Abstract
Different forms of learning and memory depend on functionally and anatomically separable neural circuits [Squire, L. R. (1992) Psychol. Rev. 99, 195–231]. Declarative memory relies on a medial temporal lobe system, whereas habit learning relies on the striatum [Cohen, N. J. & Eichenbaum, H. (1993) Memory, Amnesia, and the Hippocampal System (MIT Press, Cambridge, MA)]. How these systems are engaged to optimize learning and behavior is not clear. Here, we present results from functional neuroimaging showing that the presence of a demanding secondary task during learning modulates the degree to which subjects solve a problem using either declarative memory or habit learning. Dual-task conditions did not reduce accuracy but reduced the amount of declarative learning about the task. Medial temporal lobe activity was correlated with task performance and declarative knowledge after learning under single-task conditions, whereas performance was correlated with striatal activity after dual-task learning conditions. These results demonstrate a fundamental difference in these memory systems in their sensitivity to concurrent distraction. The results are consistent with the notion that declarative and habit learning compete to mediate task performance, and they suggest that the presence of distraction can bias this competition. These results have implications for learning in multitask situations, suggesting that, even if distraction does not decrease the overall level of learning, it can result in the acquisition of knowledge that can be applied less flexibly in new situations.
Keywords: hippocampus, learning, striatum
Evidence from humans and other animals has shown that there are multiple memory systems defined by different neural substrates and functional demands (1, 2). Declarative memory supports the acquisition of flexibly accessible knowledge and relies on the medial temporal lobe (MTL), whereas habit learning involves the gradual acquisition of behavioral tendencies and relies on the striatum (3, 4). An important question about these memory systems is how their engagement is modulated to optimize learning and behavior. A hypothesis of competition between memory systems has been proposed based on lesion studies in experimental animals (4). This hypothesis predicts that either the striatum or MTL can support learning, depending on task conditions. In experimental animals, the contributions of separate learning mechanisms to performance have been probed by using a plus-maze: If “place” learning (which depends on the hippocampus) is supporting performance, the behavior on a probe test will be different from that supported by “response” learning (which depends on the striatum) (5). Furthermore, the engagement of learning mechanisms supported by either the hippocampus (declarative memory) or the striatum (habit learning) can be modulated pharmacologically (6, 7) to show that performance on a particular task can rely on either system. Analogs of maze tasks and navigation tasks have been used to study memory systems in humans (8–11). In studies of human navigation, different types of tasks typically engage separate learning mechanisms, or different strategies lead to different levels of performance. However, it has been difficult to directly manipulate the engagement of these systems in humans within a single task or show differential engagement beyond navigation tasks.
Habit learning has been examined in humans by using a probabilistic classification task (PCT) (3, 12). In this task (Fig. 1), subjects learn to classify stimuli into two categories, based on trial-by-trial feedback. The striatum has been shown to contribute to PCT performance: Learning is impaired in patients with basal ganglia disorders (13, 14), and performance is associated with activation of the striatum and midbrain (substantia nigra/ventral tegmental area) in normal subjects (15–18). Although the MTL may not be necessary for some degree of learning in the PCT (12), there is evidence that healthy people do engage MTL-dependent learning mechanisms: These subjects exhibit flexible declarative knowledge about the task, whereas amnesic patients with MTL lesions are impaired at acquiring flexible knowledge, even though their overall classification performance may be normal (19). Flexible knowledge refers to knowledge that can be applied in a novel situation outside the training context. Furthermore, mild Parkinson’s disease patients are able to learn the PCT to some extent, but they engage the MTL more during PCT performance than do healthy controls (18). The fact that these two types of learning, which depend on separate memory systems, may be acquired within the same task makes the PCT a useful tool for investigating interactions between memory systems.
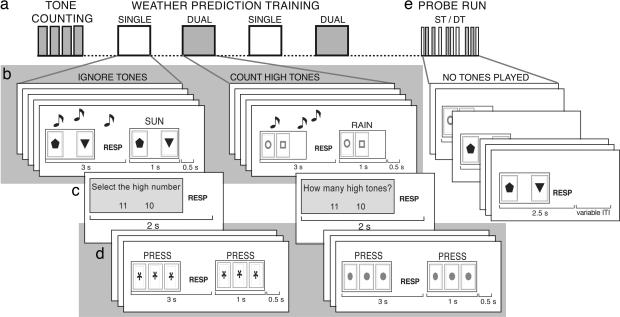
Fig. 1.
