some pathophysiological conditions where CTL killing is beneficial, NO inhibition of cytolytic T cell killing may be harmful to the host. For example, infection with influenza A virus induces iNOS expression in mice, but iNOS expression is detrimental to the host (47). Genetic deficiency of iNOS protects infected mice from lung inflammation and death. Perhaps NO blocks CTLs from killing virally infected cells (47, 48).
In other diseases where CTLs play a harmful role, NO suppression of cytolytic lymphocyte killing may be beneficial. For example, NO appears to decrease inflammation in mouse models of autoimmunity, such as experimental autoimmune encephalitis (15, 16, 49, 50). Endogenous NO also suppresses airway inflammation in murine models of asthma (51, 52). Finally, endogenous NO also protects allografts against transplant vasculopathy (18, 19, 53). Perhaps the production of NO suppresses inflammation in part by decreasing cell death that is due to cytolytic killing.
These data suggest that manipulation of NO synthesis may be a useful therapeutic strategy in selected inflammatory diseases in which an excess of cytolytic killing contributes to inflammation.
Materials and Methods
Reagents.
IL-2 was from Chiron (Emeryville, CA). The NO donor DETA-NONOate was from Cayman Chemical (Ann Arbor, MI). Ficoll-paque-PLUS was from Amersham Pharmacia Biosciences (Piscataway, NJ). The antibodies to CD3, PARP, and granzyme B were from BD Biosciences (San Jose, CA). Rabbit antibodies to phospho-Raf (Ser-259), c-Raf, phospho-ERK1/2, ERK1/2, phospho MEK, and MEK were all purchased from Cell Signaling Technology (Beverly, MA). ELISA kits (PeliKine Compact) for detection of granzyme A and B were from Sanquin Research (Flanders, NJ), and the ELISA kit for perforin was from Cell Sciences (Canton, MA). The Ras activation kit was from Upstate USA (Chicago, IL). For annexin V and propidium iodide staining, the Vybrant Apoptosis Assay Kit was used (Molecular Probes, Carlsbad, CA).
Assay for LAK Cell Exocytosis of Granzyme A and B and Perforin.
Exocytosis of granzyme A and B was measured by stimulating LAK cells with K562 cells or with antibody to CD3 in a 96-well plate coated with anti-CD3 (10 μg/ml). Granzyme A or B or perforin was detected by using ELISA kits, following the manufacturer’s instructions but with the addition of a blocking step using 7.5% milk.
RNA Extraction and RT-PCR.
RT-PCR was performed with the SuperScript First-Strand Synthesis System according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) by using primers as follows. N-Ras: sense, 5′-GATACAAAACAAGCCCACGAACTG-3′; antisense, 5′-TCAGACAGCCAAGTGAGGAGGTAG-3′ (403). K-Ras: sense, 5′-GACACAAAACAGGCTCAGGACTTAG-3′; antisense, 5′-CTCTGGGAATACTGGCACTTCG-3′ (389). H-Ras: sense, 5′-AAGCAGGTGGTCATTGATGGG-3′; antisense, 5′-GACTTGGTGTTGTTGATGGCAAAC-3′ (143). β2-Microglobulin: sense, 5′-TGAGTATGCCTGCCGTGTGAAC-3′; antisense, 5′-TCTCTGCTCCCCACCTCTAAGTTG-3′ (327).
Denaturing was carried out at 94°C for 35 s, annealing was carried out at 57°C for 40 s, and extension was carried out at 72°C for 40 s.
FACS Analysis.
We measured apoptosis of K562 cells with the Vybrant Apoptosis Assay Kit. LAK cells were pretreated with DETA-NONOate for 18 h, washed, and incubated with K562 for 3 h. Cells were washed and stained with Alexa Fluor 488 annexin V and propidium iodide (PI) and immediately analyzed by FACS with CellQuest analysis software at 530 nm and 575 nm. Gated K562 cells were measured for annexin V and PI staining.
Data Analysis.
Statistical significance was determined by one-way ANOVA with Fischer’s post hoc correction. P < 0.05 was considered significant.
For additional information, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Dr. James Mahoney (The Johns Hopkins University School of Medicine) for advice and assistance with preparation of LAK cells and cytotoxic assays, Angela H. McFillin and Polly Robarts for assistance with collecting blood, and Drs. Anthony Rosen and Stephen Desiderio (both of The Johns Hopkins University School of Medicine) for advice and assistance. This work was supported by National Institutes of Health Grants R01 HL63706, R01 HL074061, P01 HL65608, P01 HL56091 (to C.J.L.), HL72518 (to N.F.), and HL70929 (to K.I.); American Heart Association Grant EIG 0140210N; the Pfizer International HDL Research Awards Program; the Ciccarone Center; and the John and Cora H. Davis Foundation (C.J.L.).
