Abstract
The management of infectious diseases is an increasingly important public health issue, the effective implementation of which is often complicated by difficulties in teasing apart the relative roles of extrinsic and intrinsic factors influencing transmission. Dengue, a vector-borne strain polymorphic disease, is one such infection where transmission dynamics are affected by environmental variables as well as immune-mediated serotype interactions. To understand how alternative hypotheses concerning dengue infection and transmission may explain observed multiannual cycles in disease incidence, we adopt a theoretical approach that combines both ecological and immunological mechanisms. We demonstrate that, contrary to perceived wisdom, patterns generated solely by antibody-dependent enhancement or heterogeneity in virus virulence are not consistent with serotype-specific notification data in important ways. Furthermore, to generate epidemics with the characteristic signatures observed in data, we find that a combination of seasonal variation in vector demography and, crucially, a short-lived period of cross-immunity is sufficient. We then show how understanding the persistence and eradication of dengue serotypes critically depends on the alternative assumed mechanisms.
Keywords: antibody-dependent enhancement, multistrain dynamics, transient cross-immunity, vector-transmitted disease
Cyclic dynamics are a ubiquitous feature of epidemiological data. The many potential drivers, both extrinsic, such as climate (1, 2), and intrinsic, such as host immunity (3), are often difficult to disentangle from the web of causal mechanisms (4), especially for vector-borne multistrain diseases. Dengue is a mosquito-transmitted pathogen that is estimated to infect 50 million people worldwide per year. It results in a spectrum of illness from the subclinical to classic dengue fever to the severe, and sometimes fatal, dengue hemorrhagic fever (DHF) and dengue shock syndrome. Rapid and unplanned urbanization, increased human movement, and ineffective mosquito control are among the many factors that have likely contributed to dramatic dengue resurgence. Over the past 30 years, cocirculation of the four antigenically related but distinct serotypes has become widespread, which has coincided with a marked increase in the global number of reported dengue cases and almost a 6-fold increase in the number of countries reporting DHF (5).
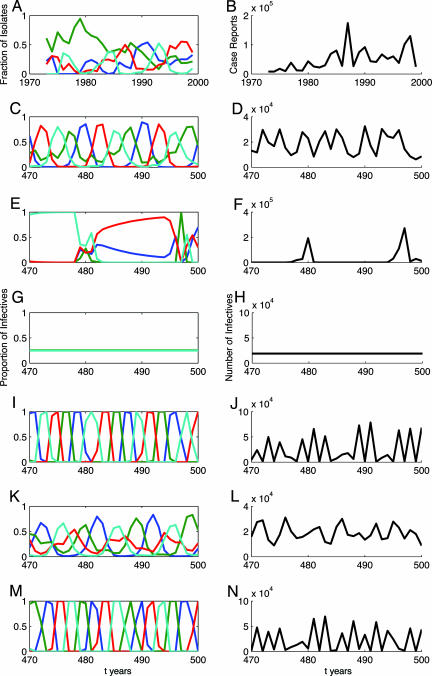
In Thailand, where all four serotypes have been detected since the early 1970s, empirical studies have demonstrated intriguing patterns in dengue incidence. Both temporal (6) and spatiotemporal (7) analyses of aggregated dengue or DHF incidence (encompassing all serotypes) have revealed fluctuations with a multiannual signature (≈3 years) but with a strong seasonal component (Fig. 1B and Fig. 4, which is published as supporting information on the PNAS web site). Although such data mask individual serotype dynamics, analysis of viral isolates from dengue patients admitted to hospital provides a proxy for quantifying the relative contributions of each serotype. A long-term study of clinical dengue in Thai children (8) revealed longer multiannual cycles (≈9 years for Den-1, -2, and -4) in individual serotype incidence, with the dominant serotype being displaced on a regular basis since 1985 (Fig. 1A). There remains, however, considerable speculation over the precise mechanisms responsible for generating these dynamics (6, 7, 9).
Fig. 1.
