Abstract
Previous attempts to identify residues that line the pore of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel have utilized cysteine-substituted channels in conjunction with impermeant, thiol-reactive reagents like MTSET+ and MTSES−. We report here that the permeant, pseudohalide anion [Au(CN)2]− can also react with a cysteine engineered into the pore of the CFTR channel. Exposure of Xenopus oocytes expressing the T338C CFTR channel to as little as 100 nM [Au(CN)2]− produced a profound reduction in conductance that was not reversed by washing but was reversed by exposing the oocytes to a competing thiol like DTT (dithiothreitol) and 2-ME (2-mercaptoethanol). In detached, inside out patches single-channel currents were abolished by [Au(CN)2]− and activity was not restored by washing [Au(CN)2]− from the bath. Both single-channel and macroscopic currents were restored, however, by exposing [Au(CN)2]−-blocked channels to excess [CN]−. The results are consistent with the hypothesis that [Au(CN)2]− can participate in a ligand exchange reaction with the cysteine thiolate at 338 such that the mixed-ligand complex, with a charge of −1, blocks the anion conduction pathway.
INTRODUCTION
In previous studies we and others have used cysteine-substituted cystic fibrosis transmembrane conductance regulator (CFTR) channels in conjunction with thiol-reactive reagents and changes in bath pH in an attempt to define the outward-facing, water-accessible surface of the CFTR protein (1–3). The size and permanent charge of reagents like MTSET+ and MTSES− make them ideally suited to this purpose as they are not likely to cross the cell membrane or to translocate through the channel (4–6). These same properties, however, may preclude access of such compounds to cysteines substituted for residues that lie in more physically constrained regions of the conduction path. An ideal probe would be a permeant anion that reacted with substituted cysteines to form a covalent adduct that would obstruct or otherwise alter the anion conduction path. Such a molecule could, in principle, be used to identify “pore-lining” residues along the entire length of the conduction pathway. We report here that the highly stable coordination compound, [Au(CN)2]−, possesses precisely these attributes.
We showed previously that [Au(CN)2]−, a pseudohalide anion, was a useful probe of the wild-type (wt) CFTR conduction path. The low free energy of hydration of [Au(CN)2]− and other psuedohalides (compared to Cl−) is a major determinant of their permeation properties. Because [Au(CN)2]− escapes water more readily than Cl− the permeability ratio, P[Au(CN)2]/PCl (determined from shifts in reversal potential), is greater than unity, typically ∼7 in wt CFTR. In addition, however, free energies of hydration also determine the rank order of “binding” within the channel (7). Anions like [Au(CN)2]− see an energy well within the channel that deepens in proportion to the decreased hydration energy and hence exhibit a smaller dissociation constant when anion binding is assayed by comparing the blockade of Cl− currents (7). The dominant influence of hydration energy on anion binding is consistent with the observation that block by [Au(CN)2]−, as well as other pseudohalides, is readily reversed by washing. [Au(CN)2]−, however, is known to react with thiols by means of ligand exchange so we reasoned that [Au(CN)2]− might react with thiols engineered into the pore of CFTR. To test this hypothesis we used a CFTR construct with a cysteine substituted at position 338, T338C CFTR. We recently reported that the functional effects of both covalent and noncovalent modification of T338C CFTR were consistent with the hypothesis that a cysteine substituted at this location lies within the anion conduction path (8). Here we report that exposure of CFTR constructs containing a cysteine at position 338 to [Au(CN)2]− produces a profound decrease in anion conduction that is not reversed by removing [Au(CN)2]− from the bathing solution but is reversed by adding a ligand that competes for Au(I). The results are consistent with the hypothesis that [Au(CN)2]− reacts with the thiolate anion to form a negatively charged, thiolate-gold-cyanide adduct that blocks the pore.
MATERIALS AND METHODS
Mutagenesis and in vitro transcription
The Cys-less CFTR construct (C76S, C126S, C225S, C276S, C343S, C491S, C524S, C590L, C592L, C657S, C832S, C866S, C1344S, C1355S, C1395S, C1400S, C1410S, C1458S) was a gift from Drs. Martin Mense and David Gadsby and was used in their pGEMHE vector previously described (9). Point mutations to both wt CFTR and the Cys-less CFTR constructs were generated using the QuikChange site-directed mutagenesis kit from Stratagene (La Jolla, CA) as described previously (3,9). Mutations were confirmed by direct DNA sequencing.