Schematic of experimental design and task structure. (a) Tone-counting task: Participants kept a running count of the number of high tones in a stream of high- and low-pitched tones. This task was subsequently used as the secondary task. (b) Participants learned to predict weather outcomes (rain or sun) for two different cities. Three seconds were allowed for responding, after which feedback was provided. During training, high and low tones were played. Participants ignored the tones on the ST blocks and counted the high tones on DT blocks. (c) After five trials of weather prediction, subjects indicated how many high tones they had counted (or selected the high number on ST blocks). ST and DT order was counterbalanced across participants. (d) On baseline trials, participants pressed with their index finger in response to the stimulus. One run of weather prediction consisted of 10 cycles of five weather trials and three baseline trials. (e) During the probe test, participants predicted weather, as during training, but did not receive feedback. No tones were played during the probe trials. During the probe run, trials of task learned under ST and DT conditions were intermixed. RESP, response; ITI, intertrial interval.
We hypothesized that the contribution of these memory systems to performance could be modulated by distraction. Learning of habits is associated with automaticity, such that performance does not require effortful attention or working memory, whereas declarative memory tasks are generally sensitive to the presence of a distracting secondary task that does engage these processes (20). Declarative memory performance is aided by elaborative encoding and effortful retrieval, processes that depend on prefrontal cortex and working memory resources. Therefore, we hypothesized that one factor modulating these systems might be the presence of a distracting secondary task. By occupying working memory, a secondary task should decrease declarative memory encoding and, thus, bias the competition in favor of habit memory mechanisms mediating performance.
To determine whether a secondary task modulates the neural systems involved in performing the PCT, we trained 14 participants on two different classification problems while they were scanned by using functional MRI (fMRI). Participants were trained on one problem under single-task (ST) conditions and on the other problem while performing a concurrent tone-counting task. During training, subjects learned the categories based on trial-by-trial feedback. After training, subjects received an additional block of probe trials using a mixed event-related (ER) fMRI paradigm, during which they classified items that had been trained under either ST or dual-task (DT) conditions. To measure how well participants had learned under each condition, no feedback was presented during the probe block, and all items were presented under ST conditions. After scanning, subjects completed an assessment of their declarative knowledge about the cue–outcome associations, which required flexible use of their knowledge about the task (19).
Results
Behavior.
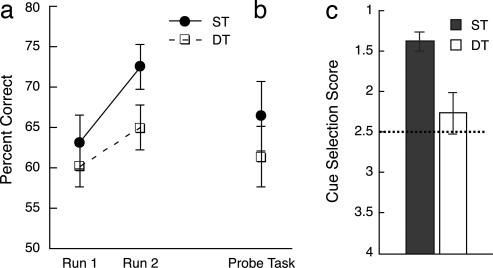
The secondary task impaired classification performance numerically during training, although this difference was only marginally significant in run 2 [t(12) = 1.973, P = 0.072]. In a 2(ST/DT) × 2(run 1/run 2) × 2(order) ANOVA, there was a main effect of run, F(1, 11) = 6.53, P = 0.027. No other effects were significant, P > 0.05 (Fig. 2a). These results are consistent with previous data showing that learning in the PCT can proceed normally under DT conditions (21). Performance on the secondary task during training was consistently high (significantly above chance, P > 0.001) but below ceiling levels (accuracy: run 1, 78%; run 2, 85%; see Table 2, which is published as supporting information on the PNAS web site). One subject was excluded from analyses because of poor classification performance (≈22% correct) on ST items during the probe task. On the probe test, where both tasks were performed under ST conditions, accuracy and response times were not significantly different for items trained under ST versus DT conditions, showing that DT conditions did not impair classification learning [accuracy, t(12) = 0.98, P = 0.35; see Fig. 2b; response time, M = 1.39 s, and mean = 1.41 s, t(12) = −0.40, P = 0.698]. Although performance was numerically higher for items learned under ST conditions, approximately half of subjects (6 of 13) performed better on the DT items than ST, and the other half showed the opposite pattern, consistent with our previous findings of no effect of DT conditions on learning in this task.
Fig. 2.
Behavioral results. The percentages of correct responses are shown. (a) PCT performance during training runs 1 and 2 for ST and DT. (b) PCT performance during the probe test. (c) Cue-selection scores. Scores ranged from 1 to 4 (chance = 2.5). Error bars are standard errors.
