Abstract
Ceramides are known to have a regulatory function in apoptosis, including the release of cytochrome c and other proapoptotic factors from the mitochondrial intermembrane space. Ceramides can form large, stable channels in the outer mitochondrial membrane, leading to the proposal that ceramide channels are the pathway through which these proteins are released. Here, we report that sphingosine, a product of ceramide hydrolysis by ceramidase, is capable of destabilizing ceramide channels, leading to their disassembly. Sphingosine is directly responsible for the disassembly of ceramide channels in planar membrane experiments and markedly reduces the ability of ceramide to induce the release of intermembrane space proteins from mitochondria in vitro. Low concentrations of both L and D sphingosine potentiate the release of intermembrane space proteins by long-chain ceramide and channel formation in liposomes. These results provide evidence for a mechanism by which the disassembly of ceramide channels, as initiated by ceramidase, could be accelerated by the direct interaction of the hydrolysis product with the ceramide channels themselves. This mechanism therefore could form a positive feedback loop for rapid shut-down of ceramide channels. However, potentiation of ceramide channel formation is also possible and thus both effects could influence the propensity for mitochondria-mediated apoptosis.
INTRODUCTION
Apoptosis, or programmed cell death, plays an important role in the development of multi-cellular organisms and the response to cellular damage. The mitochondrion is known to act as a major hub for the regulation of apoptosis. The key event of this mitochondrial pathway is the efflux of apoptosis-inducing intermembrane space proteins such as cytochrome c into the cytoplasm. The release of these proteins leads to the activation of the caspases that carry out the execution phase of apoptosis (1,2).
A number of mechanisms have been proposed for the protein permeation pathway. Candidates for the composition of this pore include Bax oligomers (3–5), the mitochondrial apoptosis-induced channel MAC (6), lipidic pores induced by Bax (7), lipidic pores induced by BH3/Bax/lipid interactions (8,9), and ceramide channels (10–12).
Ceramide has been known, for a long time, to induce apoptosis (13). The addition of ceramide to isolated mitochondria results in the release of cytochrome c, apoptosis-inducing factor AIF, AK-2, and adenylate kinase (11,14–17). Both the short-chain model compound, N-acetyl-D-erythro-sphingosine (C2-ceramide), and a typical naturally occurring long-chain ceramide, N-hexanoyl-D-erythro-sphingosine (C16-ceramide), release these proteins. The mechanism of this release is most likely the formation of large channels in the mitochondrial outer membrane (MOM) since a whole range of intermembrane space proteins are released up to a molecular mass cutoff of ∼60 kDa (11). The exact nature of these large channels is unknown but the same ceramides form large channels >10 nm in diameter in phospholipids membranes lacking any proteins demonstrating that these lipids are sufficient, but other components are likely to be involved. The properties of ceramide channels are consistent with a barrel-stave structure (10,12).
Bolstering the conclusion that channels formed in the MOM are composed of ceramide molecules as opposed to ceramide merely acting as a trigger for the formation of another pore was the observation that the impermeability of the MOM to proteins could be restored by removing ceramide. Fatty-acid-depleted albumin effectively reversed the permeabilization of the MOM by short-chain ceramide. Albumin binds this form of ceramide and thus the reversal indicates a dynamic equilibrium between ceramides in the channel with the rest of the ceramide in the membrane and, when ceramide is removed by binding to the albumin, the channels disassemble (11). The fact that the same result was not achieved with C16-ceramide is understandable due to the exceedingly low aqueous solubility of long-chain ceramide. In many respects, C2-ceramide and C16-ceramide behave similarly. For example, the permeabilization of the MOM was remarkably similar whether C2-ceramide or C16-ceramide was used in the experiment (11).
One evidence of physiological relevance is the finding that ceramide levels become elevated early in the apoptotic process. The mitochondrial concentration of ceramide is known to increase in response to apoptosis-inducing stimuli (18–20). A typical level at the early stages is 4 pmol ceramide/nmole phospholipids. At this molar ratio, ceramide effectively permeabilizes the outer membrane to cytochrome c (21). Finally, the enzymes catalyzing the metabolic pathways leading to both ceramide synthesis and breakdown are known to be present in mitochondria (22–24). Taken together, these facts indicate that the ceramide channel is the permeability pathway through the mitochondrial outer membrane for protein flux.
The structurally related lipid, sphingosine, has also been shown to induce apoptosis (25–30). Although sphingosine does have the ability to form channels, it was found that these channels are not sufficiently large to allow the passage of intermembrane space proteins and that sphingosine itself may not induce apoptosis by permeabilizing the outer membrane to proteins (31). Experiments with whole cells indicate that sphingosine induces apoptosis by acting on specific proteins (32). Nevertheless, addition of sphingosine to mitochondria results in rapid conversion to ceramide (24,31). Interestingly, Siskind et al. (31) also noted that the ability of ceramide, generated from added sphingosine, to cause the release of cytochrome c was less than when the same quantities of ceramide were introduced into the mitochondrial membranes by direct addition of ceramide to the medium (no exogenous sphingosine). This observation suggested that sphingosine destabilizes ceramide channels. If this were true, it would provide evidence for a regulatory pathway in which sphingosine, a product of ceramide hydrolysis by ceramidase, feeds back to accelerate the closure of ceramide channels by directly destabilizing them. The experiments described in this work demonstrate that sphingosine is capable of directly disassembling ceramide channels suggesting the existence of this novel anti-apoptotic regulatory step.
