Abstract
Aldehyde dehydrogenase (ALDH) is an enzyme that is expressed in the liver and is required for the conversion of retinol (vitamin A) to retinoic acids. ALDH is also highly enriched in hematopoietic stem cells (HSCs) and is considered a selectable marker of human HSCs, although its contribution to stem cell fate remains unknown. In this study, we demonstrate that ALDH is a key regulator of HSC differentiation. Inhibition of ALDH with diethylaminobenzaldehyde (DEAB) delayed the differentiation of human HSCs that otherwise occurred in response to cytokines. Moreover, short-term culture with DEAB caused a 3.4-fold expansion in the most primitive assayable human cells, the nonobese diabetic/severe combined immunodeficiency mouse repopulating cells, compared with day 0 CD34+CD38−lin− cells. The effects of DEAB on HSC differentiation could be reversed by the coadministration of the retinoic acid receptor agonist, all-trans-retinoic acid, suggesting that the ability of ALDH to generate retinoic acids is important in determining HSC fate. DEAB treatment also caused a decrease in retinoic acid receptor-mediated signaling within human HSCs, suggesting directly that inhibition of ALDH promotes HSC self-renewal via reduction of retinoic acid activity. Modulation of ALDH activity and retinoid signaling is a previously unrecognized and effective strategy to amplify human HSCs.
Keywords: retinoic acid, self-renewal, diethylaminobenzaldehyde, long-term repopulating cells
Hematopoietic stem cells (HSCs) possess the unique capacity to self-renew and give rise to all mature lymphohematopoietic progeny throughout the lifetime of an individual (1, 2). Several molecular pathways that regulate HSC self-renewal have now been identified, including Notch (3), HOXB4 (4), Wnt (5), and bone morphogenetic protein signaling pathways (6). The osteoblastic niche for HSCs within the bone marrow (BM) has also been characterized (7, 8). Despite these advances in understanding HSC biology, clinical methods to amplify human HSCs have yet to be realized, and characterization of the pathways that regulate HSC self-renewal continues to evolve.
Two decades ago, Colvin et al. (9, 10) demonstrated that the intracellular enzyme, aldehyde dehydrogenase (ALDH), protected BM progenitors from the cytotoxic effects of cyclophosphamide by deactivation of its metabolite, 4-hydroxycyclophosphamide (9, 10). Several isoforms of ALDH have been identified, with ALDH1 being the primary isoform expressed within human hematopoietic progenitors (11, 12). Recent studies have shown that human and murine hematopoietic progenitors can be isolated by using a fluorescently labeled dye specific for ALDH activity (13–16) and cord blood (CB) ALDHbrlin− cells are enriched for nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse repopulating cells [SCID-repopulating cells (SRCs)] (15, 16). Although these data demonstrate that ALDH is a selectable marker for human stem/progenitor cells, the HSC-specific function of ALDH remains unknown. In the liver, ALDH1 contributes primarily to the metabolism of retinol (vitamin A) into retinoic acid (17). Because ALDH1 is also highly concentrated in HSCs, it is plausible that the primary function of ALDH1 in HSCs relates to its production of retinoids.
The biological actions of retinoids are mediated by the retinoic acid receptor (RAR) and retinoid X receptor (RXR), ligand-dependent transcription factors that are expressed in the nuclei of target cells (18–20). Through its actions on these receptors, all-trans-retinoic acid (ATRA) induces cellular differentiation, tissue patterning, and embryonic development in vertebrates (18–22). ATRA is also used therapeutically to induce the differentiation of acute promyelocytic leukemia cells, in which the characteristic 15;17 translocation results in a fusion protein (PML-RARα) that harbors dominant negative activity against the RARα receptor (23). In light of these data, we postulated that HSC differentiation might depend on retinoid signaling and that inhibition of ALDH, which is required for production of retinoic acids, could interfere with HSC differentiation. In this study, we demonstrate that ALDH activity is necessary for normal HSC differentiation to occur in response to cytokines and that inhibition of ALDH, coupled with early acting cytokines, is sufficient to induce the quantitative expansion of human SRCs. Our findings indicate that inhibition of ALDH activity and retinoid signaling can impart a robust expansion of HSCs.
Results
Inhibition of ALDH Delays the Differentiation of HSCs in Culture.