Although probe performance was similar for the two training conditions, the tests of declarative knowledge showed that performance of the secondary task effectively impaired acquisition of flexible knowledge about cue–outcome associations. Subjects were significantly better at selecting which cues were associated with a particular outcome for items learned under ST conditions (t(11) = −3.36, P = 0.006; Fig. 2c). In the cue-estimation task, scores were numerically less accurate (i.e., farther from the true target value), but this difference was not significant (t(12) = −1.65, P = 0.12). These results demonstrate a dissociation between classification accuracy (which was not affected by DT conditions at encoding) and declarative knowledge of cue–outcome associations (which was affected).
fMRI Results.
fMRI of training runs.
Comparison of fMRI signals during DT versus ST learning conditions showed a number of differences, consistent with the fact that DT learning conditions were more demanding (e.g., there was greater dorsolateral prefrontal cortex activity during DT conditions than during ST conditions, see Supporting Text and Table 3, which are published as supporting information on the PNAS web site). However, activity in the striatum did not differ between conditions, consistent with the equal engagement of striatal learning mechanisms regardless of learning conditions. Other work has also suggested that striatal learning mechanisms are equally active across implicit and explicit learning conditions during motor-sequence learning (22), consistent with the notion that habit-learning mechanisms are automatically and obligatorily engaged when a task is performed.
fMRI of probe run.
fMRI signals during the probe test were compared to see whether the different characteristics of what had been learned in each condition, as seen in the behavioral results, were reflected in differential engagement of separate memory systems. The probe task data were examined to compare items learned under either ST or DT conditions. Although classification accuracy did not differ between conditions, there were significant differences in neural activity between conditions (see Table 1). These data suggested that the memory systems supporting task performance differed, depending on the conditions under which the subject learned the particular problem.
Table 1.
Activations during probe test (correct trials only)
| Brain region | Max Z | x, mm | y, mm | z, mm | Cluster extent, voxels |
|---|---|---|---|---|---|
| ST correct > DT correct | |||||
| R lingual gyrus | 3.92 | 8 | −66 | 0 | 1,831 |
| L cuneus | 3.82 | −6 | −72 | 4 | |
| L posterior cingulate | 3.64 | −10 | −70 | 10 | |
| L precuneus | 3.63 | −2 | −64 | 38 | |
| R posterior cingulate | 3.48 | 4 | −62 | 4 | |
| L cuneus | 3.44 | −4 | −66 | 6 | |
| R caudate body | 3.52 | 16 | −8 | 26 | 756 |
| R anterior cingulate | 3.46 | 6 | 14 | −10 | |
| R thalamus | 3.39 | 10 | −22 | 14 | |
| R nucleus accumbens | 3.19 | 10 | 6 | −10 | |
| R globus pallidus | 3.16 | 10 | 4 | −2 | |
| R thalamus | 3.14 | 24 | −28 | 10 | |
| L thalamus | 3.96 | −6 | −22 | 14 | 629 |
| L thalamus | 3.95 | −16 | −14 | 0 | |
| L thalamus | 3.55 | −18 | −24 | 16 | |
| L thalamus | 3.54 | −14 | −14 | 4 | |
| L cingulate cortex | 3.12 | −18 | −12 | 28 | |
| L caudate body | 3.12 | −16 | −6 | 24 | |
Three top significant clusters from the whole-brain analysis. Five local maxima are included. L, left; R, right. DT correct > ST correct, no significant clusters.
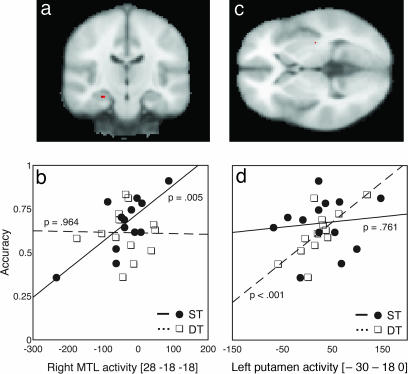
This result was further investigated by examining the relation between behavioral performance and brain activity during probe-task performance, even when overall levels of performance did not differ between the training conditions. Measures of accuracy (and declarative knowledge) were entered into regression analyses to find areas where brain activity during correct probe test performance was correlated with performance measures. These analyses revealed separate regions in the MTL and striatum that were differentially correlated with performance on items learned under ST and DT conditions [small volume correction (SVC), P < 0.05; Fig. 3 a and c]. To examine whether these results represented a dissociation, we extracted a measure of the brain activity in these regions for items learned under each condition, allowing direct comparison of the relationship between brain activity and performance using robust regression analyses. Activity in the right hippocampus was significantly correlated with performance on items learned under ST conditions (r = 0.746, P = 0.003) but not for items learned under DT conditions (r = −0.017, P = 0.955; Fig. 3b), representing a dissociation between items learned under ST and DT conditions in the MTL region. The putamen showed the opposite pattern, with activity level correlated with performance on items learned under DT conditions (r = 0.729, P = 0.005) but not ST conditions (r = 0.117, P = 0.704; Fig. 3d), representing a dissociation between items learned under ST and DT conditions in the striatal region. Each pair of correlations was significantly different (Ps < 0.05), and they were in opposite directions, thus representing a double dissociation. A double dissociation between the two regions was confirmed by a regression analysis that compared the two differences in correlation (P = 0.017). These results show that distraction during learning modulated the degree to which the MTL or striatum was involved in later task performance.