Comparison of annual dengue data for Thailand with model output. (A and B) Annual serotype-specific [each serotype is denoted by a different color (Den-1, blue; Den-2, green; Den-3, red; and Den-4, cyan) (Left)] and aggregate dengue fever and DHF case report (Right) data for Thailand (taken from ref. 8). The remaining time series are generated by the deterministic model with four serotypes: temporary cross-immunity (C and D); ADE (E and F); asymmetry in virulence (G and H); temporary cross-immunity and ADE (I and J); temporary cross-immunity and asymmetry in virulence (K and L); and temporary cross-immunity, ADE and asymmetry in virulence (M and N). Spectral analysis of the detrended aggregated output (B, D, F, H, J, L, and N) reveals a dominant period of 3.4 (data), 3.4, 15.5, 1, 2.1, 5.2, and 2.1 years, respectively. Model parameters (symmetric for all serotypes i): NH = 20 million, μH = 0.02 per year, k = 2, μV = 26.1 per year, a = 0.05, σH = 73 per year, σV = 36.5 per year, γi = 60.8 per year, βi = αi = 70 per year (R0i = 3.5), ρ1 = ρ3 = ρ4 = ρx = 0, ξi = 0, ϕi = 0. (C and D) δi = 3 per year, χi = 1, ρ2 = 0. (E and F) δi = 365 per year, χi = 3, ρ2 = 0. (G and H) δi = 365 per year, χi = 1, ρ2 = 0.05. (I and J) δi = 3 per year, χi = 3, ρ2 = 0. (K and L) δi = 3 per year, χi = 1, ρ2 = 0.05. (M and N) δi = 3 per year, χi = 3, ρ2 = 0.05. Nonzero initial conditions: S0 = 0.29NH, S1234 = 0.71NH, VSi = kNH, λH1 = β1, λH2 = 2β2, λH3 = 3β3, λH4 = 4β4.
Alternative Hypotheses
Much of the debate has focused on the immune response to dengue infection and its role in the emergence of DHF. The interaction of dengue serotypes in human hosts is mediated, in part, by the immune system’s antibody response to infection. Although the exact nature of this response is not fully understood, results from experiments on human volunteers suggest there is a relatively short period (2–9 months) during which cross-reactive antibody levels are elevated and confer cross-immunity to other serotypes (homologous immunity appears to be lifelong; refs. 10 and 11). This period of temporary cross-immunity is analogous to the convalescent period in models of interaction between sympatric childhood diseases (12). Transient strain-transcending immunity has also been found to be essential to explain key aspects of influenza dynamics (13), whereas short-lived partially cross-reactive immune responses are thought to play a significant role in generating antigenic variation in malaria (14). After the period of transient cross-protection, a second episode of infection with a heterotypic dengue virus may then lead to a process known as antibody-dependent enhancement (ADE; refs. 15–17). ADE occurs when cross-reactive antibodies stimulated by a prior infection wane to levels that no longer neutralize the heterotypic virus. Instead of preventing infection, the binding of antibody to virus at subneutralizing concentrations can result in enhanced viral replication by increased infection of cells bearing the IgG receptor (11). The presence of subneutralizing antibody levels is also thought to be temporary, although how long such levels persist is unknown. Epidemiological evidence in support of ADE is provided by studies reporting that the preexistence of dengue virus antibodies is a significant risk factor for severe disease (17, 18), although this is not always the case (19). A cascade of other immune responses initiated by memory T lymphocytes has also been implicated in the immunopathogenesis of dengue virus infection, but sequential infection with different serotypes appears to be the key trigger.
An alternative view of serotype interaction is based upon variation in virulence among and within dengue serotypes: each serotype exhibits extensive genetic variation, and it is postulated that certain “virulent” virus genotypes are associated with the manifestation of severe disease (20, 21). In particular, Dengue-2 strains originating in southeast Asia appear to be more pathogenic than their American counterparts (22, 23). Evidence as to whether ADE or variation in viral virulence, or both, is consistent with the temporal patterns of dengue incidence remains equivocal (24). One important unresolved issue is whether ADE or virulent strains are phenomena that purely increase the severity of symptoms (and increase mortality) or whether they also imply increased transmission by enhanced viral replication and an increase in viral titers. Previous theoretical work has demonstrated that if ADE is assumed to increase transmission, then coexistence and cyclic, possibly chaotic, strain fluctuations are promoted (25, 26). However, if ADE is instead assumed to increase mortality and shorten the effective infectious period, it may decrease persistence (27).
