Abstract
We have demonstrated that cytokine thymic stromal lymphopoietin (TSLP), whose expression is rapidly induced upon keratinocyte-selective ablation of retinoid X receptors (RXRs) -α and -β in the mouse (RXRαβep−/− mice), plays a key role in initiating a skin and systemic atopic dermatitis-like phenotype. We show here that topical application of the physiologically active ligand [1α,25-(OH)2D3; calcitriol] of the vitamin D receptor, or of its low-calcemic analog MC903 (calcipotriol; Dovonex), induces TSLP expression in epidermal keratinocytes, which results in an atopic dermatitis-like syndrome mimicking that seen in RXRαβep−/− mutants and transgenic mice overexpressing TSLP in keratinocytes. Furthermore, topical application of retinoic acid receptor RARγ-selective agonist BMS961 also induces TSLP expression either on its own or synergistically with 1α,25-(OH)2D3. Our data demonstrate that RXR/vitamin D receptor and RXR/retinoic acid receptor-γ heterodimers and their ligands cell-autonomously control the expression of TSLP in epidermal keratinocytes of the mouse. We propose molecular mechanisms through which vitamin D3 and retinoic acid signalings could be involved in the pathogenesis of atopic diseases.
Keywords: retinoic acid, vitamin D receptor, retinoid X receptor, retinoic acid receptor, skin
Nuclear receptors (NRs) belong to a superfamily of ligand-dependent transcriptional regulators (1, 2). Within this superfamily, retinoid X receptors (RXRs) -α, -β, and -γ play a key role through heterodimerization with some 15 NR partners, e.g., retinoic acid receptors (RARs), vitamin D receptor (VDR), peroxisome proliferator-activated receptors, and liver X receptors (1, 2). We reported (3) that selective ablation of RXRα and RXRβ in adult mouse epidermal keratinocytes (RXRαβep−/− mice) triggers a skin and systemic syndrome similar to human atopic dermatitis (AD), a chronic skin inflammatory disease with a strong genetic component that affects children (10–20%) and adults (1–3%) (4). These mice exhibit the major features of the human AD syndrome that include (i) skin eczematous-like lesions with xerosis and pruritus, associated with a skin inflammatory infiltrate mainly composed of CD4+ T helper (Th) type 2 cells, dendritic cells, eosinophils, and mast cells and (ii) systemic abnormalities, including elevated serum IgE and IgG levels and blood and tissue eosinophilia.
We found that expression of the cytokine thymic stromal lymphopoietin (TSLP), known to be produced in epidermal keratinocytes of AD patients (5), is rapidly induced in keratinocytes of RXRαβep−/− mice. Furthermore, we showed that K14-TSLP transgenic mice overexpressing TSLP in keratinocytes exhibit an AD-like phenotype similar to that of RXRαβep−/− mice (3), demonstrating that TSLP can act as an initiating cytokine at the top of a chain of immunological events that lead to an AD-like phenotype, in keeping with other recent studies on mouse models of human allergic inflammatory diseases (asthma and AD) (6–9).
We suggested that up-regulation of keratinocytic TSLP expression upon RXRα and -β ablation could be due to the relief of a transcriptional repression mediated by RXR/NR heterodimers (3). This study was aimed at revealing the identity of the possible NR partner(s) of RXRα and RXRβ.
Results
Topical Application of 1α,25-(OH)2D3 or Its Low-Calcemic Analog MC903 Activates TSLP Expression in Epidermal Keratinocytes.
Because RXR/NR heterodimers in which an agonistic ligand is not bound to the NR partner can act as transcriptional repressors (10), we examined whether TSLP expression could be induced by NR agonists. Four nanomoles of ligands were topically applied to whole ears of WT mice for 4 consecutive days (days 1–4), and TSLP RNA levels were determined on day 5. Application of 1α,25-(OH)2D3 (the physiologically active vitamin D3) led to a dramatic increase (>300-fold) in TSLP transcripts at day 5, whereas they were modestly but significantly increased (5-fold) upon application of a RARγ-selective agonist (BMS961) (11) (Fig. 1a). In contrast, agonists for RXRs (BMS649), proliferator-activated receptor (PPAR)α (fenofibrate), PPARβ (GW501516), PPARγ (rosiglitazone), and liver X-activated receptors (25-hydroxycholesterol) had no effect on TSLP expression (Fig. 1a).
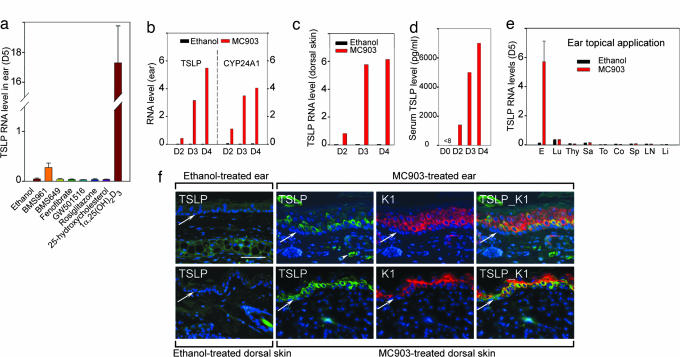
Fig. 1.