The CFTR cRNAs for Xenopus oocyte injection were synthesized using the mMessage mMachine in vitro transcription kit from Ambion (Austin, TX) as described previously (3,9). To achieve necessary surface expression of the Cys-less CFTR constructs, we increased the translation efficiency of the transcripts by using an alternative cap analog in our in vitro transcription reaction (Anti-Reverse Cap Analog, Ambion). After transcription, poly(A) tails were added to the Cys-less transcripts using Escherichia coli Poly(A) polymerase as described in the Ambion mMessage mMachine T7 Ultra transcription kit.
Cys-less CFTR channels
The functional properties of Cys-less CFTR, in which all of the 18 endogenous cysteines present in wt CFTR were replaced by serines and leucines, are remarkably similar to those of wt CFTR, including significant expression in Xenopus oocytes, activation by maneuvers that raise cytosolic cAMP, and sensitivity to blocking anions like [Au(CN)2]− and [SCN]−. In detached patches Cys-less CFTR channels are activated by protein kinase A (PKA) and ATP and exhibit a single-channel conductance in 200 mM symmetric Cl− of ∼7 pS at pH 7.4. Most importantly, the construct used in the experiments reported here, T338C on a Cys-less background (T338C/Cys-less), behaved in a manner that was virtually identical to that of T338C/wt; including similar pH titration of macroscopic conductance (8). Placing the cysteine at 338 on a Cys-less background allowed us to eliminate the possible confounding effects of other reactive cysteines particularly in recordings from detached, inside out patches (10).
Preparation and microinjection of oocytes
The preparation and microinjection of Xenopus laevis oocytes were performed using methods previously described in detail (11–13). The follicular membranes were removed by mechanical agitation (1–2 h) in a Ca2+-free solution containing (mM): 82.5 NaCl, 2 KCl, 1 MgCl2, 5 HEPES (hemiNa), pH 7.5, with 0.2 Wünsch units/mL Liberase Blendzyme 3 (Roche Molecular Biochemicals, Indianapolis, IN). Defolliculated oocytes were washed and maintained in a modified Barth's solution containing (mM): 88 NaCl, 1 KCl, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.41 CaCl2, 2.4 NaHCO3, 10 HEPES (hemiNa), and 250 mg/L Amikacin at pH 7.5. Stage VI oocytes were injected with CFTR cRNA (diluted to yield 50–200 μS of stimulated conductance: ∼0.2 ng/oocyte in a 50 nL volume for most constructs, Cys-less variants required 5–10 ng cRNA per oocyte) and cRNA encoding the human β2-adrenergic receptor.
Chemical states and reactivity of the cysteine at 338
We recently presented evidence that the chemical state of a cysteine substituted at position 338 and its reactivity toward thiol-directed reagents vary spontaneously (13). It appears that the thiol may participate in the coordination of trace amounts of copper. Although metal binding can be reversed by exposure of the oocyte to competing thiols such as 2-mercaptoethanol (2-ME) or dithiothreitol (DTT), it appears that metal coordination may lead, in a variable portion of the channels, to irreversible oxidation of the thiol. This variable reactivity is preserved in the T338C/Cys-less construct and cannot, therefore, be attributed to an intrapeptide disulfide bond. Accordingly, in the experiments here, care was taken to promote the simple thiol state of the cysteine by pretreating oocytes with 1 mM 2-ME or DTT before or during recordings. Although the observed reactivity of this construct toward reagents such as MTSET+ and MTSES− (described in detail in Liu et al. (13)) suggested that this strategy reversed metal binding, results presented here confirm those reported in Liu et al. (8,13), namely that a variable fraction of the channels remain unreactive after oocytes are exposed to 2-ME or DTT. This nonrecoverable fraction of the channel population appears to be unreactive toward [Au(CN)2]− as well as mixed disulfides and alkylating agents.