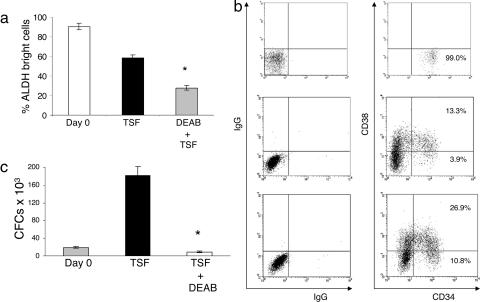
We first determined whether inhibition of ALDH activity in primary BM and CB CD34+CD38−lin− cells affected the differentiation of HSCs when cultured for 7 days in the presence of thrombopoietin (20 ng/ml), stem cell factor (SCF; 100 ng/ml), and flt-3 ligand (50 ng/ml), a cytokine combination which we have found optimizes the proliferation of human HSCs in culture (24). FACS-sorted human CD34+CD38−lin− cells were used because this population has been shown to be the most highly enriched population for human HSCs (25). Treatment with 100 μM diethylaminobenzaldehyde (DEAB) + TSF significantly reduced ALDH activity in CD34+CD38− cells compared with day 0 or after treatment with TSF alone, and >90% of day 0 CB CD34+CD38−lin− cells demonstrated ALDH activity (Fig. 1a, P < 0.001 and P < 0.001). The progeny of CB and BM CD34+CD38−lin− cells after 7 days of culture with DEAB + TSF contained significantly higher percentages of primitive CD34+CD38− cells compared with the day 7 progeny of TSF alone (Fig. 1b and Fig. 5a, which is published as supporting information on the PNAS web site; P = 0.02 and P = 0.01, respectively). DEAB + TSF cultures supported a mean 4-fold total cell expansion and a maintenance of absolute numbers of BM CD34+CD38− cells compared with day 0. Conversely, TSF culture supported a mean 15-fold increase in total cells, but this increase was associated with a 5-fold decrease in BM CD34+CD38− cell numbers compared with input (P = 0.01). Table 1 summarizes the effect of DEAB on HSC expansion in vitro. As expected, HSC-enriched day 0 CD34+CD38−lin− cells demonstrated little colony-forming cell (CFC) content (Fig. 1c). Seven days of culture of CD34+CD38−lin− cells with TSF alone caused a 5-fold increase in CFCs compared with input, indicating HSC differentiation during culture. Conversely, the progeny of CD34+CD38−lin− cells cultured with DEAB + TSF contained little CFC content, indicating that DEAB impeded HSC maturation during culture (P = 0.002). As further confirmation of the inhibitory effect of DEAB on HSC maturation in vitro, extended 14-day cultures of CB CD34+CD38−lin− cells with DEAB + TSF continued to maintain a discrete population of CD34+CD38− cells at day 14 (Fig. 5b; mean 17.5% ± 6.1). Morphologic examination of the progeny of DEAB + TSF cultures revealed a predominance of cells with high nuclear:cytoplasmic ratios and prominent nucleoli, whereas TSF-cultured progeny contained primarily bands and myelocytes, suggesting that DEAB treatment maintained more immature progenitors during culture (data not shown). To determine the effect of DEAB alone on human HSCs, CB CD34+CD38−lin− cells were also placed in culture with 100 μM DEAB in the absence of TSF and, at day 7, no viable cells were detected in culture, indicating that DEAB alone did not induce the proliferation or self-renewal of human HSCs in the absence of hematopoietic cytokines. Taken together, these results indicated that inhibition of ALDH with DEAB impeded HSC differentiation that otherwise occurred in response to cytokines.
Fig. 1.
ALDH inhibition impedes the differentiation of human HSCs. FACS-sorted CB CD34+CD38−lin− cells were analyzed for ALDH activity levels and compared with the ALDH activity level in the CD34+CD38− progeny of 7-day cultures with thrombopoietin, SCF, and flt3-ligand (TSF) alone versus TSF + DEAB (a). (b) The surface expression of CD34 and CD38 on day 0 CB CD34+CD38−lin− cells (Top) and their progeny after culture with TSF alone (Middle) versus TSF + DEAB (Bottom) is shown. Culture with TSF alone caused a marked increase in CFC content compared with day 0 CD34+CD38−lin− cells, whereas the progeny of DEAB + TSF cultures contained little CFC activity, indicating an inhibition of HSC differentiation during culture (c). ∗ indicates a statistically significant difference between the DEAB + TSF treated group versus TSF alone.
Table 1.
Expansion of CD34+ CD38−lin− cells after treatment with DEAB and ATRA
| Culture condition | Day 0 | Day 7 | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | Cell count (×103) | Total (×103) | CD34+ (×103) | CD34+CD38− (×103) | ||||
| Cell count | Fold change | Cell count | Fold change | Cell count | Fold change | |||
| TSF | BM | 5 | 71.4 ± 4 | 15.0 | 26.4 ± 1 | 5.5 | 1.1 ± 0.1 | −5 |
| TSF + DEAB | BM | 5 | 19.2 ± 2 | 4.0 | 14.4 ± 1 | 3.0 | 4.3 ± 0.3 | — |
| TSF | CB | 5 | 950.0 ± 80 | 190.0 | 100.0 ± 10 | 20.0 | 80.0 ± 1.0 | 16.0 |
| TSF + DEAB | CB | 5 | 220.0 ± 60 | 44.0 | 83.0 ± 2 | 16.6 | 58.0 ± 2.0 | 11.6 |
| TSF + ATRA | CB | 5 | 290.0 ± 70 | 58.0 | 10.0 ± 1 | 2.0 | 0.0 | — |
Primary human BM or CB CD34+CD38−lin− cells were placed in culture with either TSF alone, TSF + DEAB, or TSF + ATRA. At day 7, the number of total cells, CD34+ cells, and CD34+CD38− cells in each culture condition was quantified and compared with input.
For each culture condition, 3–6 experiments were performed.
Inhibition of ALDH Increases the Number of Human SRCs in Short-Term Culture.