Skin topical application of 1α,25-(OH)2D3 and MC903 activates TSLP expression in epidermal keratinocytes. (a) TSLP RNA at day 5 in ears topically treated with NR agonists (4 nmol). (b–e) Induction of TSLP expression is a rapid and local skin effect. TSLP and CYP24A1 RNA levels in an ethanol-treated left ear and MC903-treated right ear (b), TSLP RNA levels in ethanol-treated and MC903-treated dorsal skin at days 2, 3, and 4 (c), and increased serum TSLP levels at days 2, 3, and 4 (d), in contrast to undetectable level (<8 pg/ml) at day 0 (before treatment). Data are representative of three independent experiments. D, day. (e) TSLP RNA levels at day 5 in ear (E), lung (Lu), thymus (Thy), salivary gland (Sa), tongue (To), colon (Co), spleen (Sp), lymph node (LN), and liver (Li) of mice topically treated by ethanol or MC903 on ears. (f) IHC of TSLP (green) at day 4 in sections of ear (Upper) and dorsal skin (Lower) topically treated with ethanol or MC903. The same sections from MC903-treated skin were stained with keratin 1 antibody (K1, red), and overlaid images of TSLP and K1 staining are shown, as indicated. Blue corresponds to DAPI staining of nuclei. The white arrowhead points to autofluorescent erythrocytes, and white arrows point to the dermal/epidermal junction. (Scale bar, 50 μm.)
Because, at this dose, 1α,25-(OH)2D3 application resulted in hypercalcemia and death of the mice, we applied its analog MC903 (calcipotriol; Dovonex) (12) that exhibits a low-calcemic activity and is used for psoriasis treatment (13). MC903 and ethanol (vehicle) were applied to WT mouse right and left ears, respectively. Two shaved areas (1 cm2 each) of dorsal skin were also treated with MC903 or ethanol. One day after the first application (day 2), TSLP RNA levels were increased in right ears and further increased on days 3 and 4, whereas no increase occurred in left ears (Fig. 1b Left). Transcripts of CYP24A1, a 1α,25-(OH)2D3-inducible gene (14), were increased upon MC903 application, as expected (Fig. 1b, Right). TSLP RNA was also increased in MC903-treated dorsal skin (Fig. 1c), and serum TSLP levels were increased at days 2–4, whereas undetectable at day 0 (before treatment) (Fig. 1d). Increasing doses of MC903 (0.4, 1, or 4 nmol per ear) led to a dose-dependent increase of TSLP transcripts, which was similarly observed with other low-calcemic analogs of 1α,25-(OH)2D3, including EB1089 and KH1060 (12, 15) (data not shown).
To examine whether MC903-induced expression of TSLP was skin-restricted, various other organs were analyzed at day 5. No increase in TSLP transcripts was observed in these organs (Fig. 1e). Immunohistochemistry (IHC) did not reveal TSLP expression in epidermis or dermis of ethanol-treated ear and dorsal skin, whereas it was readily detected at day 4 upon MC903 treatment (Fig. 1f; ear skin, Upper; dorsal skin, Lower). Double IHC for TSLP and keratin 1 (K1), a suprabasal keratinocyte marker, showed that TSLP was mainly located in these keratinocytes of both MC903-treated ear and dorsal skin, whereas it could also be detected at a lower level in basal keratinocytes (expressing keratin 14) (Fig. 1f, and data not shown).
Topical Application of MC903 Triggers an AD-Like Syndrome.
Because TSLP expression appears to be critically involved in the initiation of AD-like dermatitis in the mouse (3, 8), we investigated whether a MC903 long-term treatment could induce an AD-like phenotype. MC903 (4 nmol) was applied daily for 16 days to ears of WT mice. No hypercalcemia or overall health impairment and weight loss was observed. Ethanol application did not cause any change in ear appearance, whereas reddening and swelling that worsened with time were observed from day 5 on MC903-treated ears (data not shown). At day 17, these ears were red, scaly, swollen, and crusted (Fig. 2, compare a and b), and frequent ear scratching (data not shown) suggested a pruritus. Histological analysis revealed epidermal hyperplasia and a heavy dermal cell infiltrate, in which numerous eosinophils were easily identified upon hematoxylin/eosin-staining (Fig. 2d and Inset). Their identity was confirmed with Luna’s staining (data not shown). In contrast, no eosinophils were found in ethanol-treated ears (Fig. 2c). IHC with an anti-GR1 antibody (recognizing granulocytes and monocytes) revealed a large number of positive cells in dermis, of which eosinophils, but not neutrophils, were a major component (Fig. 2f and data not shown). Numerous T lymphocytes (CD3+) were observed in MC903-treated dermis (Fig. 2h), whereas only a few resident T lymphocytes could be detected in ethanol-treated ears (Fig. 2g). Most of the infiltrated T cells were CD4+ helper T cells (Fig. 2j), and only a few CD8+ cytotoxic T cells were found (Fig. 2l). A large increase in CD11c+ dermal dendritic cells was also observed in MC903-treated ears (Fig. 2 m and n), whereas mast cells were 4-fold increased in the dermis (Fig. 2 o and p and data not shown).
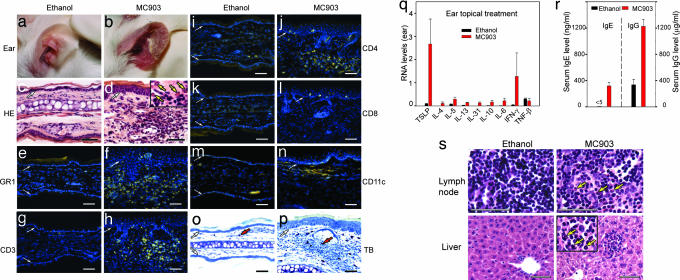
Fig. 2.