MATERIALS AND METHODS
Electrophysiological recordings
Planar phospholipid membranes were produced by the monolayer method (33) as modified (34), across a 100-μm-diameter hole in a Saran partition. Monolayers were produced using a solution of 0.5% w/v asolectin, 0.5% w/v DiPhyPC, 0.1% w/v cholesterol in hexane. This technique produces solvent-free phospholipids membranes whose lipid composition (focusing on the polar head-groups) is similar to that found in the mitochondrial outer membrane. It differs from the natural membrane in lacking proteins.
The aqueous solutions contained 1 mM MgCl2, and 5 mM Pipes pH 6.8 with KCl varying from 0.10 M to 1.0 M. The KCl concentration on one side of the membrane, referred to as the trans side, was always 0.10 M, whereas the other side the cis side was adjusted as needed. The transmembrane voltage was electronically clamped and the current through the membrane was recorded. The voltage values indicated are the voltage differences across the membrane cis side minus trans side.
C2-ceramide was stirred into the aqueous solution on each side of the membrane from a solution in DMSO, generally 0.5 mg/ml. Single or multiple additions, typically 20 μl each, yielding a final concentration of 2 μg/ml, were made to achieve a desired level of conductance. Sphingosine additions of 5 μl were made from a solution in DMSO (4 mg/mL) yielding a final concentration of 4 μg/mL.
Adenylate kinase release assay
Rat liver mitochondria were isolated by differential centrifugation of tissue homogenate as described previously (35). The preparation yielded a mitochondrial suspension containing ∼10 mg of protein per mL. 20 μl of mitochondrial suspension were diluted into 1.2 ml of 70 mM sucrose, 210 mM mannitol, 0.1 mM EGTA, 1 mM Tris-HCl, pH 7.4 yielding a final protein conc. of 0.15 mg/mL. The mitochondria were incubated for 10 min at room temperature with varying amounts of ceramide added from a 4 mg/ml DMSO solution to determine the amount of ceramide that resulted in ∼50% release of adenylate kinase. The mitochondria were then pelleted at 14,000g for 5 min at 4° C and the supernatant was kept on ice until assayed. 100 μl of supernatant was added to 700 μl of adenylate kinase reaction mixture: 50 mM Tris-HCl, pH 7.5, 5 mM MgSO4, 10 mM glucose, 5 mM ADP, 0.2 mM NADP, 10 units of hexokinase, and 10 units of glucose-6-phosphate dehydrogenase (36). The enzymes were added to the rest of the reaction mixture 1 min before the addition of the mitochondrial supernatant to allow a trace of ATP to be consumed. The activity of adenylate kinase was detected as an increase in absorbance at 340 nm. Since the activity of the kinase decays with time, even on ice, experiments were performed in sets and the activity of the first supernatant was assayed again at the end of the set. The values were fitted to a first order decay and all values within the set were corrected for the decay of the activity, based on the time delay before assay.
To determine the effect of sphingosine pretreatment on ceramide permeabilization of the mitochondrial outer membrane, mitochondria were incubated at room temperature in the presence of varying concentrations of sphingosine for 5 min before addition of ceramide and incubation for 10 min. Untreated mitochondria and mitochondria hypotonically lysed (20 μl into 1.2 ml water) served as negative and positive controls, respectively.
Experiments with C16-ceramide were performed in a similar way except as follows. The mitochondria were suspended in the above medium except that the 1 mM Tris buffer was replaced by 5 mM HEPES. To 1.0 mL of this medium at room temperature (RT) was added 17 μL of mitochondrial suspension that had been kept on ice at a concentration of ∼4 mg/mL. The final mitochondrial protein concentration during the experiment was ∼80 μg/mL. In the case of shocked mitochondria, the 1.0 mL of medium was replaced by water. Sphingosine was added, where appropriate, from a 0.25 mg/mL solution in DMSO. Typically 4–7 incubations were run in parallel. All samples were incubated for 5 min at RT. Then some received 0.4 mL of fatty acid depleted bovine serum albumin (BSA), 25 mg/mL, followed immediately by 20 μL of C16-ceramide dissolved in isopropanol at 2 mg/mL. After a 10-min incubation at RT, 10 μL of 4 mM PMSF was added followed by centrifugation. In these experiments 0.3 mL of mitochondrial supernatant was added to the adenylate kinase reaction mixture.