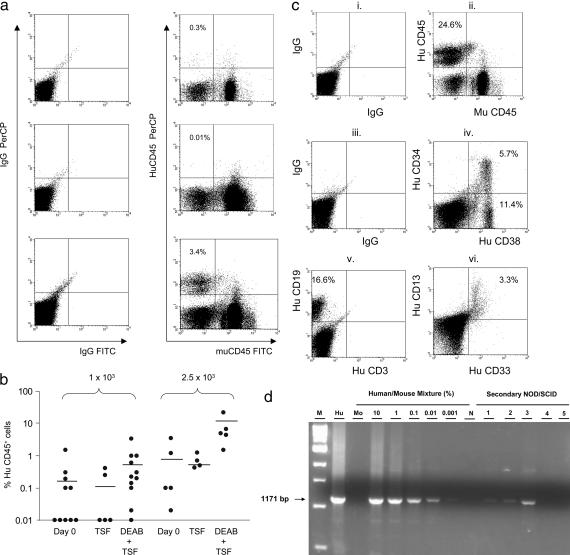
To determine whether inhibition of ALDH promoted the self-renewal of HSCs in culture, we performed limiting dilution repopulation assays as we and others have described (24–27) to estimate the SRC frequency in day 0 CB CD34+CD38−lin− cells or their progeny after culture with DEAB. Analysis of NOD/SCID mice at 8 weeks posttransplant demonstrated that the progeny of CB CD34+CD38−lin− cells cultured with DEAB + TSF contained significantly increased SRC capacity than either day 0 CB CD34+CD38−lin− cells or the progeny of cells cultured with TSF alone (Fig. 2a). At a dose of 0.5 × 103, no mice transplanted with day 0 CB CD34+CD38−lin− cells or their progeny after culture with TSF alone or TSF + DEAB demonstrated human hematopoietic cell engraftment. At doses of 1–2.5 × 103 cells, only 3 of 15 mice (20%) transplanted with day 0 CD34+CD38−lin− cells showed huCD45+ cell engraftment (Fig. 2b). Similarly, only 1 of 9 mice transplanted with the progeny of this dose after culture with TSF alone demonstrated human engraftment. In contrast, 8 of 16 mice (50%) transplanted with the progeny of CD34+CD38−lin− cells cultured with DEAB + TSF demonstrated human cell engraftment (Fig. 2b). Mice engrafted with DEAB-cultured progeny (2.5 × 103 dose) also demonstrated 1 log higher repopulation (mean 8.0% huCD45+ cells, range 1.5–21.6%) as compared with mice transplanted with day 0 CB CD34+CD38−lin− cells (mean 0.9%, range 0.02–3.5%, P = 0.048) or their progeny after culture with TSF (0.7%, range 0.4–1.3%), indicating that DEAB-cultured progeny also had a higher in vivo proliferative potential than the day 0 HSCs or TSF-cultured cells. Poisson statistical analysis (24–28) indicated that the SRC frequency within day 0 CB CD34+CD38−lin− cells was 1 in 7,500 cells (95% confidence interval of 1/2,900 to 1/30,000). The SRC frequency within the progeny of TSF-cultured CB CD34+CD38−lin− cells was more than 2-fold reduced at 1 in 17,000 cells (confidence interval of 1/3,800 to 1/290,000). In contrast, the SRC frequency within the progeny of DEAB + TSF-cultured CB CD34+CD38−lin− cells was 1 in 2,200 cells (confidence interval of 1/1,100 to 1/4,900), which was 3.4-fold higher than day 0 CB CD34+CD38−lin− cells and 7.7-fold higher than TSF-cultured progeny (P = 0.056 and P = 0.011, respectively). The human cell engraftment observed in all primary transplanted NOD/SCID mice is summarized in Table 2, which is published as supporting information on the PNAS web site. These data demonstrate that inhibition of ALDH not only inhibited the differentiation of HSCs but also promoted the amplification of HSCs in culture. Detailed flow cytometric analysis revealed extensive CD34+ progenitor cell, CD19+ B lymphoid, and CD33/13+ myeloid differentiation in mice transplanted with DEAB + TSF-cultured cells, demonstrating that a pluripotent repopulating cell was sustained during culture with DEAB (Fig. 2c). Of note, the ratio of human CD19+ B lymphoid cells to CD33/CD13+ myeloid cells detectable at 8 weeks in mice transplanted with day 0 CD34+CD38−lin− cells was ≈2:1 (mean 3.2% CD19+ cells to mean 1.7% CD13/33+ cells), whereas this ratio was 2.7:1 in mice transplanted with the progeny of CD34+CD38−lin− cells after culture with DEAB + TSF. These data suggest that inhibition of ALDH may have modestly inhibited the myeloid differentiation capacity of HSCs expanded under this condition.
Fig. 2.