Topical treatment with MC903 triggers an AD-like skin inflammation. (a and b) Appearance of ethanol- and MC903-treated ears at day 17. (c and d) Hematoxylin and eosin-stained ear sections of ethanol- and MC903-treated mice at day 17. Eosinophils displaying cytoplasmic red staining are indicated by yellow arrows in d Inset. (e–n) IHC performed on ear sections from ethanol- or MC903-treated mice at day 17, with antibodies against GR1 (e and f), CD3 (g and h), CD4 (i and j), CD8 (k and l), and CD11c (m and n). Yellow corresponds to staining of antibodies, whereas blue corresponds to DAPI staining of nuclei. (o and p) Toluidin blue (TB) staining of ear sections. Red arrows point to one of the mast cells with intense blue in the dermis. White arrows in c–p point to the dermal/epidermal junction. (q) Cytokine RNA levels in ethanol- and MC903-treated ears at day 17. (r) Serum IgE and IgG levels of ethanol- and MC903-treated mice at day 17. (s) Hematoxylin and eosin-stained sections of ear-draining lymph node and liver of ethanol- and MC903-treated mice at day 17. Yellow arrows point to three of many eosinophils (red cytoplasmic staining) in sections of lymph node and liver of MC903-treated mice. (Scale bars, 50 μm.)
Because topical application of 1α,25-(OH)2D3 at a dose of 4 nmol per ear resulted in mouse death within 7 days, WT mice were thus treated every other day at a dose of 0.25 nmol per ear, to examine whether the treatment would result in a skin inflammation similar to that generated with MC903. At this dose, TSLP expression was significantly induced at day 18, and an inflammatory infiltrate comprising CD4+ T lymphocytes, dendritic cells, eosinophils, and mast cells could also be observed (data not shown).
Taken together, these data indicated that the inflammatory cell infiltrate observed in skin of MC903- and 1α,25-(OH)2D3-treated ears had the characteristics of an AD-like skin inflammation (3). This finding was fully supported by analysis of cytokines expressed in MC903-treated ears. At day 16, TSLP transcripts were markedly increased (Fig. 2q), and Th2-type cytokine transcripts (IL-4, -5, -13, -31, -10, and -6) (3) were all significantly increased (Fig. 2q). Expression of the Th1-type cytokine IFN-γ was also enhanced, whereas that of TNF-β, another Th1-type cytokine, was unchanged. Importantly, this cytokine profile, which is essentially that of a Th2-type inflammation, was similar to those observed in skins of RXRαβep−/− and K14-TSLP transgenic mice (3), indicating that the increase of these cytokines was most probably due to enhanced TSLP production in keratinocytes.
Systemic abnormalities, including elevated serum IgE and IgG levels, associated with blood and tissue eosinophilia, have been observed in RXRαβep−/− and K14-TSLP mice, exhibiting similarities to those observed in AD patients (3). Serum IgE and IgG levels were increased in mice to which MC903 was topically applied for 16 days on ears (Fig. 2r). Moreover, at day 16, MC903-treated mice exhibited an increased number of eosinophils in ear-draining lymph nodes, liver, and spleen (Fig. 2s and data not shown). Differential blood cell counts also revealed a marked increase in eosinophils in MC903-treated mice (693 ± 220 cells per μl, versus 204 ± 134 cells per μl in ethanol-treated mice). Thus, MC903 topical application leads to a skin and systemic phenotype mimicking that of human AD.
Both Keratinocytic VDR and RXR Are Required for Induction of TSLP Expression and Generation of an AD-Like Skin Inflammation Upon MC903 Treatment.
To investigate whether the MC903-induced TSLP expression and appearance of an AD-like skin inflammation were mediated through VDR, MC903 was topically applied on ears of “floxed” VDR control (CT) mice (VDRL2/L2 mice in which both VDR alleles bear LoxP sites) and of their VDRep−/− littermates [K14-Cre(tg/0)/VDRL2/L2 mice] in which the VDR alleles are selectively ablated in keratinocytes (ref. 16 and our unpublished data). At day 17 of MC903 treatment, an inflammation was obvious on ears of VDR CT mice, whereas VDRep−/− ears did not show any sign of inflammation (Fig. 3a and b). Accordingly, a massive dermal infiltrate of inflammatory cells, including eosinophils, CD4+ Th cells, dendritic cells, and mast cells was detected in ear sections of MC903-treated VDR CT (Fig. 3c and data not shown) but not in those of VDRep−/− mice (Fig. 3d). Similarly, no AD-like skin inflammation was developed upon topical MC903 treatment of VDR−/− (germ-line knockout) mice (17) (data not shown).