Whole-cell recordings
Whole-cell recording methods were similar to those described by Mansoura et al. (14). Briefly, individual oocytes were placed in the recording chamber and continuously perfused with Frog Ringer's solution. The Ringer's solution contained (in mM): 98 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 HEPES-HemiNa, at pH 7.4. The TEVC-200 amplifier (Dagan, Minneapolis, MN) and the pClamp 8 data acquisition program (Axon Instruments, Foster City, CA) were used for data acquisition. Oocytes were maintained in the open circuit condition, and the membrane potential was periodically ramped from −120 to +60 mV over 1.8 s to construct the whole-cell I/V plots.
Single-channel recording
Single CFTR channels were studied using excised, inside out patches as described previously (8). The pipette solution contained (in mM) 196 NMDG-Cl, 2 MgCl2, and 5 MES (2-(N-morpholino)-ethanesulphonic acid) HemiNa, pH 6.0. Channels were activated by exposure to the catalytic subunit of PKA (Promega, Madison, WI) after excision into intracellular solution (in mM) 196 NMDG-Cl, 2 MgCl2, 0.5 Tris-EGTA, 5 HEPES HemiNa, and 1 Mg ATP, pH 7.4.
We used an apparent open probability (Po*) as a measure of channel gating. For multi-channel patches we defined Po* as the ratio of NPo for all levels divided by N, where NPo-for-all-levels was obtained after single-channel searching using the event detection features in Clampfit 9 (Axon Instruments, Foster City, CA) and N is the apparent number of channels in a patch. The amplitude histograms (amplitude distribution versus amplitude) were made from all-point amplitude histograms using the normalization feature of Clampfit 9 in which data were normalized so that the area under the curve equals one.
Reagents
The experiments presented here were conducted using 10 μM isoproterenol and 1 mM 3-isobutyl-1-methy xanthine (IBMX) as the stimulating cocktail (Isop+IBMX). Metanethiosulfonate reagents (MTSET+, MTSES−) were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). 2-ME, DTT, N-ethlymaleimide (NEM), 3-maleimidopropionic acid (MPA), iodoacetamide (IAM), KAu(CN)2, KC(CN)3, and NaSCN were obtained from Sigma (St. Louis, MO). KCN was obtained from Fisher Chemicals (Fairlawn, NJ).
RESULTS
T338C CFTR displays enhanced affinity for [Au(CN)2]−
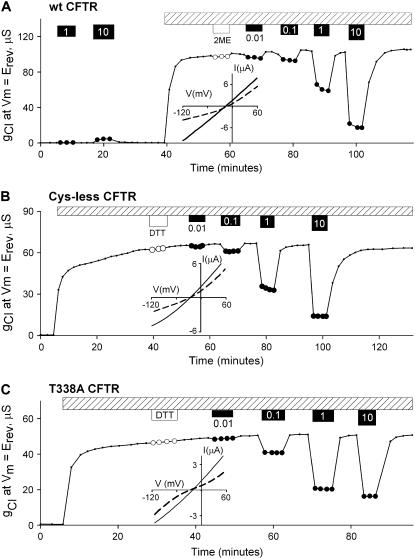
We reported previously that wt CFTR is reversibly blocked by [Au(CN)2]− (7), a result that was confirmed and extended to several CFTR mutants by Linsdell et al. (15–17). Fig. 1 A contains the results of a representative control experiment demonstrating that wt CFTR conductance was reduced by exposure to [Au(CN)2]− in concentrations ranging from 10 μM to 10 mM, but the effect was rapidly reversed by perfusion with a stimulatory cocktail free of [Au(CN)2]−. Fig. 1 B contains the results of a representative control experiment demonstrating that a Cys-less CFTR construct behaved in a similar fashion. The Cys-less CFTR conductance was reduced by exposure to [Au(CN)2]− with a K1/2 value of ∼1 mM, consistent with results obtained from wt CFTR. We refer to this relatively low affinity, rapidly reversible inhibition by [Au(CN)2]− as “lyotropic block” because the relative affinity for similar anions is determined primarily by their relative hydration energies. Similar responses to [Au(CN)2]− were observed using oocytes expressing T338A CFTR (Fig. 1 C), and the apparent dissociation constants (in mM) were similar for the three constructs: Kwt = 0.754, KCys-less = 0.813, KT338A = 0.754.