Model Framework
To distinguish among ADE, variation in virulence, and the novel hypothesis of temporary heterologous cross-immunity, we formulated a general model for vector-borne multistrain pathogens and applied it to dengue. Our model is based on the traditional susceptible-exposed-infectious-recovered classes for the human host and susceptible-exposed-infectious classes for the mosquito host (28). A key addition is that cross-immunity and ADE are assumed to be related to cross-reactive antibody levels that wane over time, so that any period of heterotypic cross-immunity or enhancement is temporary (29). We explore alternative assumptions concerning the effects of temporary cross-immunity, ADE, and variation in serotype virulence, with the effects on mortality and transmission considered independently. Our model includes an important ecological ingredient that is often ignored: seasonal variation in vector recruitment, which gives rise to temporal variation in transmission. (Further details about how ADE, viral virulence, and seasonality are incorporated in the model are given in Methods.)
Temporal Dynamics
This model is used to examine ecological and immunological mechanisms that generate time series consistent with data. Specifically, we compare model dynamics with serological time series from a long-term study of dengue in Thai children (8) together with total dengue cases reported to the Thai Ministry of Public Health (Fig. 1 A and B). The aggregate data reveal fluctuations of between 3 and 4 years in dengue case reports, whereas individual serotypes generally cycle in and out of phase with longer periods than the aggregate data (Fig. 5A, which is published as supporting information on the PNAS web site). Temporary cross-immunity alone (Fig. 1 C and D) generates model dynamics in which serotype outbreaks are asynchronous and aggregate incidence exhibits a period of ≈3 years. ADE alone results in a complex pattern of serotype dynamics with aggregate numbers of infectives that have a period exceeding 10 years (Fig. 1 E and F). Asymmetry in serotype virulence results in annual dynamics and a constant prevalence of each serotype (Fig. 1 G and H). The combination of ADE and temporary cross-immunity gives rise to out-of-phase serotype fluctuations and aggregate time series with a period of 2 years (Fig. 1 I and J), whereas asymmetry in virulence and temporary cross-immunity generate periods of ≈5 years (Fig. 1 K and L). Finally, incorporating all three mechanisms, results in a similar time series to that including just ADE and temporary cross-immunity (Fig. 1 M and N). Thus, for a fixed amplitude of seasonality, a short period of heterologous cross-immunity alone is sufficient to generate dengue dynamics consistent with observed patterns. Moreover, when strong ADE or asymmetry in virulence is included, the dynamics are generally inconsistent with data, independent of assumptions about temporary cross-immunity.
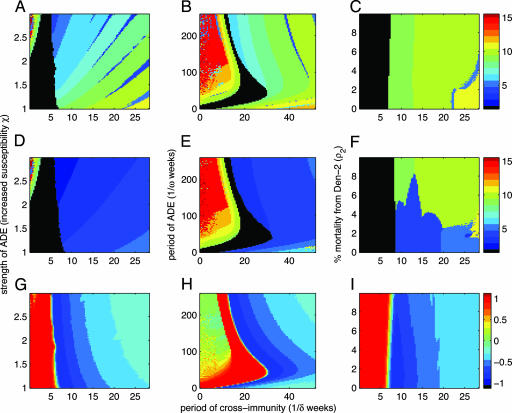
The dynamical consequences of different assumptions can be explored in a more systematic manner for the simpler case of two serotypes. We find that seasonality is necessary to explain intraannual variation in monthly dengue incidence, but it has a lesser impact on interannual dynamics (see Figs. 6 and 7, which are published as supporting information on the PNAS web site). We therefore investigate the immunological mechanisms with a fixed amplitude of seasonality. In Fig. 2, we examine how a combination of temporary cross-immunity and strength of ADE (Fig. 2 A, D, and G), or duration of ADE (Fig. 2 B, E, and H), or asymmetry in virulence (Fig. 2 C, F, and I) influences individual serotype outbreaks (Fig. 2 A–C), aggregate dynamics (Fig. 2 D–F), and the correlation between serotypes (Fig. 2 G–I). For very short periods (<2 months) of cross-immunity, annual variation in vector recruitment drives annual cycles in serotype incidence (black region, Fig. 2A), unless ADE is significantly increased, which leads to multiannual synchronized serotype cycles. For more realistic periods of cross-immunity, increasing ADE reduces the intrinsic epidemiological period for individual serotypes, and the aggregate data have an even shorter periodic signature because individual serotype epidemics do not coincide (blue region, Fig. 2D). The important conclusion to emerge from Fig. 2 A, D, and G is that a minimum period of cross-immunity is necessary to obtain the empirically observed periods of 3 years in the aggregate data; ADE alone gives rise to much longer cycles in dengue incidence. Fig. 2 B, E, and H illustrate that the same qualitative patterns emerge if ADE is a transient process.