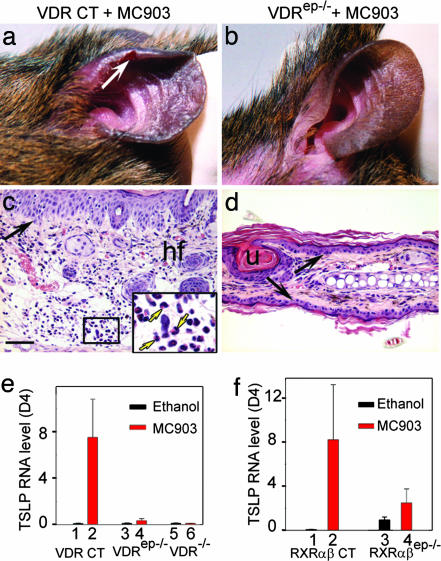
Fig. 3.
Keratinocytic VDR and RXR are required for generation of an AD-like skin inflammation and induction of TSLP expression upon MC903 treatment. Appearance of MC903-treated ears of VDR CT (a) and VDRep−/− mice (b) at day 17. White arrow in a points to lesioned skin. (c and d) Hematoxylin and eosin-stained ear sections. Yellow arrows in c Inset point to three of many eosinophils (red cytoplasmic staining) in MC903-treated CT skin. Black arrows point to dermal/epidermal junction. hf, hair follicle; u, utriculi (resulting from hair follicle degeneration in VDRep−/− mice). (Scale bar, 50 μm.) (e) TSLP RNA levels at day 4 in ethanol- and MC903-treated ears of VDR CT (lanes 1 and 2), VDRep−/− (lanes 3 and 4), and VDR−/− (lanes 5 and 6) mice. (f) TSLP RNA levels at day 4 in ethanol- and MC903-treated ears of RXRαβ CT (lanes 1 and 2) and RXRαβep −/− (lanes 3 and 4) mice.
TSLP expression was strongly induced in MC903-treated skin of VDR CT mice (Fig. 3e, lanes 1 and 2) but not at all in MC903-treated skin of VDR−/− mice (lanes 5 and 6), whereas it was weakly increased in MC903-treated skin of VDRep−/− mutants (lanes 3 and 4). This latter increase may reflect a faint response to MC903 in nonkeratinocytic skin cells. In any event, our data clearly demonstrated that induction of TSLP expression in keratinocytes upon MC903 application is a VDR-dependent cell-autonomous event.
To examine whether the effect of MC903 was transduced through RXR/VDR heterodimers, ears of RXRαβep−/− mice (3) as well as their control littermates (RXRαβ CT) were topically treated with MC903 (Fig. 3f). As expected (3), selective RXRαβ ablation in keratinocytes of adult mice led to increased TSLP expression (lanes 1 and 3). However, the further induction of TSLP by MC903 was severely reduced in RXRαβep−/− skin (lanes 2 and 4), indicating an essential function of keratinocytic RXRs in TSLP induction by VDR agonists, most probably reflecting the involvement of RXR/VDR heterodimers.
VDR and RARγ Agonistic Ligands Synergize to Induce Skin TSLP Expression.
Although much less potent than that of a VDR agonist, application of a RARγ-selective agonist (BMS961) on mouse ear skin led to significant increase of TSLP transcripts (Fig. 1a), and topical application of retinoic acid (RA) resulted in a similar induction (data not shown). To examine whether VDR and RARγ agonists could synergize in up-regulating TSLP expression, WT mouse ears were topically treated for 3 days with ethanol, BMS961 (4 nmol), a limiting dose of 1α,25-(OH)2D3 (0.4 nmol), or a combination of the two ligands. Ear TSLP transcripts and serum TSLP were determined at day 4. A clear synergism was observed between the effects of BMS961 and 1α,25-(OH)2D3 (Fig. 4a), indicating a synergistic involvement of RAR- and VDR-mediated events in transcriptional activation of TSLP expression.
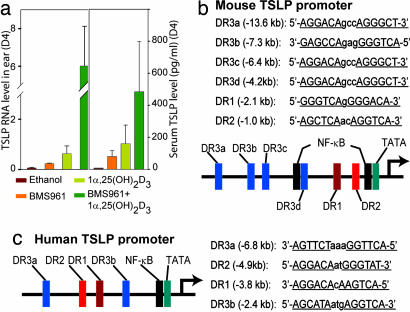
Fig. 4.
Agonist-liganded VDR and RARγ synergistically induce TSLP expression. (a) Mouse ears were treated with ethanol, BMS961 (4 nmol), 1α,25-(OH)2D3 (0.4 nm), or BMS961 (4 nmol) plus 1α,25-(OH)2D3 (0.4 nmol), as indicated. TSLP RNA levels in the ears (Left) and serum TSLP levels (Right) were measured at day 4. (b) Putative VDREs (DR3) and RAREs (DR2 and DR1) upstream of the mouse TSLP promoter. (c) Putative VDREs (DR3) and RAREs (DR2 and DR1) upstream of the human TSLP promoter.
TSLP Production and Skin Inflammation Induced by MC903 Are T and B Lymphocyte-Independent.