Liposome experiments
The polar extract of soybean phospholipids (Avanti Polar Lipids, Alabaster, AL) and cholesterol (Sigma Chemical, St. Louis, MO) were mixed in a 93:7 molar ratio in chloroform and dried under nitrogen followed by drying in vacuo overnight. This mixture resembles the lipid content of mammalian mitochondrial outer membranes. Five mg of this mixture was dispersed in 1 mL of 39 mM NaCl, 6 mM DPX (Molecular Probes, Eugene, OR), 1.5 mM carboxyfluorescein (Acros Organics, Geel,Belgium) and subjected to four freeze-thaw/sonication cycles. After extrusion through a 0.2 μm filter (Avanti Polar Lipids) for 13 passes it was run through a gel filtration column equilibrated and eluted with 50 mM NaCl, 10 mM HEPES, 1 mM EDTA, pH 7.0. The liposomes were used within 30 h. Twenty-five microliters of liposomes were dispersed into 2.0 mL of the elution buffer and placed into a quartz fluorescence cuvette. The fluorescence was monitored using a Deltascan Spectrofluorometer (Photon Technology Instruments, West Sussex, UK) using excitation at 495 nm and emission at 520 nm. Forty microliters of C16-ceramide (2 mg/mL of isopropanol) were added while stirring. Sphingosine was added from either a 1 mg/mL or a 0.1 mg/mL solution in DMSO.
RESULTS AND DISCUSSION
Sphingosine influences ceramide-dependent release of adenylate kinase from isolated mitochondria
Ceramide has been previously shown to increase the permeability of the MOM to small intermembrane space proteins such as cytochrome c and adenylate kinase (11,14–17). Sphingosine has been reported to form channels but these are small and incapable of allowing proteins to cross membranes (31). Thus the presence of both ceramide and sphingosine in a membrane may result in hybrid structures that may or may not be conductive. The release of adenylate kinase was used as a measure of MOM permeabilization to small proteins.
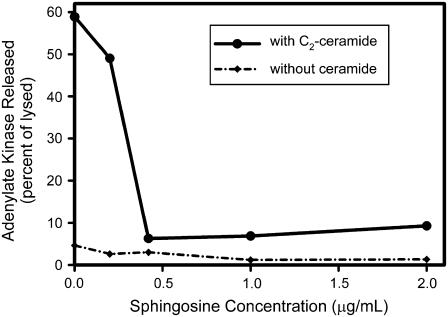
To look for interaction between sphingosine and ceramide, isolated rat liver mitochondria were treated with varying levels of sphingosine for 5 min, and then exposed to ceramide for 10 min at RT. The mitochondria were sedimented and the supernatant assayed for adenylate kinase activity. The initial rates of these reactions were plotted in Fig. 1 after normalization for the amount of adenylate kinase activity released after hypotonic shock. Essentially total inhibition was observed by exposure to 0.3–0.6 μg/mL sphingosine, depending on the batch of isolated mitochondria. In the concentration range of sphingosine examined (up to 6 μg/mL), sphingosine alone did not induce the release of adenylate kinase (Fig. 1). Thus sphingosine could either destabilize ceramide channels or convert them to much smaller structures, incapable of allowing the passage of proteins.
FIGURE 1.
Sphingosine inhibits the ceramide-dependent release of adenylate kinase from mitochondria. Mitochondria were isolated and prepared as described in Materials and Methods. They were then incubated with the indicated concentration of sphingosine for 5 min. C2-ceramide was added to each aliquot (29 μg/mL final) and they were incubated for another 10 min. Note: under these conditions only ∼1% of this ceramide inserts into mitochondria (21,31). The release of adenylate kinase is expressed as a percentage of that released by hypotonic shock. The enzyme activity was recorded as the initial rate of NADPH production. The experiment was repeated without the addition of ceramide, and this data is also presented for comparison. The data shown is one representative example of three independent experiments.
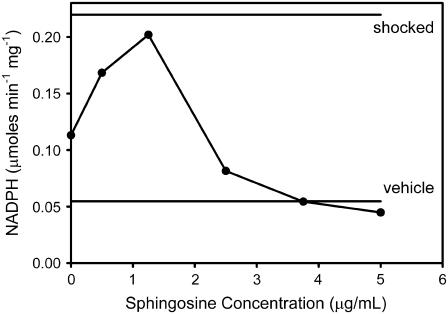
These results, obtained with C2-ceramide, differ somewhat from the results obtained with C16-ceramide. As shown in Fig. 2, the use of C16-ceramide resulted in a biphasic relationship for the permeabilization of the mitochondrial outer membrane and release of adenylate kinase. At low concentration, sphingosine potentiated the permeabilization effect of ceramide whereas at higher concentrations it inhibited the formation of the ceramide channels, as in the case of C2-ceramide. Here the actual initial rate of NADPH production was plotted and the rates observed with vehicle alone and after hypotonic shock are shown as horizontal lines.
FIGURE 2.
Sphingosine inhibits the release of adenylate kinase from mitochondria induced by C16-ceramide. Adenylate kinase activity was determined by measuring the initial rate of increase in the level of NADPH in the medium. Mitochondria were preincubated with the indicated amount of sphingosine for 5 min. This was followed by the addition of C16-ceramide to a final total concentration of 40 μg/mL. After a 10-min incubation, the supernatant was assayed for adenylate kinase activity as indicated in Materials and Methods. The levels of adenylate kinase activity released by hypotonic shock or after addition of the vehicle alone are indicated as horizontal lines. This is a typical result of four experiments performed on different batches of mitochondria.
The potentiation might arise from metabolic conversion of sphingosine to ceramide by mitochondria. It has been reported that nearly half of the added sphingosine is converted to ceramide (31), probably by the action of reverse ceramidase. Thus, the ceramide produced from the sphingosine might combine with the added ceramide to reach a critical level resulting in channel formation. Excess sphingosine overwhelms the system resulting in the same inhibition observed with C2-ceramide.