DEAB treatment promotes the amplification of human SRCs. (a) Representative flow cytometric analysis of human CD45 versus murine CD45 surface staining in NOD/SCID mice is shown at week 8 in mice transplanted with 1 × 103 day 0 CB CD34+CD38−lin− cells (Top), their TSF-cultured progeny (Middle), or their progeny after DEAB + TSF culture (Bottom). (b) A scatter plot of human CD45+ cell engraftment in NOD/SCID mice at 8 weeks posttransplantation is shown with each individual point representing a single transplanted mouse. Mice transplanted with the progeny of CB CD34+CD38−lin− cells cultured with DEAB + TSF demonstrated significantly increased frequency of human engraftment (≥1.0%) and percent huCD45+ cell repopulation compared with day 0 CB CD34+CD38−lin− cells or their progeny after culture with TSF alone. The mean levels of huCD45+ cells per culture condition are indicated by horizontal lines. (c) Multilineage engraftment of CD45+ cells, CD34+ progenitor cells, CD19+ B cells, and CD33/13+ myeloid cells is shown in the BM of a representative NOD/SCID mouse transplanted with the progeny of 2.5 × 103 CB CD34+CD38−lin− cells after DEAB + TSF culture, demonstrating multilineage in vivo differentiation of DEAB treated cells. Shown are IgGPerCP versus IgGFITC control staining (i), huCD45 versus muCD45 staining (ii), IgGFITC versus IgG-phycoerythrin control staining (iii), huCD34 versus huCD38 staining (iv), huCD19 versus huCD3 staining (v), and huCD13 versus huCD33 staining (vi). (d) PCR analysis for a 1,171-bp segment of the human chromosome 17-specific α-satellite region demonstrates engraftment of human cells in secondary mice transplanted with BM cells from primary mice that had been transplanted with limiting dilutions of DEAB + TSF-cultured CB CD34+CD38−lin− cells. Human engraftment is evident at the 0.001–0.1% levels in three of the five mice analyzed. The label “M” represents a 1-kB DNA marker; “Hu” identifies 100% human CB cell DNA; “Mo” identifies 100% mouse BM cell DNA; and “N” identifies the no template control (dH20).
Although our primary limiting dilution NOD/SCID transplantation studies were not designed to achieve the necessary high levels (20–50%) of human CD45+ cell chimerism to allow for secondary transplantation studies, we did perform a limited number of secondary transplants to assess for the presence of long-term repopulating stem cells within the DEAB-cultured populations. At 12 weeks after tail-vein transplantation of 75% of the total BM cells from primary recipients of DEAB + TSF-cultured cells (2.5 × 103 dose), 3 of the 5 mice analyzed demonstrated human hematopoietic cell engraftment via PCR analysis for a 1,171-bp fragment of human chromosome 17-specific α-satellite region (Fig. 2d) (29). These results confirmed that inhibition of ALDH activity promoted the differential maintenance of long-term repopulating stem cells in culture.
DEAB Specifically Inhibits ALDH1 and Decreases Retinoic Acid Activity in HSCs.
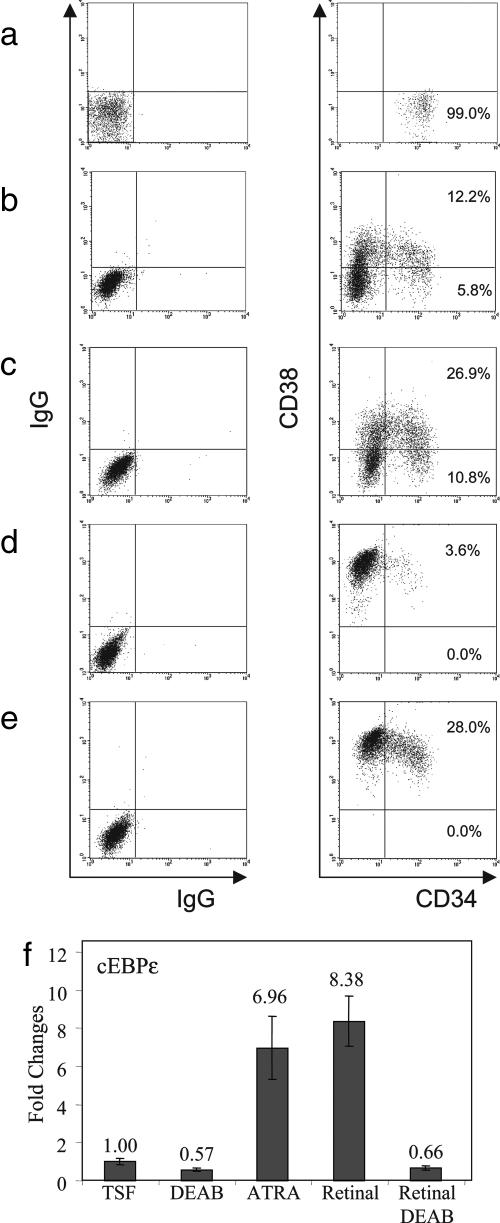
To further test our hypothesis that ALDH contributes to HSC differentiation through the production of retinoic acids, we first evaluated the effects of ATRA on primary CB CD34+CD38−lin− cells in culture. In contrast to DEAB treatment, which maintained a population of CD34+CD38− cells in culture at day 7 (Fig. 3a–c), treatment of CB CD34+CD38−lin− cells with TSF + 1 μM ATRA resulted in a marked decline in CD34+ cells in culture compared with TSF alone, and no CD34+CD38− cells were detectable at day 7 (Fig. 3d). Moreover, when we cultured CB CD34+CD38−lin− cells with 1 μM ATRA + 100 μM DEAB + TSF, phenotypic differentiation of CD34+CD38− cells again occurred, indicating that provision of extracellular retinoids overcame the effects of DEAB-induced inhibition of ALDH (Fig. 3e). Taken together, these results suggested that ALDH might contribute to HSC differentiation through its production of intracellular retinoids. Analysis of CFC content within cultures treated with ATRA + TSF demonstrated a >10-fold reduction in CFCs within ATRA-treated cultures compared with the progeny of TSF alone, indicating that ATRA either inhibited CFC formation or promoted terminal progenitor cell differentiation, thereby reducing CFC numbers (data not shown). As further support of the relationship between ALDH activity and retinoid production in HSCs, we also cultured human CB CD34+CD38−lin− cells with 100 nM of 4-(E-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl) benzoic acid (TTNPB), an RAR-specific agonist, with TSF and observed a marked differentiation of cells by day 7 of culture. The effect of TTNPB also overcame the effect of DEAB toward inhibiting HSC differentiation (Fig. 6 a and b, which is published as supporting information on the PNAS web site). The addition of 100 μM of LGD101268, a synthetic agonist of the RXR (courtesy of Ligand Pharmaceuticals, San Diego, CA), also completely overcame the inhibitory effect of DEAB on HSC differentiation during culture (Fig. 6 c and d). Lastly, the addition of 100 nM of 1,25-dihydroxyvitamin D3 (vitamin D), which has been shown to induce the differentiation of human hematopoietic progenitor cells (30), also overcame the effects of DEAB on HSCs in culture (Fig. 6 e and f). Interestingly, the addition of DEAB did appear to increase the maintenance of CD34+ progenitor cells in culture when combined with either the RAR agonist, the RXR agonist, or vitamin D, suggesting that inhibition of ALDH activity moderately slowed the differentiation of HSCs that occurred in response to these three ligands (Fig. 6 a–f). Nonetheless, these data demonstrated that the addition of exogenous retinoids, rexinoids, or vitamin D generally overcame the effect of DEAB toward inhibiting HSC differentiation, further supporting our hypothesis that ALDH mediates its effects on HSC fate via its contribution to retinoid production.