As a direct effect of 1α,25-(OH)2D3 on naïve CD4+ T cells may enhance the development of Th2 cells (18), we investigated whether CD4+ T cells are required for development and progression of the active vitamin D3-induced AD-like phenotype. Ethanol or MC903 was applied on ears of RAG1−/− mice, which are devoid of mature B and T lymphocytes (19). As observed in WT mice, keratinocytic TSLP expression at day 4 was induced in MC903-treated, but not in ethanol-treated, RAG1−/− mice (see Fig. 6 a, b, and k, which is published as supporting information on the PNAS web site). At day 16, MC903-treated RAG1−/− mice exhibited thickened and scaly red ears (Fig. 6d), associated with a dermal cell infiltrate and an epidermal hyperplasia (Fig. 6f), whereas ethanol-treated RAG1−/− ears had a normal appearance (Fig. 6 c and e). As expected, lymphocytes were absent in RAG1−/− skin sections (data not shown). However, we detected an increased number of infiltrated eosinophils (Fig. 6 f vs. e), mast cells (Fig. 6 h vs. g), and dermal dendritic cells (Fig. 6 j vs. i) in MC903-treated RAG1−/− skin. Thus, enhancement of TSLP expression and generation of a skin inflammation upon MC903-treatment do not require the presence of mature B and T lymphocytes.
Moreover, whereas transcripts of Th2-type cytokines IL-5, -13, and -31 were not increased in MC903-treated RAG1−/− skin (as compared with ethanol treatment), an increase in transcripts of other Th2-type cytokines IL-4, -6, and -10 was observed (Fig. 6k), indicating that these cytokines can be produced by nonlymphocytic cells upon topical MC903 treatment. A marginal increase of IFN-γ was also observed (Fig. 6k). Eosinophilia in blood and some tissues (e.g., ear-associated lymph nodes, liver, and spleen) was also observed in MC903-treated RAG1−/− mice (data not shown), whereas no immunoglobulins could be detected in sera (data not shown), indicating that increased levels of IgE or IgG were not indispensable for generating an AD-like syndrome.
Discussion
Selective ablation of both RXRα and -β in mouse skin keratinocytes results in a marked increase of TSLP expression that leads to the development of an AD-like phenotype (3). Because (i) putative NR response elements are present in mouse and human (20) TSLP promoters (see Fig. 4 b and c); (ii) TSLP repression did not require the AF-2 activation function of RXRs; and (iii) TSLP induction could not be triggered by an RXR agonist (Fig. 1a), we proposed (3) that this TSLP overexpression could reflect the relief of a transcriptional repression exerted by nonpermissive RXR/NR(s) heterodimers (21) in which the NR partner is unliganded. We demonstrate here that RXRα(β)/VDR heterodimers are such heterodimers, because topical treatment of mouse skin with 1α,25-(OH)2D3 or its low-calcemic analogs strongly induces TSLP expression in skin keratinocytes and triggers a skin and systemic AD-like syndrome mimicking that observed in RXRαβep−/− mutant and K14-TSLP transgenic mice (3). Moreover, the induction of TSLP by active vitamin D3 is a cell-autonomous event, because it is abolished upon keratinocyte-selective ablation of either VDR or RXRα and -β. We also show that, although less efficiently than RXRα(β)/VDR heterodimers, RXRα(β)/RARγ heterodimers liganded with RA or the RARγ-selective agonist BMS961, also mediate induction of TSLP expression but to a level too low to trigger, on its own, an overt AD-like phenotype. In keeping with these data, TSLP expression can be induced by 1α,25-(OH)2D3 and RA in a mouse epithelial tumor cell line (C1271, derived from a mammary carcinoma) (our unpublished data); TSLP was also shown to be induced by 1α,25-(OH)2D3 in a human epithelial tumor cell line (SCC25, derived from a tongue squamous cell carcinoma) (20). Furthermore, that agonists of VDR and RARγ could induce TSLP expression either on their own or synergistically indicates that the corresponding RXR heterodimers bind to distinct cognate response elements. In this respect, it is noteworthy that both mouse and human TSLP promoter regions contain putative response elements (Fig. 4 b and c) that may bind RXR/VDR heterodimers [vitamin D response element (VDRE):DR3] or RXR/RAR heterodimers [RA response element (RARE):DR2 and DR1] (22).
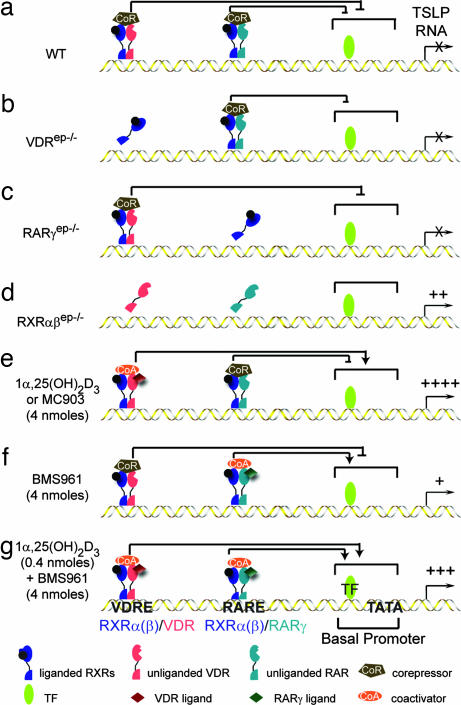
Based on this evidence, we propose a model accounting for the modulation of TSLP promoter activity by RXRα(β)/VDR and RXRα(β)/RARγ heterodimers (schematized in Fig. 5). The promoter region of mouse and human TLSP genes includes a TATA box element and proximal elements (e.g., NF-κB-binding sites) (the basal promoter) as well as putative VDREs and RAREs (Fig. 4 b and c). Because, under homeostatic conditions in vivo, there is no RA and very little, if any, active vitamin D3 (see below) in epidermal keratinocytes, the TSLP promoter basal activity is silenced by unliganded RXRα(β)/VDR and RXRα(β)/RARγ heterodimers associated with corepressors (10) (Fig. 5a). This repression can be efficiently exerted by either RXRα(β)/VDR or RXRα(β)/RARγ heterodimers, because it cannot be relieved by ablation of either VDR or RARγ (Fig. 3e and data not shown; Fig. 5 b and c). RXRα and -β ablation (Fig. 5d), which releases both heterodimers from their binding sites, abolishes this repression and allows basal promoter-bound transcription factors to stimulate TSLP transcription to a basal activity (see Fig. 3f, lane 3) that is sufficient to trigger the generation of an AD-like phenotype (3). Topical application of either active vitamin D3 or a low-calcemic analog (MC903) (Fig. 5e) generates RXR/VDR-coactivator complexes whose transcriptional activity (see Fig. 3f, lane 2) is efficient enough to not only relieve the repression exerted by RXR/RARγ corepressor complexes but also to further enhance the basal promoter activity. Interestingly, the RXR/RARγ coactivator complexes formed upon application of BMS961 are much less efficient (Fig. 5f), because they generate lower TSLP transcript levels (Fig. 1a) than those resulting from the basal promoter activity, as observed in keratinocytes ablated for RXRα and -β (see Fig. 3f, lane 3). However, upon cotreatment with BMS961 and a limiting dose of 1α,25-(OH)2D3 (Fig. 5g), liganded RXR/RARγ and RXR/VDR heterodimers can efficiently synergize to enhance the activity of TSLP basal promoter.