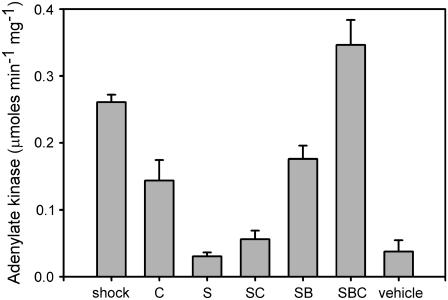
This hypothesis was tested by determining whether sphingosine converted to ceramide would be capable of permeabilizing the MOM to adenylate kinase. Mitochondria were incubated with 2.4 μg/mL sphingosine for 5 min followed by addition of 10 mg of fatty acid depleted bovine serum albumin BSA to remove the unconverted sphingosine. The release of adenylate kinase is evident in Fig. 3 (S versus SB) indicating that the removal of excess sphingosine allowed the ceramide formed to permeabilize the MOM. Note that the combination of added ceramide and sphingosine converted to ceramide (SBC…after BSA was used to remove excess sphingosine) resulted in complete release of adenylate kinase. The long-chain ceramide produced from sphingosine cannot be removed from the mitochondrial membranes by BSA addition (11). BSA alone did not result in any release of adenylate kinase. The addition of BSA to intact mitochondria or mitochondria treated with osmotic shock did not alter the level of adenylate kinase activity (data not shown) showing that BSA does not influence either adenylate kinase or the coupled enzyme assay.
FIGURE 3.
Removal of sphingosine by BSA reverses the inhibition by sphingosine of C16-ceramide permeabilization of the mitochondrial outer membrane. The experiments were performed as in Fig. 2. BSA, fatty acid depleted, was added just before addition of C16-ceramide. The treatments were as follows: “shock”, hypotonic shock only; “C”, ceramide added only 40 μg/mL; “S”, sphingosine 2.5 μg/mL; “SC”, sphingosine 2.5 μg/mL followed by C16-ceramide 40 μg/mL; “SB”, sphingosine 2.5 μg/mL for 5 min followed by BSA 7 mg/mL; “SBC”, sphingosine 2.5 μg/mL for 5 min followed by BSA 7 mg/mL and C16-ceramide 40 μg/mL; and “vehicle”, instead of ceramide isopropanol was added. See Materials and Methods for details. The error bars are mean ± SE of three independent experiments except for shock n = 7, S n = 5, and SB n = 5. Student's t-tests showed that “SC” and “SBC” differ at the 99% confidence level; “S” and “SB” at the 99.9% level; “C” and “SC” at the 95% level; and “C” and “SCB” at the 95% level.
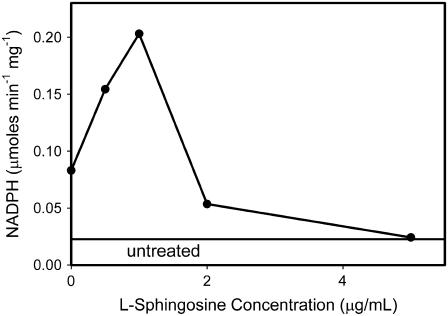
An alternative hypothesis for the ability of low levels of sphingosine to enhance ceramide permeabilization of the MOM is a direct effect of low-levels of sphingosine on ceramide channel stabilization. To test this hypothesis, L-threo sphingosine was used instead of the natural D-erythro sphingosine. This optical isomer is not metabolized as readily as the natural isomer. Preincubation with the L-threo sphingosine resulted in the same biphasic effect on ceramide permeabilization of the MOM (Fig. 4). Thus the conversion to ceramide may not be necessary for the potentiating effect of sphingosine. Conversion was checked by removing excess L-threo sphingosine with BSA to see if there was a permeabilization of the MOM as observed with the same treatment with the D isomer. In this case, no permeabilization of the MOM to adenylate kinase was observed. This is consistent with the conclusion that the D but not the L isomer is converted to ceramide and that both the L and D isomers act to favor ceramide permeabilization of the MOM when present at low concentrations.
FIGURE 4.
Experiments were performed as described in Fig. 2 except that the L isomer of sphingosine was used instead of the naturally occurring D isomer. “Untreated” refers to the level of adenylate kinase detected in the supernatant when no reagent was added. Otherwise the mitochondria were handled in exactly the same way as those treated with sphingosine.
Sphingosine enhances the ability of ceramide to permeabilize liposomal membranes
If the ability of low levels of sphingosine to enhance ceramide channel formation is a direct action on the channels, this influence should also be observed on ceramide channels formed in liposomes lacking proteins. In these liposomes there is no possibility of metabolism or indirect effects on protein factors.