FIGURE 1.
[Au(CN)2]− reversibly blocked gCl in oocytes expressing a CFTR construct lacking cysteines at position 338. (A) A representative experiment showing the effect of [Au(CN)2]− on wt CFTR conductance. Before activation, the oocyte was exposed to 1 and 10 mM [Au(CN)2]− (solid circles) to evaluate the effects of the pseudohalide on background currents. The result was a modest increase in background conductance. After activation by a stimulatory cocktail (Isop+IBMX, hatched bar), oocyte was exposed to 1 mM 2-ME (open circles) and then to 10 μM, 100 μM, 1 mM, and 10 mM [Au(CN)2]− with a wash in between. (B) A representative experiment showing the effect of [Au(CN)2]− on Cys-less CFTR conductance. After activation by a stimulatory cocktail (Isop+IBMX, hatched bar), an oocyte expressing Cys-less CFTR channels was exposed to 1 mM DTT (open circles) and then to 10 μM, 100 μM, 1 mM, and 10 mM of [Au(CN)2]− (solid circles). (C) A representative experiment showing the effect of [Au(CN)2]− on T338A CFTR conductance. After activation by a stimulatory cocktail (Isop+IBMX, hatched bar), an oocyte expressing T338A CFTR channels was exposed to 1 mM DTT (open circles) and then to 10 μM, 100 μM, 1 mM, and 10 mM of [Au(CN)2]− (solid circles). Insets show the current-voltage relationships (I/V plots) in absence of (solid lines) and presence of 1 mM external [Au(CN)2]− (dashed lines). Note that ECl sometimes depolarizes (Fig. 1 A, inset) in long experiments apparently due to a slow increase in cellular chloride concentration.
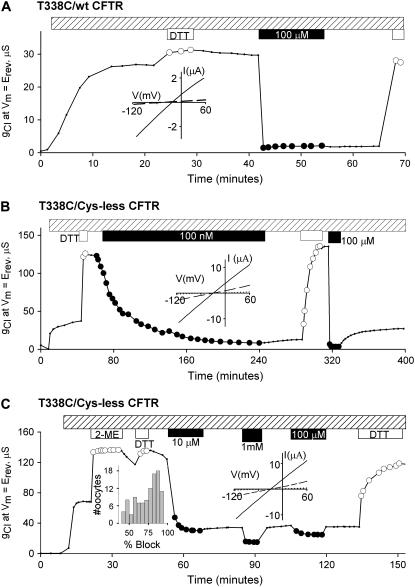
The response to [Au(CN)2]− of oocytes expressing either of two CFTR constructs containing a cysteine substituted at position 338 differed strikingly from lyotropic block. In the experiment depicted in Fig. 2 A, after exposure to DTT, exposure of T338C/wt to 100 μM [Au(CN)2]−, a concentration of the pseudohalide that produced only modest inhibition of Cys-less CFTR conductance, virtually abolished the T338C/wt CFTR conductance. Furthermore, the inhibition was not reversed by perfusing the oocytes with a [Au(CN)2]−-free solution. The inhibition was rapidly reversed, however, by exposing the oocyte to a [Au(CN)2]−-free solution containing a competing thiol, in this case DTT. As indicated in Fig. 2 B, profound inhibition of conductance due to T338C/Cys-less CFTR that was not reversed by washing could be achieved with as little as 100 nM [Au(CN)2]−, albeit at a slower rate. These results indicate that the cysteine at 338 is necessary and sufficient for “high affinity” inhibition of CFTR by [Au(CN)2]− and that, in the absence of a competing thiol, the inhibition is essentially irreversible (see also below). I/V plots (Fig. 2 B, inset) suggest that the block was not highly voltage dependent, as expected for an irreversible, blocker-channel interaction. The data summarized in Fig. 3 demonstrate that between 0.1 and 1 μM the rate of block was a linear function of concentration, consistent with a second-order rate coefficient of 4001 M−1 s−1.