Abbreviations
- CTL
cytotoxic T lymphocyte
- LAK
lymphokine-activated killer
- NOS
NO synthase
- iNOS
inducible NOS
- MEK
MAPK/ERK kinase
- PARP
poly(ADP-ribose) polymerase
- DETA-NONOate
(Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fang F. C. Nat. Rev. Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 3.Fang F. C. J. Clin. Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacMicking J., Xie Q. W., Nathan C. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 5.Michel T., Feron O. J. Clin. Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan C., Xie Q. W. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 7.Nathan C., Shiloh M. U. Proc. Natl. Acad. Sci. USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacMicking J. D., North R. J., LaCourse R., Mudgett J. S., Shah S. K., Nathan C. F. Proc. Natl. Acad. Sci. USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray H. W., Nathan C. F. J. Exp. Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaragoza C., Ocampo C. J., Saura M., Bao C., Leppo M., Lafond-Walker A., Thiemann D. R., Hruban R., Lowenstein C. J. J. Immunol. 1999;163:5497–5504. [PubMed] [Google Scholar]
- 11.Saura M., Zaragoza C., McMillan A., Quick R. A., Hohenadl C., Lowenstein J. M., Lowenstein C. J. Immunity. 1999;10:21–28. doi: 10.1016/S1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 13.MacMicking J. D., Nathan C., Hom G., Chartrain N., Fletcher D. S., Trumbauer M., Stevens K., Xie Q. W., Sokol K., Hutchinson N., et al. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 14.Gilkeson G. S., Mudgett J. S., Seldin M. F., Ruiz P., Alexander A. A., Misukonis M. A., Pisetsky D. S., Weinberg J. B. J. Exp. Med. 1997;186:365–373. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahrbacher U. C., Lechner F., Eugster H. P., Frei K., Lassmann H., Fontana A. Eur. J. Immunol. 1998;28:1332–1338. doi: 10.1002/(SICI)1521-4141(199804)28:04<1332::AID-IMMU1332>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Fenyk-Melody J. E., Garrison A. E., Brunnert S. R., Weidner J. R., Shen F., Shelton B. A., Mudgett J. S. J. Immunol. 1998;160:2940–2946. [PubMed] [Google Scholar]
- 17.Gabbai F. B., Boggiano C., Peter T., Khang S., Archer C., Gold D. P., Kelly C. J. J. Immunol. 1997;159:6266–6275. [PubMed] [Google Scholar]
- 18.Qian Z., Gelzer-Bell R., Yang Sx S. X., Cao W., Ohnishi T., Wasowska B. A., Hruban R. H., Rodriguez E. R., Baldwin W. M., III, Lowenstein C. J. Circulation. 2001;104:2369–2375. doi: 10.1161/hc4401.098471. [DOI] [PubMed] [Google Scholar]
- 19.Koglin J., Glysing-Jensen T., Mudgett J. S., Russell M. E. Circulation. 1998;97:2059–2065. doi: 10.1161/01.cir.97.20.2059. [DOI] [PubMed] [Google Scholar]
- 20.Petros A., Bennett D., Vallance P. Lancet. 1991;338:1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- 21.Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. Proc. Natl. Acad. Sci. USA. 1990;87:3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita K., Morrell C. N., Cambien B., Yang S. X., Yamakuchi M., Bao C., Hara M. R., Quick R. A., Cao W., O’Rourke B., et al. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman J. Nat. Rev. Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 24.Vivier E., Nunes J. A., Vely F. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 25.Samelson L. E. Annu. Rev. Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 26.Kurose I., Miura S., Saito H., Tada S., Fukumura D., Higuchi H., Ishii H. Gastroenterology. 1995;109:1958–1968. doi: 10.1016/0016-5085(95)90764-5. [DOI] [PubMed] [Google Scholar]
- 27.Samlowski W. E., Yim C. Y., McGregor J. R. Nitric Oxide. 1998;2:45–56. doi: 10.1006/niox.1998.0169. [DOI] [PubMed] [Google Scholar]
- 28.Salvucci O., Kolb J. P., Dugas B., Dugas N., Chouaib S. Blood. 1998;92:2093–2102. [PubMed] [Google Scholar]