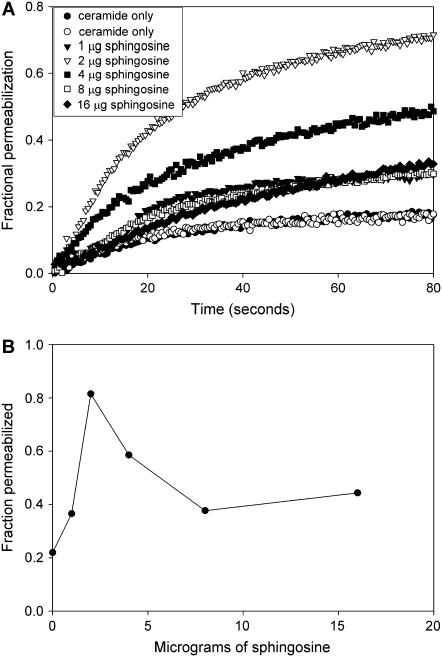
Fig. 5 A shows the fluorescence increase resulting from the release of carboxyfluorescein from liposomes after the addition of C16-ceramide. Pretreatment with sphingosine causes a biphasic enhancement of the ceramide-induced permeabilization. At the low doses of sphingosine the formation of sphingosine channels is sufficiently minimal, allowing ceramide channel formation to dominate. At the higher doses the sphingosine-induced carboxyfluorescein release is high enough to mask the expected inhibition of ceramide channel formation. In any case, the biphasic enhancement is evident and closely mirrors the results obtained with mitochondria. The defined nature of the liposome experiments allows one to conclude that the observed effects of sphingosine on ceramide channel formation arise from a direct interaction of sphingosine with the ceramide in the membrane.
FIGURE 5.
Sphingosine influences ceramide channel formation in liposomes in a biphasic fashion. (A) The release of carboxyfluorescein from liposomes by C16-ceramide (80 μg) alone or after the liposomes (90 μg of lipid) were pretreated with the indicated amounts of sphingosine (see Materials and Methods). The release is expressed as a fraction of the release achieved with 0.08% Triton X100. (B) The fluorescence level achieved in the experiments described in A 100 s after ceramide addition. Results are representative of two experiments.
The ability of sphingosine to potentiate channel formation by long-chain ceramide but not by short-chain ceramide may lie in the fundamental structure of the channel. Molecular dynamics simulations (37) have provided evidence for dual curvature in ceramide monomers packed into the channel. Negative curvature in the plane of the membrane allows for formation of the annulus. Positive curvature normal to that plane allows for effective articulation between ceramides and the phospholipid bilayer. This articulation involves distortion of both ceramides and the phospholipids and would be ameliorated by the presence of lipids with positive curvature. Short-chain ceramide already has the low hydrocarbon bulk that would aid in generating the positive curvature. Perhaps low concentrations of sphingosine would serve the same function. At the high concentrations, sphingosine may intercalate into the ceramide channels resulting in their destabilization because sphingosine lacks the amide linkage (believed to be critical to channel stability) and has a net charge that would lead to electrostatic repulsion.
Sphingosine promotes disassembly of ceramide channels in planar membranes
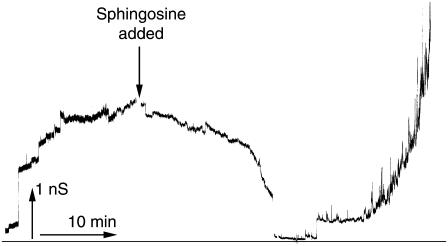
The observations of sphingosine induced suppression of ceramide permeabilization of mitochondrial and liposomal membranes could have a variety of mechanistic explanations. The higher levels of sphingosine might prevent the formation of ceramide channels from monomers (e.g., by forming nonconducting complexes with these monomers) or sphingosine might induce the disassembly of ceramide channels. Direct demonstration of sphingosine-induced ceramide channel disassembly was achieved by experiments on channel-formation in planar phospholipids membranes. Ceramide channel conductance increases in a stepwise fashion, and these conductance increases have been demonstrated to represent the growth of a single channel in the membrane (12) as opposed to many small channels acting in parallel (38). The increases in conductance in the left half of Fig. 6 are thus increases in the size of a single channel. The typical ceramide channel grows in this way over a period of time until it reaches a fairly stable size. At this point, sphingosine was added (Fig. 6) and the membrane conductance decreased over the next 15–20 min. The conductance declined until it reached the level of the unmodified membrane, indicating that the ceramide channel was completely disassembled. Six minutes later, the conductance increased once again but the nature of this conductance differed from that of a typical ceramide channel.
FIGURE 6.
Changes in conductance demonstrate ceramide channel disassembly. A ceramide channel was allowed to form after the addition of C2-ceramide (9 μg/mL) to each side of the membrane. Once channel growth had slowed, sphingosine (4 μg/mL) was added to the cis side of the membrane, at the time indicated. The figure shows the continuous recording of conductance across the membrane. The applied voltage was clamped at 10 mV.
The signal observed during the earlier half of this experiment, representing the flow of current through the membrane, is characteristic of ceramide channels. These characteristics include the growth of the channel in a steady, stepwise fashion and a relatively high signal/noise. These characteristics continued through the phase of the experiment during which the conductance was decreasing, with the channel shrinking steadily, periodically punctuated with sharp decrements, and with the current signal showing a low level of noise. In contrast to this, the increase in conductance that followed showed no large increments and was highly noisy. The appearance of this particular type of noise is characteristic of sphingosine channels (31), reflecting the fact that sphingosine forms multiple channels in the planar membrane and that these channels exist only transiently, thus explaining the wide fluctuations in the current.