Fig. 5.
Schematic model of RXRα(β)/VDR- and RXRα(β)/RAR-mediated regulation of TSLP expression in mouse keratinocytes (see Discussion). As concluded from our study (25), keratinocytic RXRs are shown bound to a non-RA-agonistic ligand.
It should be stressed that, even though the present model accounts for all of our present observations, its refinement will require additional genetic (selective ablation of both VDR and RARγ in epidermal keratinocytes; mutation of the putative VDRE and RARE in the mouse) and biochemical (e.g., ChIP assays) studies to determine to which elements the RXR/VDR and RXR/RAR heterodimers bind, and whether VDR or RARγ preferentially heterodimerize with RXRα or RXRβ. In any event, the rapid and regulable induction of TSLP in mouse keratinocytes upon topical treatment with low-calcemic vitamin D3 analogs (e.g., MC903) provides a highly convenient AD preclinical model for exploring therapeutic avenues as well as a mouse model allowing characterization of various aspects of AD pathogenesis from its onset to the fully established disease phenotype. In this respect, we have found that epidermal thickening and dermal infiltration are observed in MC903-treated skin of both WT and RAG1−/− mice lacking mature T and B cells, showing that, even though 1α,25-(OH)2D3 could directly enhance the formation of Th2 cells from naïve CD4+ T cells (18), these cells are actually not required for TSLP-induced development of an AD-like inflammation. Thus, the presence of eosinophils, mast cells, and dermal dendritic cells in MC903-treated RAG1−/− mice suggests that TSLP could act directly on these and other myeloid-derived cells to initiate an atopic inflammation, whereas the additional accumulation of CD4+ Th2 cells in MC903-treated WT mice may correspond to a secondary effect of TSLP expression that serves for further progression of the AD-like phenotype. A similar conclusion was recently reached by Yoo et al. (8) using TCRβ−/− mice that lack T cells. As expected, no IgE could be detected in both MC903-treated and untreated RAG1−/− mice, indicating that IgE are dispensable for development of the AD-like phenotype, in keeping with the observation that, although elevated serum IgE is a frequently associated clinical feature in AD, ≈20% of AD patients have a normal serum IgE level (4).
Recent reports (5–9), including ours (3), have shown that TLSP represents a master switch of allergic inflammation and established in the mouse a direct link between TLSP expression in keratinocytes and airway epithelial cells and the pathogenesis of atopic dermatitis and asthma, respectively. However, how TLSP expression is triggered in these cells upon allergen exposure remains to be unveiled. Our previous (3) and present reports indicate that RXR heterodimerized with VDR and RARγ actively suppress TSLP expression, whereas active vitamin D3 and, to a lesser extent, RA can relieve this repression, thus raising the question of whether and how these ligands could be instrumental in triggering TSLP production in vivo. In this respect, a possible direct involvement of vitamin D signaling in atopy is supported by the observation that VDR-null mutant mice fail to develop symptoms of experimental asthma (23). Furthermore, vitamin A deficiency is known to diminish Th2-mediated responses, whereas high dietary vitamin A enhances them (24), which may reflect a role of RA-liganded RAR in TSLP induction.