Fig. 3.
DEAB inhibits ALDH1 activity and decreases retinoid signaling in HSCs. FACS-sorted CB CD34+CD38−lin− cells (a) were cultured with TSF alone (b), and their phenotype was compared with the progeny of cells cultured with TSF + DEAB (c). For further comparison, CD34+CD38−lin− cells were cultured with ATRA + TSF (d), which induced a marked loss of CD34+ cells and CD34+CD38− cells in culture as compared with input or TSF culture, consistent with accelerated differentiation during culture. The addition of ATRA to TSF also overcame the inhibitory effects of DEAB on HSC differentiation (e), suggesting that exogenous retinoids can overcome the effect of ALDH inhibition on HSCs. (f) Real-time PCR analysis demonstrated a 50% reduction in cEBPε expression in DEAB + TSF-treated cells at day 7 compared with TSF alone. Whereas addition of the substrate for ALDH1, retinaldehyde, markedly increased cEBPε expression in HSCs, the addition of DEAB completely blocked this effect, suggesting that DEAB inhibited ALDH1 activity, thereby impeding retinoid signaling in HSCs.
Because several isoforms of ALDH exist (31), we sought to determine whether DEAB was specifically inhibiting ALDH1, which is known to be highly expressed in HSCs (11, 14, 32) and is the predominant regulator of retinoic acid synthesis in mammals (31), versus other isoforms. First, we observed that treatment of HSCs with DEAB for 7 days caused a 2-fold decrease in the expression of cEBPε (Fig. 3f), which is under the control of RAR signaling (33), confirming that inhibition of ALDH activity with DEAB caused a decrease in retinoid activity in human HSCs. Secondly, the addition of 1 μM retinaldehyde, which is a specific substrate for ALDH1, to HSC cultures caused an 8.4-fold increase in cEBPε expression in HSCs, confirming the contribution of ALDH1 to retinoic acid activity in these cells. However, when DEAB was added to HSC cultures supplemented with retinaldehyde, the effect of retinaldehyde on cEBPε expression was significantly reduced, demonstrating that DEAB significantly inhibited the activity of ALDH1 in HSCs, which consequently caused a marked decrease in retinoid signaling in these primitive cells (Fig. 3f).
Inhibition of ALDH Activity Up-Regulates HOXB4 Expression in Human HSCs.
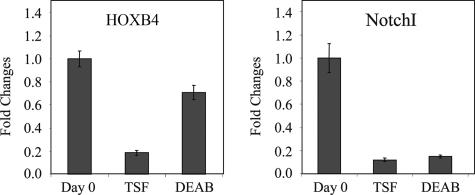
Because HOXB4 and Notch have established roles in HSC self-renewal (3, 4), we sought to determine whether ALDH inhibition might regulate HSC self-renewal by altering the transcription of either of these target genes. Interestingly, culture of primary CB CD34+CD38−lin− cells with TSF alone caused a 5-fold decrease in HOXB4 transcription compared with day 0 CB CD34+CD38−lin− cells, whereas the addition of DEAB to TSF maintained HOXB4 expression at 70% of input levels (Fig. 4). Conversely, treatment with DEAB did not alter Notch transcription compared with TSF alone. Taken together, these data indicate that inhibition of ALDH may also promote HSC self-renewal via discrete interactions with other established pathways, such as HOXB4, although the organization of these signals is yet to be elucidated.
Fig. 4.
Treatment with DEAB sustains HOXB4 expression in HSCs. RNA was isolated from multiple replicates of FACS-sorted CB CD34+CD38−lin− cells at day 0 and their progeny after culture with TSF alone or DEAB + TSF. The RNA was reverse-transcribed, and the expression of HOXB4 and Notch 1 was analyzed by quantitative real-time PCR. (Left) The expression of HOXB4 in CD34+CD38− cells was significantly reduced after short-term culture with TSF, whereas treatment with DEAB prevented the down-regulation of HOXB4 expression over time. (Right) The expression of Notch was also significantly reduced after TSF culture and was not altered by treatment with DEAB.