Fig. 2.
ADE or variation in virulence; the dominant period and correlation of two dengue serotypes from deterministic model simulations. A, D, and G explore the effects of permanent ADE, as defined by increased susceptibility to one serotype after a primary infection with the other, whereas B, E, and H fix χ and explore the effects of a temporary period of ADE. C, F, and I show the effects of increasing the mortality due to infection with only one of the serotypes. In all cases, these effects are studied as the temporary period of cross-immunity (after infection with one serotype and before infection with the other) is lengthened. A–C present the dominant period of incidence of a single serotype, D–F present the dominant period of aggregated dengue incidence (black represents annual cycles), and G–I illustrate the correlation between the dynamics of the two serotypes. See Fig. 8, which is published as supporting information on the PNAS web site, for significance associated with the dominant period. Model parameters are as in Fig. 1, except: NH = 1 million, δ1 = δ2 = δ, χ1 = χ2 = χ, in B, E, and H, χ = 2, and in C, F, and I, χ = 1 and ρ1 = 0. It is interesting to note that if ADE is asymmetric, i.e., primary infection with only one of the serotypes induces ADE (38) (χ1 = 1, χ2 ≥ 1), then for short periods of temporary cross-immunity ADE does not result in the multiannual cycles shown here; instead, only annual fluctuations are generated by the model.
When considering differential serotype virulence, we focus on increased mortality due to a primary or secondary infection with a particular serotype. As is the case for ADE, without a period of temporary cross-immunity, increases in mortality do not result in the observed cyclic incidence of dengue (Fig. 2 C, F, and I). With temporary cross-immunity, cycles of 4 years are observed for very small relative increases in mortality, which is consistent with dengue mortality statistics. In summary, a short-lived period of cross-immunity induced by infection with another serotype seems to be sufficient to match epidemiological observations. Additional moderate amounts of ADE and increased virulence are also compatible but not necessary. Of course, this does not rule out a role for ADE or increased virulence in causing DHF, but these mechanisms alone cannot explain total dengue population dynamics. Although more complex, these results generally hold for the same systematic exploration of the four-strain system (see Fig. 9, which is published as supporting information on the PNAS web site). In particular, transient cross-immunity among the four serotypes gives rise to more complex phase relationships in which individual serotypes fluctuate in and out of phase with one another, a pattern observed in the serotype-specific data (Fig. 7A).
Public Health Implications
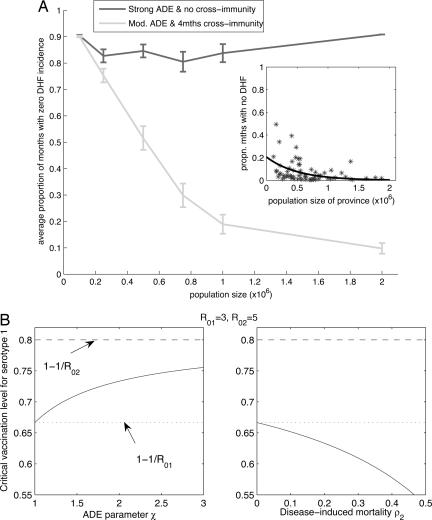
In addition to influencing interepidemic periods, accurate assessment of the roles of ADE, variation in serotype virulence, and temporary cross-immunity have important public health consequences, namely for infection persistence and eradication. We use an event-driven stochastic formulation of our model to explore how dengue persistence changes with population size (see Methods). This analysis is carried out for two sets of parameter values: one assuming almost no cross-immunity and strong ADE and one assuming 4 months of cross-immunity and moderate ADE (parameters that generate deterministic time series consistent with the data). As shown in Fig. 3A, we find that, assuming strong ADE and no cross-immunity, there is no systematic reduction in extinctions even when the population size exceeds 2 million. In contrast, moderate ADE and 4 months of cross-immunity generate a trend consistent with data from the Thai provinces: local dengue extinctions become rare as the population size increases to >1 million (Fig. 3A Inset).
Fig. 3.