Under homeostatic conditions in vivo, epidermal keratinocytes lack RA (11, 25). In keeping with a very low level of TSLP transcripts, others (refs. 14 and 26 and references therein) have shown that there is very little, if any, active vitamin D3 in these cells in which the enzyme 25(OH)D3-1α-hydroxylase, required for synthesis of 1α,25-(OH)2D3 from 25(OH)D3, is apparently not expressed. What could then be the origin of active vitamin D3 that induces TSLP expression in atopic dermatitis? Interestingly, it has been recently reported that, upon microbe-derived ligand activation of their Toll-like receptors (TLRs) that mediate the synthesis of antimicrobial peptides involved in the innate immune response, human, but not mouse, macrophages can produce 25(OH)D3-1α-hydroxylase and synthesize 1α,25-(OH)2D3 (27, 28). We suggest that similar activation by allergen-derived ligands, of skin macrophage, dendritic cell, or, possibly, keratinocyte TLRs (29–31) may provide 1α,25-(OH)2D3 to keratinocytes in either a paracrine or autocrine manner. Thus, upon exposure of skin to an allergen, TSLP production might be triggered through a TLR-mediated production of active vitamin D3, raising the interesting possibility that genetic or acquired defects of TLRs’ function could be implicated in the pathogenesis of AD. Whether, upon allergen exposure, a TLR-mediated mechanism might also be involved in the production of RA is unknown.
That vitamin D could be instrumental to the pathogenesis of atopic diseases in humans is supported by an association between vitamin D supplementation in infancy and an increased risk of atopy later in life (32, 33). Our present study indicates that this association could be related to an increase of TSLP production in epithelial cells upon exposure to allergens during the perinatal period, which may ultimately result in an increase of allergen-specific Th2 memory cells which could be instrumental to triggering allergic reactions later in life. Interestingly, genetic investigations have also indicated that vitamin D signaling could be implicated in the pathogenesis of atopic diseases, as shown by association of VDR genetic variants with childhood and adult asthma, and atopic response (34, 35). These observations suggest that genetic predisposition to atopic disorders may implicate alteration of other components of vitamin D (and possibly vitamin A) signaling, which will ultimately result in TSLP overproduction. Dysregulation of vitamin D signaling may therefore be a key contributor to both genetic and environmental factors that underlie atopic diseases.
The therapeutic use of vitamins D and A has been considered for AD patients (36–39). Our present data indicate that, on the contrary, administration of these vitamins may exacerbate AD and, very likely, asthma by promoting expression of TSLP in skin and lungs. On the other hand, topical administration of vitamins D and/or A antagonists to AD patients may be beneficial, because it could enhance the repression exerted by RXR/VDR and RXR/RARγ heterodimers (40) on TSLP expression. It has also been recently suggested that low-calcemic VDR agonists (e.g., MC903) could be used to boost the innate immune response (through enhanced antimicrobial peptide production), for instance, to protect against infections and to accelerate wound healing, notably in the case of chronic ulcerated skin wounds (28, 39, 41, 42). Our results indicate that such therapy may not be beneficial to patients prone to atopy. Finally, our study also provides insight into the molecular mechanism that could underlie the use of low-calcemic vitamin D analogs in the treatment of psoriasis (13), a Th1 cell-driven skin disease. Interestingly, the most-common side effect of this treatment is a skin irritation (red, dry, and itchy skin) (43) exhibiting similarities with AD lesions. What our data, therefore, suggest is that a Th1 to Th2 skewing of Th cell differentiation triggered by induction of TSLP expression in keratinocytes may contribute to the therapeutic effect of topical MC903 on psoriatic lesions.
Methods
Experimental Animals and Skin Topical Application.
VDR−/− (17), RXRαβep−/− (tamoxifen-treated K14-Cre-ERT2(tg/0)/RXRαL2/L2/RXRβL2/L2) (3), and RAG1−/− mice (The Jackson Laboratory) were as described. VDRep−/− (K14-Cre(tg/0)/VDRL2/L2) mice were obtained by crossing K14-Cre(tg/0) transgenic mice (16) with floxed VDRL2/L2 mice (our unpublished data). 1α,25-(OH)2D3 (Biomol), MC903 (12), and BMS961 (11) were dissolved in ethanol and topically applied on ears or shaved dorsal skin (1 cm2) of either 6- to 8-week-old female CD1 WT mice or mice with indicated genotypes.
Other Methods.
Histopathology, IHC, RNA analysis, serum cytokine and Ig determination, hematology assays, and statistic analysis are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank the staff of the mouse, histopathology, hematology, and transgenic facilities and the Secretariat of the Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC) and Institut Clinique de la Souris for their kind help; Drs. N. Rochel-Guiberteau (IGBMC) and D. Moras (IGBMC) for vitamin D3 analogs; Bristol Meyer Squibb for BMS961; Dr. S. Chan (IGBMC) for RAG1−/− mice and helpful discussion; and Dr. F. Geissmann for a critical reading of the manuscript. This work was supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Collège de France, the Ministère de la Recherche, and European Community Project EUMORPHIA PLRT-CT-2001-00930.