Discussion
Improved characterization of the pathways that regulate HSC self-renewal will facilitate the development of therapies to amplify HSCs in vitro or in vivo for clinical purposes. In this study, we have characterized the novel contributions of the enzyme ALDH and retinoid signaling to human HSC differentiation and self-renewal. ALDHs are NAD(P)+-dependent enzymes that oxidize a large number of aldehydes to their corresponding carboxylic acids (17, 31). Several different ALDH isoforms (30) have been identified that are responsible for the metabolism of ethanol (34), catecholamines, (14) and the conversion of vitamin A to its active metabolite, retinoic acid (17). ALDH is also a selectable marker of human stem/progenitor cells (13, 15, 16). However, the contribution of ALDH activity to HSC function has remained unknown. In this study, we show that inhibition of ALDH activity with DEAB delayed the phenotypic and functional maturation of HSCs in response to thrombopoietin, SCF, and flt-3 ligand. ALDH inhibition, coupled with TSF, also gave rise to a 3.4-fold increase in SRCs in short-term culture, whereas treatment with TSF alone was associated with a 2-fold reduction in SRC content compared with input. Importantly, secondary transplant studies confirmed that long-term repopulating stem cells were maintained in cultures treated with DEAB. These studies indicate that ALDH plays a critical role in human HSC differentiation. Moreover, inhibition of ALDH, when combined with early acting cytokines, is sufficient to induce the amplification of human HSCs.
In light of the observed effects of ALDH inhibition on the amplification of human HSCs in culture, we also sought to determine the mechanism through which this effect occurred. Because ALDH1 is the predominant isoform within HSCs (11, 14, 32) and is the dominant isoform in mammals that regulates the conversion of retinaldehydes to retinoic acids (31), we tested whether DEAB specifically inhibited ALDH1 activity in HSCs and the effect this had on RAR-response genes. Our studies confirmed that DEAB treatment blocked the capacity for HSCs to convert retinaldehyde into retinoic acids by virtue of a marked decrease in expression of cEBPε, which is an RAR-specific response gene. Because ALDH1 is the dominant isoform required for the conversion of retinaldehyde to retinoic acids, these results also confirmed that the effect of DEAB on HSC differentiation was predominantly mediated through inhibition of ALDH1. Taken together, these data provide strong evidence that ALDH1 mediates the differentiation of HSCs via production of intracellular retinoic acids and that targeted inhibition of this enzyme promotes HSC self-renewal via inhibition of retinoic acid signaling.
The data presented here demonstrate that modulation of retinoid signaling can induce the expansion of human HSCs. There are several implications of these observations. First, the functional role of ALDH1 in HSC fate and the link between ALDH1 activity, retinoid signaling, and HSC self-renewal has not been previously described. Interestingly, Purton et al. (35, 36) reported that culture of murine c-kit+sca-1+lin− cells with ATRA for 14 days enhanced the maintenance of cells with in vivo repopulating capacity as compared with culture with cytokines alone. These results appear to contrast with our observations that inhibition of ALDH1 and retinoid signaling induces the expansion of human HSCs. These differences may be explained by differences in the contribution of ALDH1 activity to HSC fate between mice and humans or differences in the repopulating assays being performed. We have initiated additional studies to determine whether the function of ALDH1 in hematopoiesis is conserved in both humans and mice. Our data also suggest that cytokines, such as thrombopoietin, SCF, and flt-3 ligand, induce HSC differentiation via induction of retinoid signaling, perhaps mediated through increased ALDH1 activity. This hypothesis is supported by the recent observation that another cytokine, IL-3, induces hematopoietic progenitor cell differentiation via activation of Stat5 which, in turn, activates retinoid signaling (37). Our results also have implications for the development of strategies to amplify human HSCs for clinical purposes. We performed these studies on primary human HSCs, and our observations are therefore directly translatable to clinical protocols to expand human HSCs. Moreover, in contrast to other reported strategies to expand HSCs in vitro (5, 38), the approach we have described does not depend on the genetic modification of HSCs or coculture with surrogate stromal cell niches to achieve potency (39, 40). Finally, the observed in vivo multilineage differentiation of DEAB-treated HSCs transplanted in NOD/SCID mice demonstrates that ALDH inhibition does not significantly alter the normal differentiation program of human HSCs. It will be important to further augment the expansion of HSCs described here via the combination of ALDH inhibitors with other ligands capable of inhibiting HSC differentiation programs. In addition, it will also be important to directly modulate RAR and RXR signaling in primary HSCs. We have observed that a selective RXR modulator causes the enhancement of SRC content in short-term culture in a manner highly comparable to ALDH inhibition (J.P.C., D.P.M., G.G.M., and R.S., unpublished data).
In summary, our data suggest that ALDH1 functions fundamentally in HSCs to promote differentiation via the production of retinoic acids. The notion that ALDH1 is both a selectable marker of stem and progenitor cells (13, 14) and a critical regulator of stem cell differentiation appears counterintuitive. However, because a fundamental property of HSCs is the ongoing production of all mature hematopoietic cells, it is not surprising that HSCs would possess a differentiation program (e.g., ALDH1 activity, production of retinoic acids) that can be activated early in their lifespan, particularly in response to external stimuli (e.g., cytokines). Production of large numbers of HSCs lacking such an early differentiation capacity would also be potentially pathologic. Therefore, we believe it is consistent with normal hematopoiesis that HSCs might possess in vivo repopulating capacity while also carrying the critical capacity for fairly rapid differentiation in response to external signals. The results presented here demonstrate that inhibition of ALDH and retinoid activity is sufficient to induce the expansion of human HSCs.