Consequences of alternative assumptions about serotype interaction for dengue persistence and eradication. (A) Critical community size. Comparison of results from stochastic realizations with monthly DHF incidence from Thai provinces (Inset). Simulations where there is strong ADE (χ = 3) and virtually no cross-immunity (δ = 365 per year) demonstrate high extinction probabilities over all population sizes ≤2 million. Simulations where there is moderate ADE (χ = 1.5) and a 4-month period of cross-immunity (δ = 3 per year) show decreasing extinction probabilities as the population size increases above 1 million; consistent with the Thai data (7). (The black line denotes the least-squares best-fit exponential curve.) Other model parameter values are as in Fig. 1, and results are averages of 50 realizations with standard error bars. (B) Effects of ADE and difference in virulence on vaccination thresholds. If two serotypes have slightly different R0, then the serotype with the smaller R0 is either more or less difficult to eradicate in the presence of the other serotype depending on whether there is ADE or increased mortality from the other serotype. Critical vaccination level is given by the following expression, 1 − ((R02/R01 − 1)/c + 1)/R02, where c ≈ χ(1 − ρ2).
Where these epidemiological mechanisms may play a more important role is in disease eradication. Most current control efforts are focused on combating dengue through vaccination (30). Because of the importance of the pathogenesis of DHF, any vaccine will be required to protect against all four serotypes. If we consider the effects of the different mechanisms on vaccination, we find that ADE can have quite the opposite effect on eradication than increased virulence. Although neither affects the critical vaccination level to achieve eradication of all serotypes, if one serotype has a lower basic reproduction ratio (R0) than the other, then ADE increases the effort required to eradicate the serotype with lower transmission, whereas increased virulence decreases the effort (Fig. 3B). (A refractory period of a few months has no discernible effect.)
Of further interest is that, in the absence of serotype-specific data, aggregate dengue cases can still give some insight into the underlying mechanisms, because cross-protection and cross-enhancement result in different patterns of serotype synchrony and thus leave different dynamical footprints. However, to confirm our predictions on a wider scale, more serotype-specific data are essential. These would permit a more quantitative analysis of model agreement with data. In particular, stochastic simulation-based inference methods that provide likelihood estimates of model parameters could be used to compete a suite of alternative hypotheses. Such analyses would be especially beneficial in considering further the potentially asymmetric interactions between the serotypes. Correspondingly, to fully explore the variation in virulence, we require additional information, in particular molecular data, about the evolution within dengue serotypes. At present, such genetic data are restricted to a few clinical cases of dengue fever/DHF per population, so local viral diversity is often underestimated (24). Selection within both human and mosquito hosts is known to occur, but whether one is more important than the other is largely unknown (31). Our model does not explicitly consider within serotype variation, the assumption being that a particular viral strain has become fixed in the population. An open question is how this genetic variation interacts with the epidemiological dynamics of the dengue viruses.
Methods
Deterministic Model.
The full set of equations is given in Supporting Text, which is published as supporting information on the PNAS web site. Here we outline how the ecological and immunological mechanisms under investigation are incorporated into our theoretical framework.
ADE.
The precise immunological consequences of ADE are not fully understood, but there are two alternative assumptions that can be made: (i) ADE can result in increased transmission, by either increased susceptibility to infection after a primary infection (32) or increased infectiousness with a secondary infection (25), perhaps through an increase in viral titers (ref. 33; both variations lead to similar results); or (ii) ADE can result in increased mortality after a secondary infection (27). Here, we focus on assumption i: increased susceptibility to infection after a primary infection, regulated by the parameter χ. If we assume ii, and that mortality does not alter the effective infectious period (because death usually occurs after the period of viremia), then including ADE in the two-serotype model has no dynamical impact. However, when we investigated this assumption with the four-serotype model, we obtained similar results to those for differential mortality due to asymmetry in virulence (see Fig. 10, which is published as supporting information on the PNAS web site).
Variation in Serotype Virulence.
As with ADE, variation in virulence can be assumed to increase transmission (increases individual R0) or increase mortality (does not increase individual R0) after infection with a particular serotype. Because analyses of age-stratified serological data suggest there is only minor variation in the R0 of each serotype (34), we focus here on increased mortality. However, if we consider differential virulence as leading to increased transmission of one of the two serotypes, we obtain annual dynamics over a larger region of parameter space, and there is little change in the intrinsic epidemiological period, because this is generally determined by the strain with the smaller transmission rate (see Fig. 11, which is published as supporting information on the PNAS web site).