Selectivity change corresponds to transition from ceramide channel to sphingosine channels
To test the interpretation of Fig. 6, selectivity experiments were performed to distinguish between ceramide conductance and sphingosine conductance. Ceramide and sphingosine channels differ in their ion selectivities. Ceramide channels are weakly cation selective (12), and the magnitude of this selectivity decreases as the channel size conductance increases. Sphingosine channels, have a preference for anions, likely owing to the positive charge on the sphingosine molecule (31), and thus they exhibit a positive reversal potential. Therefore by monitoring the reversal potential one can distinguish between conductance arising from ceramide channels and that due to sphingosine channels.
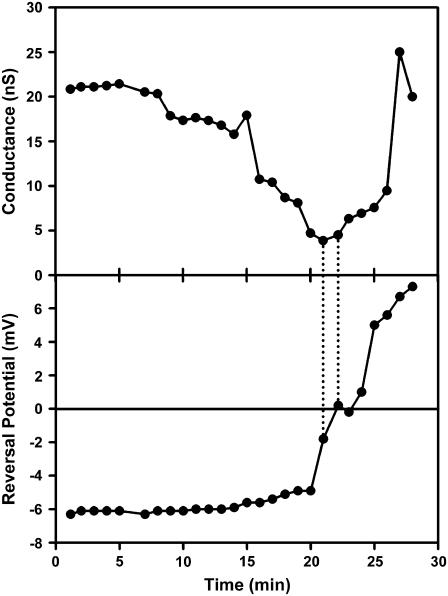
Fig. 7 shows an experiment where both the conductance and the reversal potential were measured at regular intervals. The applied voltage was changed in steps allowing the conductance to be measured. The reversal potential was calculated: reversal potential = applied voltage − [current/conductance]. The figure begins after the conductance of the ceramide channel had stabilized and sphingosine was added. The conductance declined with time reaching a minimum at ∼20 min. Then the reversal potential changed rapidly from negative to positive values indicating a change from cation to anion selectivity and thus a change from ceramide channel conductance to the conductance of sphingosine channels. Note that a further rise in conductance resulted in little change in reversal potential because the selectivity of sphingosine channels remained fairly steady irrespective of the number of channels present or the magnitude of the overall conductance.
FIGURE 7.
Changes in reversal potential indicate changes in channel composition. A ceramide channel was formed after the addition of C2-ceramide (3 μg/mL) to each side of the membrane. After channel growth slowed, KCl was added to the aqueous solution on the cis side of the membrane to a final concentration of 0.45 M. MgCl2 and Pipes concentrations remained unchanged. The channel-containing membrane was then left undisturbed for 30 min. Sphingosine was added to a final concentration of 8 μg/mL at t = 0 min. The conductance and reversal potential of the channel was measured periodically over the following 30 min. This is one of three independent experiments.
The timing of the change of reversal potential corresponded with the point at which the conductance of the membrane began to increase, as indicated by the dashed lines in Fig. 7. This behavior is indicative of a changeover in the primary source of the membrane selectivity from a single ceramide channel to many sphingosine channels.
Other features of note in the Fig. 7 provide insight into the process of channel disassembly. One would expect that the gradual loss of ceramide conductance reflects the shrinkage of the ceramide channel. As the channel becomes smaller the ion selectivity should increase as previously described (12). However, as the ceramide conductance declined the reversal potential of the membrane stayed roughly constant, and eventually moves toward zero. Therefore, the presence of sphingosine in the membrane may somehow prevent the expected selectivity increase. One possibility is that sphingosine channels may have already begun to form in parallel with the ceramide channel, and that the formation of these anion selective channels negates and eventually overcomes the increase in cation selectivity expected from the shrinking ceramide channel. Another possibility is that some of the sphingosine incorporates itself into the ceramide channels, and thus not only destabilizes them, but also changes their characteristic selectivity by introducing positive charge near the inner wall of the channel. These two possibilities are, of course, not mutually exclusive and could be occurring simultaneously. The dominance of one over the other may also depend on how much sphingosine was added.
These results demonstrate the ability of sphingosine to markedly reduce the ceramide-induced permeability of the outer mitochondrial membrane to intermembrane space proteins. In whole mitochondria this could be due to a variety of mechanisms but in view of the results obtained in planar membranes, simple disassembly of ceramide channels is the simplest explanation.
When experiments performed on mitochondria isolated from different animals are pooled, the error bars are fairly large because there is variability of the response from one mitochondrial preparation to another. However, the changes were always in the same direction and the reported effects are statistically significant. The relative response under different conditions also depended on the potency of the ceramide-induced permeabilization, again this varied from one batch of mitochondria to another.