Methods
Isolation of Human BM and CB CD34+CD38−lin− Cells.
We obtained whole BM and CB units from the Duke University Stem Cell Laboratory within 48 h of collection. CB was volume-reduced, and lineage depletion was conducted by using the Human Progenitor Enrichment Mixture (StemCell Technologies, Vancouver). For details, see Supporting Methods, which is published as supporting information on the PNAS web site.
Lin− CB or BM cells were thawed and washed once in Iscove’s modified Dulbecco’s medium (Invitrogen) containing 10% FBS and 1% penicillin/streptomycin. Immunofluorescent staining and sterile cell sorting to isolate CD34+CD38− and CD34+CD38+ subsets was performed as described in ref. 24. For additional details, see Supporting Methods.
Analysis of in Vitro Hematopoietic Activity of Human CD34+CD38−lin− Cells After Culture with DEAB.
Primary human BM and CB CD34+CD38−lin− cells were placed in culture with 20 ng/ml thrombopoietin, 100 ng/ml stem cell factor, and 50 ng/ml flt-3 ligand (TSF, R & D Systems), a cytokine combination which we have previously found to induce human stem and progenitor cell proliferation and differentiation in vitro (24). BM or CB CD34+CD38−lin− cells at a dose of 0.5–1 × 104 were cultured for 7 days with and without 100 μM DEAB (courtesy of M. Colvin, Duke University). At day 7, cell counts and immunophenotypic analysis was performed on progeny cells by using anti-human CD34 and CD38 mAbs (Becton Dickinson) and isotype control IgG mAbs and compared with day 0 (input) staining. Fourteen-day methylcellulose CFC assays were performed in triplicate as we have previously described (24, 26) (for details, see Supporting Methods).
BM and CB CD34+CD38−lin− cells were also placed in culture with TSF with and without ATRA (Sigma-Aldrich), TTNPB (Sigma-Aldrich), LGD101268, or 1-α,25-dihydroxyvitamin D3 (Sigma-Aldrich) with or without DEAB to determine the relationship between ALDH inhibition and retinoic acid signaling in human HSCs (see Supporting Methods for details).
In Vivo Long-Term Repopulating Assays in NOD/SCID Mice.
NOD/SCID mice (41) were transplanted with either day 0 FACS-sorted BM or CB CD34+CD38−lin− cells or the progeny of CD34+CD38− lin− cells cultured with TSF alone or TSF supplemented with 100 μM DEAB. Cell transplantation, analysis of human engraftment in primary and secondary mice, and SCID-repopulating cell (SRC) frequency estimates were performed as described in refs. 24 and 26. For details, see Supporting Methods.
Real-Time PCR Analysis of Gene Expression in HSCs.
Total RNA isolation from Day 0 CB CD34+CD38−lin− and the resultant day 7 progeny was conducted on 1 × 104 cells per sample by using the RNAqueous-Micro kit (Ambion, Austin, TX) following the manufacturer’s suggested protocol. See Supporting Methods for details.
Analysis of ALDH Activity.
ALDH enzyme activity in day 0 CD34+CD38−lin− cells and their progeny was assayed by using the ALDEFLUOR staining kit (StemCell Technologies) as described in ref. 16. See Supporting Methods for details.
Supplementary Material
Acknowledgments
We thank Dr. David Venzon for his critical assistance with the statistical analysis.
Abbreviations
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- BM
bone marrow
- CB
cord blood
- HSC
hematopoietic stem cell
- NOD/SCID
nonobese diabetic/severe combined immunodeficiency
- SRC
SCID-repopulating cell
- CFC
colony-forming cell
- DEAB
diethylaminobenzaldehyde
- ATRA
all-trans-retinoic acid.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Osawa M., Hanada K., Hamada H., Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 2.Sorrentino B. Nat. Rev. Immunol. 2004;4:878–888. doi: 10.1038/nri1487. [DOI] [PubMed] [Google Scholar]
- 3.Varnum-Finney B., Xu L., Brashem-Stein C., Nourigat C., Flowers D., Bakkour S., Pear W., Bernstein I. Nat. Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 4.Krosl J., Austin P., Beslu N., Kroon E., Humphries R., Savageau G. Nat. Med. 2003;9:1428–1432. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- 5.Reya T., Duncan A., Ailles L., Domen J., Scherer D., Willert K., Hintz L., Nusse R., Weissman I. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj G., Murdoch B., Wu D., Baker D., Williams K., Chadwick K., Ling L., Karanu F., Bhatia M. Nat. Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 7.Calvi L., Adams G., Weibrecht K., Weber J., Olson D., Knight M., Martin R., Schipani E., Divieti P., Bringhurst F., et al. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., Niu C., Ye L., Huang H., He X., Tong W., Ross J., Haug J., Johnson T., Feng J., et al. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 9.Colvin M., Russo J., Hilton J., Dulik D., Fenselau C. Adv. Enzyme Regul. 1988;27:211–221. doi: 10.1016/0065-2571(88)90018-0. [DOI] [PubMed] [Google Scholar]