Seasonality.
Dengue is principally vectored by the day-biting mosquito, Aedes aegypti, the life cycle of which is influenced by seasonal variation in climatic variables: adult vector density is often higher during the wet season (35), and ambient temperature is known to regulate dengue transmission through its effects on adult longevity, blood-feeding activity, and the incubation of the virus within the mosquito (36). We illustrate the seasonal component of dengue epidemics and reproduce Fig. 2 in the absence of seasonal variation and for a larger amplitude of seasonality in Figs. 9 and 10. Although we incorporate seasonality into the recruitment of adult vectors, similar results are obtained if it is placed directly in the biting rate.
Stochastic Model.
We transform our deterministic model into its stochastic analogue using Gillespie’s algorithm, or more precisely, his direct method (37). To allow us to compare our results to data on DHF incidence from Thai provinces, we binomially sample total monthly case reports from the stochastic realizations at 1%. We then plot the proportion of months with zero DHF incidence (Fig. 3A). This analysis is replicated for an alternative assumption about DHF reporting in Fig. 12, which is published as supporting information on the PNAS web site.
Parameter Values and Sensitivity Analysis.
For parameter values and sensitivity analysis, see Table 1 and Figs. 13 and 14, which are published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Steve Sait, Mark Hunter, Marc Choisy, Matt Bonds, Sonia Altizer, and two anonymous reviewers for comments on this manuscript. This work was supported by National Institutes of Health Grant R01 GM69111, National Science Foundation Grant DEB 0343176, and a New Scholar Award in Global Infectious Disease from the Ellison Medical Foundation (to P.R.).
Abbreviations
- DHF
dengue hemorrhagic fever
- ADE
antibody-dependent enhancement.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pascual M., Rodo X., Ellner S. P., Colwell R., Bouma M. J. Science. 2000;289:1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- 2.Sultan B., Labadi K., Guegan J. F., Janicot S. PLoS Med. 2005;2:43–49. doi: 10.1371/journal.pmed.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grassly N. C., Fraser C., Garnett G. P. Nature. 2005;433:417–421. doi: 10.1038/nature03072. [DOI] [PubMed] [Google Scholar]
- 4.Koelle K., Pascual M. Am. Nat. 2004;163:901–913. doi: 10.1086/420798. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie J. S., Gubler D. J., Petersen L. R. Nat. Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 6.Hay S. I., Myers M. F., Burke D. S., Vaughn D. W., Endy T., Nisalak A., Shanks G. D., Snow R. W., Rogers D. J. Proc. Natl. Acad. Sci. USA. 2000;97:9335–9339. doi: 10.1073/pnas.97.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings D. A. T., Irizarry R. A., Huang N. E., Endy T. P., Nisalak A., Ungchusak K., Burke D. S. Nature. 2004;427:344–347. doi: 10.1038/nature02225. [DOI] [PubMed] [Google Scholar]
- 8.Nisalak A., Endy T. P., Nimmannitya S., Kalayanarooj S., Thisayakorn U., Scott R. M., Burke D. S., Hoke C. H., Innis B. L., Vaughn D. W. Am. J. Trop. Med. Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 9.Cazelles B., Chavez M., McMichael A. J., Hales S. PLoS Med. 2005;2:313–318. doi: 10.1371/journal.pmed.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabin S. B. Am. J. Trop. Med. Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 11.Innis B. L. In: Dengue and Dengue Hemorrhagic Fever. Gubler D. J., Kuno G., editors. Wallingford, Oxfordshire, U.K.: CABI; 1997. [Google Scholar]
- 12.Rohani P., Earn D. J. D., Finkenstadt B. F., Grenfell B. T. Proc. R. Soc. London Ser. B; 1998. pp. 2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson N. M., Galvani A. P., Bush R. M. Nature. 2003;422:428–433. doi: 10.1038/nature01509. [DOI] [PubMed] [Google Scholar]