FIGURE 2.
[Au(CN)2]− produced a profound, irreversible reduction of gCl in oocytes expressing T338C CFTR. After activation with stimulatory cocktail (hatched bar), oocytes were exposed to 2 ME and/or DTT (1 mM, open circles) and then to [Au(CN)2]− (solid circles). (A) 100 μM [Au(CN)2]− reduced gCl to near background conductance; after [Au(CN)2]− washout the conductance remained at this level. Exposure to DTT reversed the inhibition. (Inset) I/V plot in absence (solid lines) and presence (dashed lines) of 100 μM [Au(CN)2]−. (B) Comparison of two [Au(CN)2]− concentrations; 100 nM [Au(CN)2]− reduced gCl more than 95%. Washout produced a modest rebound of gCl, and DTT (1 mM, open circles) fully reversed the inhibition. Subsequent exposure to 100 μM [Au(CN)2]− provoked a rapid reduction of gCl. Washout resulted in a rebound of ∼20%. (Inset) I/V plot in absence (solid lines) and presence (dashed lines) of 100 nM and 100 μM [Au(CN)2]− (dotted lines). (C) Multiple components of inhibition by [Au(CN)2]−. The oocyte was first exposed to 1 mM 2-ME (open circles) and then to 1 mM DTT (open circles). 10 μM [Au(CN)2]− produced a rapid and largely irreversible reduction of gCl by ∼70%. Subsequent exposure to 1 mM and 100 μM [Au(CN)2]− produced additional dose-dependent, reversible block. (Inset) I/V plot in absence (solid lines) and presence (dashed lines) of 10 μM and 1 mM [Au(CN)2]− (dotted lines). Histogram represents percentage of conductance block calculated in oocytes after exposure to 100 μM [Au(CN)2]−.
FIGURE 3.
A linear relationship between the rate of block and [Au(CN)2]− concentration, consistent with a second-order rate coefficient of 4001 M−1 s−1. Each data point represents the mean of several separate experiments indicated on the plot.
Also evident in Fig. 2 B is the rapid reversal by DTT of the inhibition induced by 100 nM [Au(CN)2]−. The subsequent inhibition by 100 μM [Au(CN)2]−, a 1000-fold greater concentration, differed in two ways. First, it was more rapid and of somewhat greater extent than the response to 100 nM. Second, washing produced a slow (t1/2 = 20 min), partial (∼20%) reversal of inhibition. Exposure of oocytes exhibiting this sort of slow partial reversal to a second thiol-reactive compound, MTSES−, produced a decrease in conductance as expected if the partial rebound of conductance produced by washing represents a reversal of the [Au(CN)2]−-thiol interaction in some portion of the CFTR channel population that rendered these thiols susceptible to thiol-disulfide exchange (not shown). Conversely, in instances in which a slow “rebound” of conductance after removal of the [Au(CN)2]− was not seen (Fig. 2 A, for example), or if the mixed disulfide reagent was added before washing out [Au(CN)2]−, the conductance was not altered by subsequent exposure to MTSES−, as expected if the [Au(CN)2]−-thiol interaction prevented the thiol-disulfide exchange reaction with MTSES− (not shown). For reasons considered in more detail below, we speculate that this variable partial reversal (compare Fig. 2, A and B) is due to the generation of a small and highly variable amount of free cyanide anion in oocytes exposed to [Au(CN)2]−.
Fig. 2 C contains an example of an oocyte expressing T338C/Cys-less CFTR in which it was possible to observe high affinity, irreversible block, and lyotropic block by [Au(CN)2]−. Here the extent of inhibition by [Au(CN)2]− was less than that depicted in Fig. 2, A and B; initial exposure to 10 μM [Au(CN)2]− produced ∼80% inhibition of the conductance. Over the course of these experiments we found that in oocytes pretreated with a reducing agent (2-ME or DTT) the extent of inhibition of T338C/(wt or Cys-less) conductance by [Au(CN)2]− varied from as much as nearly 100% to as little as 30%–40% as indicated in the histogram (Fig. 2 C, inset). We have reported previously that a cysteine at position 338 (on a wt or Cys-less background) is subject to spontaneous reactions to at least two different chemical states, one of which is not reversed by exposure to reducing agents and can render the cysteine unreactive toward methanethiosulfonate (MTS) reagents (8,13). The variability in the response to [Au(CN)2]− of oocytes expressing constructs bearing a cysteine at 338 suggests that channels that are unreactive toward MTS reagents also fail to react with the pseudohalide.