Fig. 3.
Correlations between accuracy and brain activity during the probe task. (a) Activity in the right hippocampus was significantly correlated with performance of the PCT learned under ST conditions (SVC, P < 0.05). (b) During the ST, but not the DT, activity was significantly correlated with performance. (c) Activity in the left putamen was correlated with performance of the PCT learned under DT conditions (SVC, P < 0.05). (d) During DT, but not ST, activity was significantly correlated with performance. Regression lines and P values plotted are from the robust regression results. The x axes in b and d represent signal change (arbitrary units). MNI coordinates are displayed under the figure. (L = R in images).
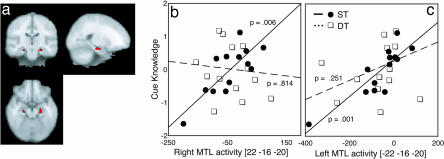
Dual-task conditions during learning also reduced the level of subsequent flexible declarative knowledge about the task (Fig. 2c). We assessed the relation between neural activity and cue–outcome knowledge to confirm the role of the MTL in flexible knowledge acquisition on this task. A simple regression analysis, as described above, identified regions that were correlated with participants’ cue-knowledge scores. During trials where subjects classified items previously learned under ST conditions, task-related activity within the MTL was significantly correlated with the measure of flexible declarative knowledge (Fig. 4a; SVC P < 0.05). No such correlation was seen during trials of the PCT learned under DT conditions. To further compare the response of the MTL during performance of each task, we extracted the mean activity for each subject from the left MTL during performance of each task and tested the correlations with explicit-knowledge scores (Fig. 4b). We repeated the same procedure for the corresponding location on the right side (Fig. 4c). The correlations were significantly different between items learned under ST versus DT conditions for both the left (ST, r = 0.854; DT, r = 0.258, P = 0.041) and right (ST, r = 0.779; DT, r = −0.131, P < 0.005) MTL (see also Table 4, which is published as supporting information on the PNAS web site).
Fig. 4.
Correlations between declarative knowledge and brain activity during the probe task. (a) Regions correlated with declarative cue knowledge of PCT learned under ST conditions (SVC, P < 0.05). Significant correlations were found in both left and right MTLs during performance on items learned under ST conditions. Correlations between declarative knowledge and brain activity for items learned under DT conditions were not significant. Regression lines and P values plotted are from the robust regression results. The x axes represent signal change (arbitrary units), and the y axes represent declarative knowledge z scores. The absolute difference between cue estimates and actual values and the cue-selection scores were transformed to z scores and averaged separately for the ST and DT scores (therefore, the significant difference between declarative knowledge for items learned under ST and DT conditions is not apparent in these figures). This composite score represented a general measure of each participant’s flexible knowledge of cue–outcome associations. (b) Activity in the right MTL correlated with cue knowledge during ST performance. (c) Activity in the left MTL correlated with cue knowledge during ST performance. MNI coordinates are displayed under the figure. (L = R in images).
Discussion
The results demonstrate that equivalent levels of learning on the PCT can be supported by either the MTL or the striatum and that distraction by a secondary task modulates the relative engagement of these systems. These data provide the most direct evidence to date for contributions from separate memory systems to performance of the same task and also demonstrate that the relative contribution from these memory systems can be modulated by task conditions. The results add to extensive evidence that declarative memory encoding depends on working memory. In contrast, habit learning in the PCT does not appear to be nearly as sensitive to available working-memory resources. The apparent lack of sensitivity to the concurrent performance of a secondary task may be a distinguishing feature of habit learning. Other forms of nondeclarative memory (e.g., priming) do appear to sometimes be affected by divided attention at study (23), suggesting that different forms of nondeclarative memory differ in terms of their reliance on available working-memory resources, just as these abilities also differ in terms of their neural substrates.