- 14.Recker M., Nee S., Bull P. C., Kinyanjui S., Marsh K., Newbold C., Gupta S. Nature. 2004;429:555–558. doi: 10.1038/nature02486. [DOI] [PubMed] [Google Scholar]
- 15.Halstead S. B. Yale J. Biol. Med. 1970;42:350–361. [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead S. B., Orourke E. J. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 17.Kliks S. C., Nisalak A., Brandt W. E., Wahl L., Burke D. S. Am. J. Trop. Med. Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 18.Burke D. S., Nisalak A., Johnson D. E., Scott R. M. Am. J. Trop. Med. Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 19.Watts D. M., Porter K. R., Putvatana P., Vasquez B., Calampa C., Hayes C. G., Halstead S. B. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 20.Rosen L. Am. J. Trop. Med. Hyg. 1977;26:337–343. doi: 10.4269/ajtmh.1977.26.337. [DOI] [PubMed] [Google Scholar]
- 21.Holmes E. C., Burch S. S. Trends Microbiol. 2000;8:74–77. doi: 10.1016/s0966-842x(99)01669-8. [DOI] [PubMed] [Google Scholar]
- 22.Rico-Hesse R. Virology. 1990;174:479–493. doi: 10.1016/0042-6822(90)90102-w. [DOI] [PubMed] [Google Scholar]
- 23.Messer W. B., Vitarana U. T., Sivananthan K., Elvtigala J., Preethimala L. D., Ramesh R., Withana N., Gubler D. J., De Silva A. M. Am. J. Trop. Med. Hyg. 2002;66:765–773. doi: 10.4269/ajtmh.2002.66.765. [DOI] [PubMed] [Google Scholar]
- 24.Chevillon C., Failloux A.-B. Trends Microbiol. 2003;11:415–421. doi: 10.1016/s0966-842x(03)00206-3. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson N. M., Anderson R., Gupta S. Proc. Natl. Acad. Sci. USA. 1999;96:790–794. doi: 10.1073/pnas.96.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings D. A. T., Schwartz I. B., Billings L., Shaw L. B., Burke D. S. Proc. Natl. Acad. Sci. USA. 2005;102:15259–15264. doi: 10.1073/pnas.0507320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi I., Sasaki A., Boots M. Proc. R. Soc. London Ser. B; 2003. pp. 2241–2247. [Google Scholar]
- 28.Anderson R. M., May R. M. Infectious Diseases of Humans: Dynamics and Control. Oxford, U.K.: Oxford Univ. Press; 1991. [Google Scholar]
- 29.Bartley L. M., Donnelly C. A., Garnett G. P. Trans. R. Soc. Trop. Med. Hyg. 2002;96:387–397. doi: 10.1016/s0035-9203(02)90371-8. [DOI] [PubMed] [Google Scholar]
- 30.Edelman R. J. Infect. Dis. 2005;191:650–653. doi: 10.1086/427784. [DOI] [PubMed] [Google Scholar]
- 31.Cologna R., Armstrong P. M., Rico-Hesse R. J. Virol. 2005;79:853–859. doi: 10.1128/JVI.79.2.853-859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Z., Velasco-Hernandez J. X. J. Math. Biol. 1997;35:523–544. doi: 10.1007/s002850050064. [DOI] [PubMed] [Google Scholar]
- 33.Vaughn D. W., Green S., Kalayanarooj S., Innis B. L., Nimmannitya S., Suntayakorn S., Endy T. P., Raengsakulrach B., Rothman A. L., Ennis F. A., Nisalak A. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson N. M., Donnelly C. A., Anderson R. M. Philos. Trans. R. Soc. London Ser. B. 1999;354:757–768. doi: 10.1098/rstb.1999.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strickman D., Kittayapong P. Am. J. Trop. Med. Hyg. 2002;67:247–259. doi: 10.4269/ajtmh.2002.67.247. [DOI] [PubMed] [Google Scholar]
- 36.Kuno G. In: Dengue and Dengue Hemorrhagic Fever. Gubler D. J., Kuno G., editors. Wallingford, Oxfordshire, U.K.: CABI; 1997. [Google Scholar]
- 37.Gillespie D. T. J. Phys. Chem. 1977;81:2340–2361. [Google Scholar]
- 38.Kochel T. J., Watts D. M., Halstead S. B., Hayes C. G., Espinoza A., Felices V., Caceda R., Bautista C. T., Montoya Y., Douglas S., Russell K. L. Lancet. 2002;360:310–312. doi: 10.1016/S0140-6736(02)09522-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.