The effects described here might be subject to a variety of criticisms. First of all one might be concerned that sphingosine might form micelles in the medium that would act as sinks for the ceramide resulting in the trapping of ceramide and thus inhibition of ceramide channel formation. However, the published CMC for sphingosine is more than 30 μg/mL (39), far greater than the amounts used. In addition, by using radiolabeled sphingosine we find that more than 80% of added sphingosine partitions into mitochondria and thus the amount of sphingosine in the medium is far less than that indicated in the figures and text. Thus the possibility of an artifact from sphingosine micelles is unrealistic. Another criticism is the amounts of sphingolipids used in these experiments as compared to levels found in cells. Again the use of radioisotopes shows that most of the ceramide added does not insert into mitochondria (21). The amount that does insert is within the physiological range 4 pmoles ceramide per nmole of phospholipids, the level measured early in apoptosis in mitochondria. The physiological levels of sphingosine in mitochondria during apoptosis are not well defined. Cellular levels increase to an equivalent of 1.5–3 μg/mL (27) but the level in the mitochondrial outer membrane is unclear. However, the action of ceramidase would generate sphingosine and local levels may indeed be high enough to favor ceramide disassembly. Note that after correcting for differences in insertion of ceramide and sphingosine, the levels used here are rather comparable as might be expected in the mitochondrial membrane when active turnover is occurring.
CONCLUSION
The observed disassembly of ceramide channels in the presence of sphingosine provides evidence for a potential amplification of the negative regulation of ceramide channels through the action of ceramidase. This enzyme, found in mitochondrial membranes, hydrolyzes ceramide to produce sphingosine (22,24), thus reducing the concentration of ceramide in the membrane and leading to channel shrinkage or disassembly. This disassembly process could be hastened by the presence of the reaction product, sphingosine, which amplifies the effect of the decreasing concentration of ceramide on the size of the ceramide channel by direct interaction with the channel to destabilize it. It depends on the local sphingosine level because if the level is too low there is potentiation rather than inhibition. As ceramidase continues to act, the concentration of sphingosine relative to ceramide continues to increase, thus accelerating the disassembly process. This putative self-amplifying regulatory mechanism thus becomes a candidate for an anti-apoptotic regulatory step.
The potentiating effect of sphingosine at lower concentrations may also be physiologically important and is consistent with the reported pro-apoptotic effect of sphingosine. The local concentration of sphingosine is key to knowing how sphingosine will act.
Acknowledgments
The assistance of Richie Gumpert is gratefully acknowledged.
This work was supported by National Institutes of Health grant NS42025.
References
- 1.Crompton, M. 1999. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 2.Susin, S. A., N. Zamzami, and G. Kroemer. 1998. Mitochondria as regulators of apoptosis: doubt no more. Biochim. Biophys. Acta. 1366:151–165. [DOI] [PubMed] [Google Scholar]
- 3.Antonsson, B., S. Montessuit, S. Lauper, R. Eskes, and J. C. Martinou. 2000. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 345:271–278. [PMC free article] [PubMed] [Google Scholar]
- 4.Antonsson, B., S. Montessuit, B. Sanchez, and J. C. Martinou. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276:11615–11623. [DOI] [PubMed] [Google Scholar]
- 5.Saito, M., S. J. Korsmeyer, and P. H. Schlesinger. 2000. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2:553–555. [DOI] [PubMed] [Google Scholar]
- 6.Pavlov, E. V., M. Priault, D. Pietkiewicz, E. H. Cheng, B. Antonsson, S. Manon, S. J. Korsmeyer, C. A. Mannella, and K. W. Kinnally. 2001. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J. Cell Biol. 155:725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basañez, G., A. Nechushtan, O. Drozhinin, A. Chanturiya, E. Choe, S. Tutt, K. A. Wood, Y. T. Hsu, J. Zimmerberg, and R. J. Youle. 1999. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA. 96:5492–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwana, T., M. R. Mackey, G. Perkins, M. H. Ellisman, M. Latterich, R. Schneiter, D. R. Green, and D. D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 111:331–342. [DOI] [PubMed] [Google Scholar]
- 9.Terrones, O., B. Antonsson, H. Yamaguchi, H.-G. Wang, J. Liu, R. M. Lee, A. Herrmann, and G. Basañez. 2004. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J. Biol. Chem. 279:30081–30091. [DOI] [PubMed] [Google Scholar]
- 10.Siskind, L. J., and M. Colombini. 2000. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 275:38640–38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siskind, L. J., R. N. Kolesnick, and M. Colombini. 2002. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 277:26796–26803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siskind, L. J., A. Davoody, N. Lewin, S. Marshall, and M. Colombini. 2003. Enlargement and contracture of C2-ceramide channels. Biophys. J. 85:1560–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obeid, L. M., C. M. Linardic, L. Karolak, and Y. A. Hannun. 1993. Programmed cell death induced by ceramide. Science. 259:1769–1771. [DOI] [PubMed] [Google Scholar]