Evidence from neuropsychological patients has demonstrated that performance on the PCT can be independent of the integrity of MTL structures (12). The present results are consistent with these findings in that they demonstrate that normal PCT performance can occur independently of MTL activation, as was shown for the DT PCT. However, the present results do indicate that, in young, neurologically intact subjects, performance on the PCT is associated with MTL activity when learning occurs under ST conditions. It appears that the PCT can be learned by using different brain systems, depending on the task demands, and it is likely that manipulations that enhance declarative learning of the cue–outcome associations, such as making the associations less probabilistic, decreasing the number of stimuli, or increasing study time, will enhance the contribution of the MTL structures to performance. In support of the idea that there are multiple routes of learning in the PCT, impaired performance has been detected in patients with MTL damage when slightly more deterministic associations were used than in previous studies (24). Furthermore, the patients and control subjects in this study were, on average, ≈20 years younger than the participants in previous work showing intact performance in amnesic patients. If declarative memory is more efficient in younger subjects, it may be that younger normal subjects are more likely to engage the MTL during performance of the PCT than older subjects.
Neuroimaging studies have consistently shown that the MTL is less active during classification learning compared with a variety of baseline tasks (15–17), a finding replicated in this study. The correlations observed here between behavior and MTL activity thus occurred against a background of relative “deactivation” in the MTL. This result suggests that the overall level of activity in the MTL may be less indicative of its role in behavior than is its relation to performance. Consistent with this view, a number of recent studies have shown that baseline tasks in functional imaging studies can be relatively active conditions for the MTL (e.g., ref. 25). Thus, relative “deactivation” of the MTL during the PCT may be more reflective of the engagement of the MTL during the baseline task than of active suppression of MTL activity during classification learning.
In humans, the MTL memory system appears to dominate learning on many tasks. Our data suggest that the striatal system is able to mediate performance in the case where some other factor (such as a secondary task) has prevented development of sufficient explicit knowledge to perform the task. It is not possible to determine from the present data whether development of explicit knowledge under ST conditions prevented habit learning or simply diminished its contributions to performance. However, the pattern of activity during learning of the tasks suggests that striatal regions associated with habit learning were engaged equally during training regardless of the secondary task (see ref. 22). This finding suggests that separate memory systems may acquire redundant information and that any potential competition between memory systems may then be occurring at a stage when the knowledge is applied rather than during acquisition of the task (see ref. 26). Thus, competition appears to be occurring at the response level. Although a habit representation in the putamen may be activated in the task, it does not appear to influence behavior when declarative representations are robust. Such an interpretation would be consistent with findings in experimental animals. Experiments querying the different types of representations acquired by rats in the plus-maze indicate that, although one type of knowledge may be governing performance, redundant representations still exist. For example, inactivation of the dorsal striatum can reinstate a response strategy that depends on the MTL (7), showing that, although the knowledge is not applied, it is still accessible.
It is important to note that, although either the MTL or striatal system was able to support performance on the weather prediction task, the nature of what is learned by each system differs (see ref. 6). Our results indicate that the learning that is supported by the MTL and by the striatum differ fundamentally in terms of their sensitivity to distraction. Whereas MTL-dependent declarative learning of the cue–outcome associations was disrupted by performance of the secondary task, the striatal-dependent habit learning was not diminished and, in fact, was more closely linked to performance than under ST conditions. The second critical difference between these systems is the degree to which they support flexible use of knowledge. Studies of amnesic patients with damage to the MTL have shown that such patients can, under some conditions, learn information that usually depends on the integrity of the MTL (27, 28). However, this learning requires extensive training; more critically, the resulting representations lack flexibility and are more characteristic of habit learning. Our results support the idea that the MTL-dependent memories are readily accessed in situations that differ from the training context (1, 2), as in the cue-knowledge tests given in this experiment. In contrast, knowledge acquired by the striatal-dependent habit-learning system appears to be encapsulated in the stimulus–response mappings acquired during learning. In sum, the present results show how the presence of distraction can change the way that a task is learned such that later expression of knowledge can rely on different brain systems.
Methods
Participants.