In the experiment depicted in Fig. 2 C, washing the oocyte with [Au(CN)2]−-free solution produced a modest, partial reversal to a level of inhibition that was essentially irreversible. Subsequent exposure to 1 mM [Au(CN)2]− further reduced the conductance, but perfusion with a [Au(CN)2]−-free solution resulted in a rapid return to the original level of inhibition in a manner reminiscent of the lyotropic block of Cys-less CFTR (Fig. 1 B). Application of 100 μM [Au(CN)2]− produced a smaller, reversible block of conductance. This result suggests that the variable conductance that remains after irreversible block of T338C/Cys-less by micromolar concentrations of [Au(CN)2]− represents CFTR channels that are not susceptible to high affinity block but remain susceptible to lyotropic block. Block of the residual conductance by [Au(CN)2]− was consistent with an apparent dissociation constant of ∼0.8 mM, comparable to that seen with wt, T338A, and Cys-less CFTR. This result is consistent with the hypothesis that those spontaneously reacting T338C CFTR channels in which the thiol cannot be recovered by exposure to 2-ME or DTT do not differ from wt CFTR as regards their susceptibility to lyotropic block.
In some oocytes, as indicated by the histogram in Fig. 2 C, inset, inhibition of CFTR conductance was virtually complete, i.e., activated conductance was reduced to the level of the background conductance. For example in four such oocytes the background conductance averaged 0.95 ± 0.12 μS, the activated conductance averaged 97 ± 13 μS, and the conductance after the exposure of 100 μM [Au(CN)2]− (followed by washing) was 1.45 ± 0.23 μS, a value not significantly different from the background conductance (p < 0.05). The results described above are consistent with the pattern of reactivity previously reported for T338C CFTR (13). T338C channels that have been previously exposed to 2-ME or DTT comprise at least two subpopulations. The larger of the two represents channels in which the substituted cysteine is in the highly reactive, thiolate form. Exposure of these channels to nanomolar concentrations of [Au(CN)2]− produces a profound, irreversible block that accounts for the majority of the inhibition of macroscopic conductance due to T338C/wt or T338C/Cys-less CFTR. In additional experiments (not shown) it was apparent that this component of block persisted even after washing the oocytes with [Au(CN)2]−-free solution for more than 2 h. A smaller population of channels are those prevented from reacting with [Au(CN)2]− by a spontaneous reaction of the cysteine thiolate that is not reversed by 2-ME or DTT. This is consistent with our previous finding that such channels are also unreactive toward mixed disulfides (MTS reagents), which also require the cysteine thiolate anion for reactivity. Channels in this latter population, however, remain susceptible to lyotropic block.
As a further test of the hypothesis that [Au(CN)2]− interacts specifically with the cysteine thiolate at position 338, we examined the effect of blocking the cysteine thiolate using IAM, an alkylating agent that forms an irreversible, thioether bond with the reactive thiolate (Fig. 4). Previous studies demonstrated that cysteines at 338 or 334 can be effectively blocked by alkylation (3,13). Exposure of an oocyte expressing T338C/wt CFTR to 100 μM [Au(CN)2]− resulted in nearly 100% inhibition of the conductance. Washing induced a slow rebound, and inhibition was reversed by exposing the oocyte to 1 mM 2-ME. In contrast, after exposure to 100 μM IAM, the oocyte responded to the pseudohalide in a manner similar to that seen in oocytes expressing Cys-less or wt CFTR, i.e., a readily reversible block with an apparent dissociation constant of 0.80 mM. High affinity block of T338C CFTR by [Au(CN)2]− was similarly abolished by exposing oocytes to either MTSET+ or MPA, a polar malemide (not shown). Exposure of an oocyte expressing a construct bearing a non-Cys substitution at position 338 (T338A CFTR) to IAM (2 mM) was without effect on reversible, lyotropic block by [Au(CN)2]− (13).