- 14.Arora, A. S., B. J. Jones, T. C. Patel, S. F. Bronk, and G. J. Gores. 1997. Ceramide induces hepatocyte cell death through disruption of mitochondrial function in the rat. Hepatology. 25:958–963. [DOI] [PubMed] [Google Scholar]
- 15.Ghafourifar, P., S. D. Klein, O. Schucht, U. Schenk, M. Pruschy, S. Rocha, and C. Richter. 1999. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J. Biol. Chem. 274:6080–6084. [DOI] [PubMed] [Google Scholar]
- 16.Di Paola, M., T. Cocco, and M. Lorusso. 2000. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 39:6660–6668. [DOI] [PubMed] [Google Scholar]
- 17.Di Paola, M., P. Zaccagnino, G. Montedoro, T. Cocco, and M. Lorusso. 2004. Ceramide induces release of pro-apoptotic proteins from mitochondria by either a Ca2+ -dependent or a Ca2+ -independent mechanism. J. Bioenerg. Biomembr. 36:165–170. [DOI] [PubMed] [Google Scholar]
- 18.Birbes, H., C. Luberto, Y. T. Hsu, S. El Bawab, Y. A. Hannun, and L. M. Obeid. 2005. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem. J. 386:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Ruiz, C., A. Colell, M. Marí, A. Morales, and J. C. Fernández-Checa. 1997. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 272:11369–11377. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Lafrasse, C., G. Alphonse, M. T. Aloy, D. Ardail, J. P. Gerard, P. Louisot, and R. Rousson. 2002. Increasing endogenous ceramide using inhibitors of sphingolipid metabolism maximizes ionizing radiation-induced mitochondrial injury and apoptotic cell killing. Int. J. Cancer. 101:589–598. [DOI] [PubMed] [Google Scholar]
- 21.Siskind, L. J., R. N. Kolesnick, and M. Colombini. 2006. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. In press. [DOI] [PMC free article] [PubMed]
- 22.El Bawab, S., P. Roddy, T. Qian, A. Bielawska, J. J. Lemasters, and Y. A. Hannun. 2000. Molecular cloning and characterization of a human mitochondrial ceramidase. J. Biol. Chem. 275:21508–21513. [DOI] [PubMed] [Google Scholar]
- 23.Shimeno, H., S. Soeda, M. Sakamoto, T. Kouchi, T. Kowakame, and T. Kihara. 1998. Partial purification and characterization of sphingosine N-acyltransferase ceramide synthase from bovine liver mitochondrion-rich fraction. Lipids. 33:601–605. [DOI] [PubMed] [Google Scholar]
- 24.Bionda, C., J. Portoukalian, D. Schmitt, C. Rodriguez-Lafrasse, and D. Ardail. 2004. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane and/or mitochondria? Biochem. J. 382:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuvillier, O., L. Edsall, and S. Spiegel. 2000. Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J. Biol. Chem. 275:15691–15700. [DOI] [PubMed] [Google Scholar]
- 26.Cuvillier, O., V. E. Nava, S. K. Murthy, L. C. Edsall, T. Levade, S. Milstien, and S. Spiegel. 2001. Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells. Cell Death Differ. 8:162–171. [DOI] [PubMed] [Google Scholar]
- 27.Cuvillier, O. 2002. Sphingosine in apoptosis signaling. Biochim. Biophys. Acta. 1585:153–162. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis, W. D., F. A. Fornari, R. S. Traylor, H. A. Martin, L. B. Kramer, R. K. Erukulla, R. Bittman, and S. Grant. 1996. Induction of apoptosis and potentiation of ceramide-mediated cytotoxicity by sphingoid bases in human myeloid leukemia cells. J. Biol. Chem. 271:8275–8284. [DOI] [PubMed] [Google Scholar]
- 29.Sweeney, E. A., J. Inokuchi, and Y. Igarashi. 1998. Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS Lett. 425:61–65. [DOI] [PubMed] [Google Scholar]
- 30.Hung, W.-C., H.-C. Chang, and L.-Y. Chuang. 1999. Activation of caspase-3-like proteases in apoptosis induced by sphingosine and other long-chain bases in Hep3B hepatoma cells. Biochem. J. 338:161–166. [PMC free article] [PubMed] [Google Scholar]
- 31.Siskind, L. J., S. Fluss, M. Bui, and M. Colombini. 2005. Sphingosine forms channels in membranes that differ greatly from those formed by ceramide. J. Bioenerg. Biomembr. 37:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki, E., K. Handa, M. S. Toledo, and S. Hakomori. 2004. Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc. Natl. Acad. Sci. USA. 10141:14788–14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montal, M., and P. Mueller. 1972. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA. 69:3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombini, M. 1987. Characterization of channels isolated from plant mitochondria. Methods Enzymol. 148:465–475. [DOI] [PubMed] [Google Scholar]
- 35.Parsons, D. F., G. R. Williams, and B. Chance. 1966. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann. N. Y. Acad. Sci. 137:643–666. [DOI] [PubMed] [Google Scholar]
- 36.Sottocasa, G. L., B. Kuylenstierna, L. Ernster, and A. Bergstrand. 1967. Separation and some enzymatic properties of the inner and outer membranes of rat liver mitochondria. Methods Enzymol. 10:448–463. [Google Scholar]
- 37.Anishkin, A., S. Sukharev, and M. Colombini. 2006. Searching for the molecular arrangement of transmembrane ceramide channels. Biophys. J. 90:2414–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaulin, Y. A., L. V. Schagina, S. M. Bezrukov, V. V. Malev, A. M. Feigin, J. Y. Takemoto, J. H. Teeter, and J. G. Brand. 1998. Cluster organization of ion channels formed by the antibiotic syringomycin E in bilayer lipid membranes. Biophys. J. 74:2918–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deguchi, H., S. Yegneswaran, and J. H. Griffin. 2004. Sphingolipids as bioactive regulators of thrombin generation. J. Biol. Chem. 279:12036–12042. [DOI] [PubMed] [Google Scholar]