Fourteen adults, recruited from the University of California Los Angeles (UCLA) community, were paid for participation in this study (mean age 25.71 yr, SD = 6.53; 5 males). Participation was limited to those between 18 and 45 yr of age, right-handed, with no reported history of neurological illness or drug abuse. All participants provided informed consent in accordance with procedures approved by the UCLA Office for the Protection of Human Research Subjects.
Behavioral Procedure.
Participants were told that they would learn to predict the weather (sun or rain) in two different cities based on sets of cues, which were colored shapes. Four pink cues were used to predict weather for one city and four green cues to predict weather for the other city. Between one and three cues could appear on each trial, yielding 14 combinations. The relative screen locations of specific cues were randomized across trials. The cue strength of each of the 14 resulting stimuli were such that the overall probability associating each cue with sun or rain was 0.756, 0.575, 0.425, and 0.244 across 100 trials (see Table 5, which is published as supporting information on the PNAS web site). These probabilities were the same as those used in several previous studies using the PCT (3, 12). A response was counted as correct if it matched the outcome most strongly associated with a stimulus (i.e., a maximizing criterion). Because the feedback was probabilistic, a participant could make an optimal prediction (which was counted as correct) yet receive feedback inconsistent with that prediction.
One hundred trials of learning on the weather-prediction task, divided into two runs, were completed for each city, resulting in four training runs. During all four training blocks, participants heard high- (1,000 Hz) and low- (500 Hz) pitched tones through headphones but were required to keep a running count of only the high-pitched tones during weather prediction in one set of blocks, the DT blocks. Assignment of cues to ST or DT conditions and the order of ST and DT blocks were counterbalanced across subjects. ST and DT training was always interleaved, such that participants performed either ST1-DT1-ST2-DT2 or DT1-ST1-DT2-ST2 (see Fig. 1). Cues were displayed for 3 s, during which participants responded. Thereafter, the outcome was displayed above the cues for 1 s. The intertrial interval was 0.5 s. After each five trials, participants were asked how many high tones they had counted and had 2 s to respond by choosing between two numbers shown on the screen (the target number and a foil, which was the target ± 1). On ST blocks, participants were instead asked to select the higher of two numbers. After the tone (or number) question, three baseline trials occurred. Each baseline trial displayed three identical symbols unrelated to the cues for predicting the weather, and participants were required to press with their index finger on each trial. Each run consisted of 10 cycles of task and baseline trials and lasted 416 s.
After learning to predict the weather in two cities, an ER probe run was completed, during which no tones were heard. Trials with pink cues and those with green cues were randomly intermixed, and no feedback was given. The probe run presented the 14 cue combinations used in each task four times for a total of 112 trials (56 in each condition). Thus, performance on this task was a measure of the weather prediction proficiency achieved during the previous training. Each trial lasted 2.5 s. The ITI varied between 0.5 and 5 s (mean = 1.75 s) and was sampled randomly from a truncated exponential distribution. The ITI was optimized for efficiency of the comparison between trials with stimuli learned under ST vs. DT conditions by using custom software in MATLAB (Mathworks, Natick, MA). The run lasted 477.75 s.
Outside the scanner, subjects completed a test of their declarative knowledge of cue–outcome associations (as in ref. 19). First, subjects were asked to estimate the probability associated with all cue combinations they had seen. In this test, participants were shown each of the 14 cue combinations for each cue set and asked how likely (in terms of percent) it would be for the outcome to become sun if this cue combination was present. The other half of subjects gave their responses in terms of the “rain” outcome. Second, subjects were asked to select, from a lineup, the cues most likely to be present given a specific outcome. Subjects were presented with all single cues and asked to pick the cue most associated with either sun or rain. This procedure was repeated for the two cue combinations and the three cue combinations. The entire test was performed a second time, with subjects asked to select the cue or combination of cues most likely to occur when the other outcome (sun or rain) was present. This test is presumed to reflect more flexible and abstract knowledge (characteristic of MTL-dependent learning) (19) and may thus be more sensitive to interference with MTL function. The absolute difference between cue estimates and actual values and the cue-selection scores were transformed to z scores and averaged. One participant’s cue-selection score was lost because of computer error, and only the cue-estimate score figured in that participant’s knowledge score. This composite score represented a general measure of each participant’s flexible knowledge of cue–outcome associations and was used as a covariate in regression analyses.
MRI Acquisition.