- 29.Langrehr J. M., Dull K. E., Ochoa J. B., Billiar T. R., Ildstad S. T., Schraut W. H., Simmons R. L., Hoffman R. A. Transplantation. 1992;53:632–640. doi: 10.1097/00007890-199203000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Medot-Pirenne M., Heilman M. J., Saxena M., McDermott P. E., Mills C. D. J. Immunol. 1999;163:5877–5882. [PubMed] [Google Scholar]
- 31.Cifone M. G., Festuccia C., Cironi L., Cavallo G., Chessa M. A., Pensa V., Tubaro E., Santoni A. Cell. Immunol. 1994;157:181–194. doi: 10.1006/cimm.1994.1215. [DOI] [PubMed] [Google Scholar]
- 32.Juretic A., Spagnoli G. C., von Bremen K., Horig H., Filgueira L., Luscher U., Babst R., Harder F., Heberer M. Cell. Immunol. 1994;157:462–477. doi: 10.1006/cimm.1994.1242. [DOI] [PubMed] [Google Scholar]
- 33.Yim C. Y., McGregor J. R., Kwon O. D., Bastian N. R., Rees M., Mori M., Hibbs J. B., Jr, Samlowski W. E. J. Immunol. 1995;155:4382–4390. [Google Scholar]
- 34.Cifone M. G., D’Alo S., Parroni R., Millimaggi D., Biordi L., Martinotti S., Santoni A. Blood. 1999;93:3876–3884. [PubMed] [Google Scholar]
- 35.Jyothi M. D., Khar A. Scand. J. Immunol. 2000;52:148–155. doi: 10.1046/j.1365-3083.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- 36.De Maria R., Cifone M. G., Trotta R., Rippo M. R., Festuccia C., Santoni A., Testi R. J. Exp. Med. 1994;180:1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. J. Exp. Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orucevic A., Lala P. K. Cell. Immunol. 1996;169:125–132. doi: 10.1006/cimm.1996.0100. [DOI] [PubMed] [Google Scholar]
- 39.Akool el S., Kleinert H., Hamada F. M., Abdelwahab M. H., Forstermann U., Pfeilschifter J., Eberhardt W. Mol. Cell. Biol. 2003;23:4901–4916. doi: 10.1128/MCB.23.14.4901-4916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lander H. M., Ogiste J. S., Pearce S. F., Levi R., Novogrodsky A. J. Biol. Chem. 1995;270:7017–7020. doi: 10.1074/jbc.270.13.7017. [DOI] [PubMed] [Google Scholar]
- 41.Lander H. M., Milbank A. J., Tauras J. M., Hajjar D. P., Hempstead B. L., Schwartz G. D., Kraemer R. T., Mirza U. A., Chait B. T., Burk S. C., Quilliam L. A. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 42.Lander H. M., Hajjar D. P., Hempstead B. L., Mirza U. A., Chait B. T., Campbell S., Quilliam L. A. J. Biol. Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 43.Deora A. A., Hajjar D. P., Lander H. M. Biochemistry. 2000;39:9901–9908. doi: 10.1021/bi992954b. [DOI] [PubMed] [Google Scholar]
- 44.Yun H. Y., Gonzalez-Zulueta M., Dawson V. L., Dawson T. M. Proc. Natl. Acad. Sci. USA. 1998;95:5773–5778. doi: 10.1073/pnas.95.10.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams J. G., Pappu K., Campbell S. L. Proc. Natl. Acad. Sci. USA. 2003;100:6376–6381. doi: 10.1073/pnas.1037299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu D., Rovira I. I., Finkel T. Dev. Cell. 2002;2:251–252. doi: 10.1016/s1534-5807(02)00132-6. [DOI] [PubMed] [Google Scholar]
- 47.Karupiah G., Chen J. H., Mahalingam S., Nathan C. F., MacMicking J. D. J. Exp. Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hesse M., Cheever A. W., Jankovic D., Wynn T. A. Am. J. Pathol. 2000;157:945–955. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalton D. K., Wittmer S. J. Neuroimmunol. 2005;160:110–121. doi: 10.1016/j.jneuroim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 50.van der Veen R. C., Dietlin T. A., Hofman F. M. J. Neuroimmunol. 2003;145:86–90. doi: 10.1016/j.jneuroim.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Xiong Y., Karupiah G., Hogan S. P., Foster P. S., Ramsay A. J. J. Immunol. 1999;162:445–452. [PubMed] [Google Scholar]
- 52.Rodriguez D., Keller A. C., Faquim-Mauro E. L., de Macedo M. S., Cunha F. Q., Lefort J., Vargaftig B. B., Russo M. J. Immunol. 2003;171:1001–1008. doi: 10.4049/jimmunol.171.2.1001. [DOI] [PubMed] [Google Scholar]
- 53.Shears L. L., Kawaharada N., Tzeng E., Billiar T. R., Watkins S. C., Kovesdi I., Lizonova A., Pham S. M. J. Clin. Invest. 1997;100:2035–2042. doi: 10.1172/JCI119736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.