FIGURE 4.
Alkylation of the cysteine at 338 abolished the irreversible inhibition by [Au(CN)2]−. An oocyte expressing T338C/wt CFTR was exposed to 1 mM 2-ME (open circles); 100 μM [Au(CN)2]− (solid circles) irreversibly reduced gCl by ∼80%. After reversing inhibition (1 mM 2-ME) and exposure to 100 μM IAM (shaded circles), the same concentration of [Au(CN)2]− induced a reversible, 20% decrease in gCl.
High affinity block of T338C CFTR conductance: evidence for a ligand-exchange reaction
The irreversible block of T338C CFTR conductance by [Au(CN)2]− suggests that the anion participates in a high affinity interaction with the substituted cysteine. Studies of the interaction of [Au(CN)2]− with human serum albumin (18) as well as simple thiols (19) provide a possible mechanism for this reaction, namely ligand exchange (or ligand substitution), i.e.,
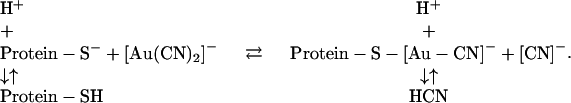 |
(1) |
In this reaction one of the [CN]− ligands of the Au exchanges with the protein thiolate (Protein-S−) to form Protein-S-[Au-CN]−. The Protein-S-[Au(CN)]− reaction product has been demonstrated for cysteine 34 of human serum albumin (18) and for glutathione (20).
If the reaction between [Au(CN)2]− and T338C CFTR occurs via the mechanism depicted above then it should be possible to reverse the reaction by supplying excess cyanide ligand. The results depicted in Fig. 5, A and B, are consistent with this hypothesis. After activating T338C/Cys-less CFTR and treating the oocyte with 2-ME, exposure to 0.5 mM KCN resulted in an 18% reduction of the conductance that was readily reversed by washing with a KCN-free solution (Fig. 5 A). After establishing “irreversible” inhibition of conductance by [Au(CN)2]−, subsequent exposure to 0.5 mM KCN reversed the inhibition, and washout of the KCN restored the conductance to its preinhibition value. The result depicted in Fig. 5 B shows that it is possible to reverse the inhibition by [Au(CN)2]− even in the continued presence of the pseudohalide. After profound inhibition of conductance by 1 μM [Au(CN)2]−, stepwise addition of 20 μM and 50 μM KCN produced partial and nearly complete reversal of the block, respectively. This result is consistent with establishment of a reversible equilibrium for the proposed ligand exchange reaction. In this context it is important to note that the pKa of HCN is 9.2 (21), so that the concentration of the free anion, [CN]−, at an ambient pH of 7.4 would be ∼1.6% of the nominal concentration of KCN, i.e., 8 μM if bath [KCN] = 0.5 mM. It is possible, however, that due to the presence of the arginine at 334, the pH in the vicinity of position 338 is even more alkaline than the bathing solution, by as much as one pH unit (8), which would imply a local concentration of [CN]− of as much as 80 μM. In any case the local concentration is likely to be much less than that of the added KCN. The sensitivity of the product of the ligand substitution reaction to ambient [CN]− is consistent with the very high stability of the gold-cyanide complex (20).
FIGURE 5.
[CN]− reversed block by [Au(CN)2]−. (A) Oocytes expressing T338C/Cys-less CFTR were activated and exposed first to 1 mM 2-ME (open circles) and then to 500 μM KCN (shaded triangles). Before [Au(CN)2]−, KCN induced a small and readily reversible inhibition of gCl. After 100 μM [Au(CN)2]− (solid circles) and subsequent washout, exposure to 500 μM KCN induced a rapid increase in gCl, followed by a further increase when KCN was washed from the perfusate. (B) In constant presence of [Au(CN)2]− increasing concentrations of [CN]− shifted the reaction to the unblocked state. After activation, oocytes expressing T338C/Cys-less CFTR were exposed to 1 mM 2-ME (open circles) and then to 1 μM [Au(CN)2]− (solid circles). In constant presence of the pseudohalide, the oocyte was exposed to 20 μM (shaded triangles) and 50 μM (open triangles) KCN. Each exposure of [CN]− rapidly induced a stable increment in conductance, consistent with the increase of the back reaction.