Imaging was performed with a 3T Siemens Allegra head-only MR scanner. Before functional scanning, the following structural images were acquired: a high-resolution T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) [repetition time (TR) = 2,300 ms; inversion time 1,100 ms; echo time (TE) = 2.93 ms; 256-mm field of view (FOV); 192 × 192 matrix; 1.33 mm × 1.33 mm pixel size; slice thickness, 1 mm] and a high-resolution T2-weighted anatomical image coplanar to the functional acquisition (TR = 5 s; TE = 33 ms; 128 × 128 matrix; 1.56 mm × 1.56 mm pixel size; 30 slices; 4-mm slice thickness plus 1-mm gap; 200 mm FOV). Blood oxygenation level-dependent-sensitive functional images were collected by using a gradient-echo echo-planar pulse sequence (TR = 2,000 ms; TE = 30 ms; 64 × 64 matrix; 3.125 mm × 3.125 mm pixel size; 30 slices; 4-mm slice thickness plus 1-mm gap; 200 mm FOV). Four images at the beginning of each run were discarded to allow T1 equilibration.
Data Processing and Analysis.
Preprocessing and statistical analysis of the data were performed by using the program FSL [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, Oxford, U.K.] (29). Motion correction was performed by using MCFLIRT (30). Each fMRI run was subjected to independent components analysis by using the FSL MELODIC tool (31). This tool provides a report displaying the spatial and temporal characteristics for each of the isolated components; these reports were examined, and components clearly related to motion or other sources of low- or high-frequency noise were removed to create a denoised data set.† (All basic analyses were performed on denoised as well as “noisy” data sets, and the results did not differ qualitatively.) Images were temporally high-pass filtered with a cutoff period of 80 s for the tone-counting run, 75 s for PCT training runs, and 66 s for the final ER run. Spatial smoothing was applied with a Gaussian kernel of 5 mm (FWHM). After preprocessing, statistical analyses were performed at the single-subject level by using the general linear model within FSL (FEAT, FMRI Expert Analysis Tool). Each event was modeled as an impulse convolved with a canonical hemodynamic response function (HRF) (a double γ-function modeling the HRF rise and following undershoot) along with its temporal derivative. For the ER probe run, trials with correct responses for each task were modeled separately. Specific comparisons of interest were tested by using linear contrasts. After analysis at the individual level, the results were spatially normalized to the Montreal Neurological Institute (MNI)-152 template by using FSL’s FLIRT registration tool. Functional images were first aligned to the coplanar high-resolution T2-weighted image, and then the coplanar was aligned to T1-weighted MP-RAGE and, finally, the MP-RAGE to the standard MNI-152 image. All transformations were carried out by using 12 degrees of freedom affine transforms (30). Mixed-effects group analyses were performed for each contrast by using FSL’s FLAME (FMRIB’s local analysis of mixed effects) module (29). Higher-level statistical maps were thresholded by using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05, according to the theory of Gaussian random fields (32).
For a priori anatomical regions, additional analyses used randomization tests across voxels limited to the region of interest (ROI) by using the FSL randomize tool; the voxel-based maximum statistic was used to correct for the volume of interest (33). ROIs for the MTL were obtained from the automated anatomical labeling (AAL) library (34), and ROIs for the striatum were created by manually tracing the caudate and putamen in the MNI-152 template.
Because of the presence of outliers in the data, robust regression was performed by using an iteratively reweighted least-squares algorithm (implemented in the MATLAB Statistics Toolbox); this method ensures against the influence of outliers on the regression solution. To directly compare brain activity associated with each task in regions identified in the regression analyses described above, we extracted mean activity in a single voxel for each task during the probe run. The resulting measures of activity during performance of each task for each subject were then entered in ordinary least-squares regression using Zellner’s seemingly unrelated regression analysis (implemented by using Stata 8.0 (Stata College Station, TX). These analyses compared the difference between the regions that were differentially associated with performance under ST and DT conditions (i.e., tested the difference between the differences in regression lines that resulted from the correlation between brain activity and performance measures).
Supplementary Material
Acknowledgments
We thank Michael Mitchell and staff (Statistical Consulting Group, Academic Technology Services, University of California, Los Angeles) for help with regression analyses and Sabrina M. Tom for help with fMRI data acquisition. This work was supported by National Science Foundation Grant BCS-0223843, a Whitehall Foundation grant (to R.A.P.), and a National Science Foundation Graduate Fellowship (to K.F.).
Abbreviations
- DT
dual-task
- ER
event-related
- fMRI
functional MRI
- MNI
Montreal Neurological Institute
- MTL
medial temporal lobe
- PCT
probabilistic classification task
- ST
single-task
- SVC
small volume correction.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The neuroimaging data have been deposited with the fMRI Data Center, www.fmridc.org (accession no. 2-2006-1225Y).