Abbreviations
- AD
atopic dermatitis
- CT
control
- IHC
immunohistochemistry
- NR
nuclear receptor
- RA
retinoic acid
- RAG1
recombination activating gene 1
- RAR
RA receptor
- RXR
retinoid X receptor
- Th
T helper
- TLR
Toll-like receptor
- TSLP
thymic stromal lymphopoietin
- VDR
vitamin D receptor.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Laudet V., Gronemeyer H. The Nuclear Receptor: Factsbook. San Diego: Academic; 2002. [Google Scholar]
- 2.Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., et al. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M., Messaddeq N., Teletin M., Pasquali J. L., Metzger D., Chambon P. Proc. Natl. Acad. Sci. USA. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung D. Y., Boguniewicz M., Howell M. D., Nomura I., Hamid Q. A. J. Clin. Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soumelis V., Reche P. A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., et al. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y. J. J. Exp. Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B., Comeau M. R., De Smedt T., Liggitt H. D., Dahl M. E., Lewis D. B., Gyarmati D., Aye T., Campbell D. J., Ziegler S. F. Nat. Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 8.Yoo J., Omori M., Gyarmati D., Zhou B., Aye T., Brewer A., Comeau M. R., Campbell D. J., Ziegler S. F. J. Exp. Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Shami A., Spolski R., Kelly J., Keane-Myers A., Leonard W. J. J. Exp. Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perissi V., Rosenfeld M. G. Nat. Rev. Mol. Cell Biol. 2005;6:542–554. doi: 10.1038/nrm1680. [DOI] [PubMed] [Google Scholar]
- 11.Chapellier B., Mark M., Messaddeq N., Calleja C., Warot X., Brocard J., Gerard C., Li M., Metzger D., Ghyselinck N. B., et al. EMBO J. 2002;21:3402–3413. doi: 10.1093/emboj/cdf331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlberg C. J. Cell. Biochem. 2003;88:274–281. doi: 10.1002/jcb.10337. [DOI] [PubMed] [Google Scholar]
- 13.Kragballe K., Iversen L. Dermatol. Clin. 1993;11:137–141. [PubMed] [Google Scholar]
- 14.Jones G., Strugnell S. A., DeLuca H. F. Physiol. Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 15.Carlberg C., Mathiasen I. S., Saurat J. H., Binderup L. J. Steroid Biochem. Mol. Biol. 1994;51:137–142. doi: 10.1016/0960-0760(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 16.Li M., Chiba H., Warot X., Messaddeq N., Gerard C., Chambon P., Metzger D. Development (Cambridge, U.K.) 2001;128:675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizawa T., Handa Y., Uematsu Y., Takeda S., Sekine K., Yoshihara Y., Kawakami T., Arioka K., Sato H., Uchiyama Y., et al. Nat. Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 18.Boonstra A., Barrat F. J., Crain C., Heath V. L., Savelkoul H. F., O’Garra A. J. Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 19.Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 20.Wang T. T., Tavera-Mendoza L. E., Laperriere D., Libby E., MacLeod N. B., Nagai Y., Bourdeau V., Konstorum A., Lallemant B., Zhang R., et al. Mol. Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 21.Chambon P. Mol. Endocrinol. 2005;19:1418–1428. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- 22.Leid M., Kastner P., Chambon P. Trends Biochem. Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- 23.Wittke A., Weaver V., Mahon B. D., August A., Cantorna M. T. J. Immunol. 2004;173:3432–3436. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 24.Stephensen C. B. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 25.Calleja C., Messaddeq N., Chapellier B., Yang H., Krezel W., Li M., Metzger D., Mascrez B., Ohta K., Kagechika H., et al. Genes Dev. 2006;20:1525–1538. doi: 10.1101/gad.368706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhooke J. L., Prahl J. M., Kimmel-Jehan C., Mendelsohn M., Danielson E. W., Healy K. D., DeLuca H. F. Proc. Natl. Acad. Sci. USA. 2006;103:75–80. doi: 10.1073/pnas.0509734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupper T. S., Fuhlbrigge R. C. Nat. Rev. Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu P. T., Stenger S., Li H., Wenzel L., Tan B. H., Krutzik S. R., Ochoa M. T., Schauber J., Wu K., Meinken C., et al. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 29.Fritsche J., Mondal K., Ehrnsperger A., Andreesen R., Kreutz M. Blood. 2003;102:3314–3316. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 30.Takeda K., Kaisho T., Akira S. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 31.Chu A. C., Morris J. F. In: Skin Immune System. Bos J. D., editor. Boca Raton, FL: CRC; 2005. pp. 77–99. [Google Scholar]
- 32.Wjst M., Dold S. Allergy. 1999;54:757–759. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 33.Hypponen E., Sovio U., Wjst M., Patel S., Pekkanen J., Hartikainen A. L., Jarvelinb M. R. Ann. N.Y. Acad. Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 34.Poon A. H., Laprise C., Lemire M., Montpetit A., Sinnett D., Schurr E., Hudson T. J. Am. J. Respir. Crit. Care Med. 2004;170:967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 35.Raby B. A., Lazarus R., Silverman E. K., Lake S., Lange C., Wjst M., Weiss S. T. Am. J. Respir. Crit. Care Med. 2004;170:1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 36.Worm M. Curr. Opin. Investig. Drugs. 2002;3:1596–1603. [PubMed] [Google Scholar]
- 37.Lehmann B., Querings K., Reichrath J. Exp. Dermatol. 2004;13(Suppl 4):11–15. doi: 10.1111/j.1600-0625.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 38.Zasloff M. J. Invest. Dermatol. 2005;125:xvi–xvii. doi: 10.1111/j.0022-202X.2005.23924.x. [DOI] [PubMed] [Google Scholar]
- 39.Zasloff M. Nat. Med. 2006;12:388–390. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 40.Germain P., Iyer J., Zechel C., Gronemeyer H. Nature. 2002;415:187–192. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- 41.Gombart A. F., Borregaard N., Koeffler H. P. Faseb J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 42.Wang T. T., Nestel F. P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J. H., Mader S., et al. J. Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb A. B. J. Am. Acad. Dermatol. 2005;53:S3–S16. doi: 10.1016/j.jaad.2005.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.