- 10.Russo J., Hilton J., Colvin O. M. Prog. Clin. Biol. Res. 1989;290:65–79. [PubMed] [Google Scholar]
- 11.Kastan M., Schlaffer E., Russo J., Colvin O. M., Civin C., Hilton J. Blood. 1990;75:1947–1950. [PubMed] [Google Scholar]
- 12.Magni M., Shammah S., Shiro R., Mellado W., Dalla-Favera R., Gianni A. Blood. 1996;87:1097–1103. [PubMed] [Google Scholar]
- 13.Storms R., Trujillo A., Springer J., Shah L., Colvin O. M., Ludeman S., Smith C. Proc. Natl. Acad. Sci. USA. 2000;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones R., Barber J., Vala M., Collector M., Kaufmann S., Ludeman S., Colvin O. M., Hilton J. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 15.Hess D., Meyerrose T., Wirthlin L., Craft T., Herrbrich P., Creer M., Nolta J. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 16.Storms R., Green P., Safford K., Niedzwieki D., Cogle C., Colvin O. M., Chao N., Rice H., Smith C. Blood. 2005;106:95–102. doi: 10.1182/blood-2004-09-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat P., Samaha H. Biochem. Pharmacol. 1999;57:195–197. doi: 10.1016/s0006-2952(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 18.Chambon P. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 19.Collins S. Leukemia. 2002;16:1896–1905. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 20.Zechel C. Mol. Endocrinol. 2005;19:1629–1645. doi: 10.1210/me.2004-0540. [DOI] [PubMed] [Google Scholar]
- 21.Zile M. J. Nutr. 2001;131:705–708. doi: 10.1093/jn/131.3.705. [DOI] [PubMed] [Google Scholar]
- 22.Tocci A., Parolini I., Gabbianelli M., Testa U., Luchetti L., Samoggia P., Masella P., Russo G., Valtieri M., Peschle C. Blood. 1996;88:2878–2888. [PubMed] [Google Scholar]
- 23.Tallman M., Anderson J., Schiffer C., Appelbaum F., Feusner J., Ogden A., Shepherd L., Willman C., Bloomfield C., Rowe J., et al. N. Engl. J. Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 24.Chute J., Muramoto G., Fung J., Oxford C. Blood. 2005;105:576–583. doi: 10.1182/blood-2004-04-1467. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia M., Bonnet D., Kapp U., Wang J., Murdoch B., Dick J. J. Exp. Med. 1997;186:619–624. doi: 10.1084/jem.186.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chute J., Saini A., Chute D., Wells M., Clark W., Harlan D., Park J., Stull M., Civin C., Davis T. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Doedens M., Dick J. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- 28.Ueda T., Tsuji K., Yoshino H., Ebihara Y., Yagasaki H., Hisakawa H., Mitsui T., Manabe A., Tanaka R., Kobayashi K., et al. J. Clin. Invest. 2000;105:1013–1021. doi: 10.1172/JCI8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yahata T., Ando K., Sato T., Miyatake H., Nakamura Y., Muguruma Y., Kato S., Hotta T. Blood. 2003;101:2905–2913. doi: 10.1182/blood-2002-07-1995. [DOI] [PubMed] [Google Scholar]
- 30.Munker R., Norman A., Koeffler H. J. Clin. Invest. 1986;78:424–430. doi: 10.1172/JCI112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haselbeck R., Hoffman I., Duester G. Dev. Genet. 1999;25:353–364. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo J., Barnes A., Berger K., Desgrosellier J., Henderson J., Kanters A., Merkov L. BMC Pharmacol. 2002;2:4–11. doi: 10.1186/1471-2210-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrella E., Gianni M., Cecconi V., Nigro E., Barzago M., Rambaldi A., Rochette-Egly C., Terao M., Garattini E. J. Biol. Chem. 2004;279:42026–42040. doi: 10.1074/jbc.M406530200. [DOI] [PubMed] [Google Scholar]
- 34.Russo J., Hilton J. Cancer Res. 1988;48:2963–2968. [PubMed] [Google Scholar]
- 35.Purton L., Bernstein I., Collins S. Blood. 1999;94:483–495. [PubMed] [Google Scholar]
- 36.Purton L., Bernstein I., Collins S. Blood. 2000;95:470–477. [PubMed] [Google Scholar]
- 37.Si J., Collins S. Blood. 2002;100:4401–4409. doi: 10.1182/blood-2001-12-0374. [DOI] [PubMed] [Google Scholar]
- 38.Schiedelmeier B., Klump H., Will E., Arman-Kalcek G., Li Z., Wang Z., Rimek A., Friel J., Baum C., Ostertag W. Blood. 2003;101:1759–1768. doi: 10.1182/blood-2002-03-0767. [DOI] [PubMed] [Google Scholar]
- 39.Kawano Y., Kobuno M., Yamaguchi M., Nakamura K., Ito Y., Sasaki K., Takahashi S., Nakamura T., Chiba H., Sato T., et al. Blood. 2003;101:532–540. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- 40.Hackney J., Charbord P., Brunk B., Stoeckert C., Lemishka I., Moore K. Proc. Natl. Acad. Sci. USA. 2002;99:13061–13066. doi: 10.1073/pnas.192124499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz L., Schweitzer P., Christianson S., Gott B., Schweitzer I., Tennent B., McKenna S., Mobraaten L., Rajan T., Greiner T., et al. J. Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.