Poldrack, R. A., Aron, A. R. & Tom, S. M. (2005). Poster presented at the International Conference on Functional Mapping of the Human Brain, Toronto, ON, Canada (June 12–16).
References
- 1.Squire L. R. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 2.Cohen N. J., Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 3.Knowlton B. J., Mangels J. A., Squire L. R. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 4.Packard M. G., Hirsh R., White N. M. J. Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poldrack R. A., Packard M. G. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 6.Packard M. G. Proc. Natl. Acad. Sci. USA. 1999;96:12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packard M. G., McGaugh J. L. Neurobiol. Learn. Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 8.Voermans N. C., Petersson K. M., Daudey L., Weber B., Van Spaendonck K. P., Kremer H. P., Fernandez G. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Iaria G., Petrides M., Dagher A., Pike B., Bohbot V. D. J. Neurosci. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire E. A., Burgess N., Donnett J. G., Frackowiak R. S., Frith C. D., O’Keefe J. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 11.Hartley T., Maguire E. A., Spiers H. J., Burgess N. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 12.Knowlton B. J., Squire L. R., Gluck M. A. Learn. Mem. 1994;1:106–120. [PubMed] [Google Scholar]
- 13.Knowlton B. J., Squire L. R., Paulsen J. S., Swerdlow N. R., Swenson M. Neuropsychology. 1996;10:538–548. [Google Scholar]
- 14.Shohamy D., Myers C. E., Grossman S., Sage J., Gluck M. A., Poldrack R. A. Brain. 2004;127:851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- 15.Poldrack R. A., Prabhakaran V., Seger C. A., Gabrieli J. D. E. Neuropsychology. 1999;13:564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- 16.Poldrack R. A., Clark J., Pare-Blagoev E. J., Shohamy D., Moyano J. C., Myers C., Gluck M. A. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- 17.Aron A. R., Shohamy D., Clark J., Myers C., Gluck M. A., Poldrack R. A. J. Neurophysiol. 2004;92:1144–1152. doi: 10.1152/jn.01209.2003. [DOI] [PubMed] [Google Scholar]
- 18.Moody T. D., Bookheimer S. Y., Vanek Z., Knowlton B. J. Behav. Neurosci. 2004;118:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- 19.Reber P. J., Knowlton B. J., Squire L. R. Behav. Neurosci. 1996;110:861–871. doi: 10.1037//0735-7044.110.5.861. [DOI] [PubMed] [Google Scholar]
- 20.Craik F. I., Govoni R., Naveh-Benjamin M., Anderson N. D. J. Exp. Psychol. Gen. 1996;125:159–180. doi: 10.1037//0096-3445.125.2.159. [DOI] [PubMed] [Google Scholar]
- 21.Foerde K., Poldrack R. A., Knowlton B. J. Mem. Cognit. doi: 10.3758/bf03193461. in press. [DOI] [PubMed] [Google Scholar]
- 22.Willingham D. B., Salidis J., Gabrieli J. D. J. Neurophysiol. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- 23.Crabb B. T., Dark V. J. Mem. Cognit. 2003;31:997–1008. doi: 10.3758/bf03196121. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins R. O., Myers C. E., Shohamy D., Grossman S., Gluck M. Neuropsychologia. 2004;42:524–535. doi: 10.1016/j.neuropsychologia.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Stark C. E., Squire L. R. Proc. Natl. Acad. Sci. USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poldrack R. A., Rodriguez P. Neurobiol. Learn. Mem. 2004;82:324–332. doi: 10.1016/j.nlm.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Bayley P. J., Frascino J. C., Squire L. R. Nature. 2005;436:550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayley P. J., Squire L. R. J. Neurosci. 2002;22:5741–5748. doi: 10.1523/JNEUROSCI.22-13-05741.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith S. M., Jenkinson M., Woolrich M. W., Beckmann C. F., Behrens T. E., Johansen-Berg H., Bannister P. R., De Luca M., Drobnjak I., Flitney D. E., et al. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson M., Bannister P., Brady M., Smith S. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 31.Beckmann C. F., Smith S. M. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 32.Friston K. J., Worsley K. J., Frakowiak R. S. J., Mazziotta J. C., Evans A. C. Hum. Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 33.Nichols T. E., Holmes A. P. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.