The observation that a small amount of [CN]− can displace the thiolate ligand may provide an explanation for the slow, partial reversal of high affinity block that is seen in some oocytes (compare Fig. 2, B and C). Results such as that depicted in Fig. 2 B suggest that the extent of this spontaneous reversal was increased at higher concentrations of [Au(CN)2]−. A direct comparison indicated a significant difference (p < 0.05) between spontaneous reversal seen at 1 μM [Au(CN)2]− (5.1% ± 1.9%, n = 8) and 100 μM (16% ± 1.7%, n = 13). Experiments such as these suggest that in some oocytes the interaction of [Au(CN)2]− with other thiols, perhaps residing in the cytosol or on the membrane surface, can generate sufficient free [CN]− to partially reverse the ligand exchange reaction. We also considered the possibility that the partial reversal could reflect contaminating KCN in the KAu(CN)2 obtained from the supplier but rejected this explanation for two reasons. First, the same batch of KAu(CN)2 resulted in partial reversal in some oocytes and not in others; and second, recrystallizing the salt did not eliminate the partial reversal phenomenon.
As a further test of a ligand-exchange hypothesis we examined the effect of two other cyanide-containing anions, tricyanomethanide ([C(CN)3]−) and thiocyanate ([SCN]−), which are not expected to participate in ligand-exchange reactions. Both anions produced only rapidly reversible, lyotropic block of T338C/Cys-less CFTR that was not affected by blocking the thiolate with IAM (not shown).
Single CFTR channels are blocked irreversibly by [Au(CN)2]−
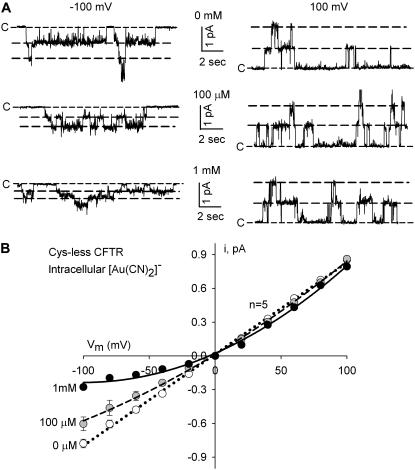
Figs. 6 and 7 compare the effects of [Au(CN)2]− on Cys-less and T338C/Cys-less CFTR channels recorded from detached, inside out patches. Using inside out patches enabled us to operationally define CFTR single-channel currents by their sensitivity to ATP/PKA and also to test accessibility of the cysteine at 338 to [Au(CN)2]− applied to the cytoplasmic side. Pipette pH was adjusted to 6 to maximize the amplitude of single-channel current in the cysteine-containing construct (8). Exposure of the Cys-less CFTR construct to cytoplasmic [Au(CN)2]− (n = 5) produced a voltage-dependent block of single-channel current similar to that reported previously by Linsdell et al. (15,17) for wt CFTR (Fig. 6). This result, as suggested by Linsdell and colleagues (15,17) and Smith et al. (7), is consistent with the reversible block of the CFTR pore by the pseudohalide. The kinetics of block is such that individual blocking events cannot be discerned and the block is manifest as an apparent decrease in single-channel conductance. Exposure of Cys-less CFTR to [Au(CN)2]− did not result in any readily discernable change in gating. At an ATP concentration of 40 μM, the apparent open probabilities, Po* (n = 3) were 0.2 ± 0.03, 0.18 ± 0.02, and 0.2 ± 0.03 for 0, 100 μM, and 1 mM intracellular [Au(CN)2]−, respectively. The apparent Po* is only an estimate of the true value of open probability, but the three values of Po* were obtained under the same ATP concentration from the same patches, and the similarity among these values suggests little or no change in gating due to [Au(CN)2]− exposure. This observation differs from that reported by Linsdell and Gong (17) for wt CFTR, suggesting that the interaction of [Au(CN)2]− with endogenous cysteines not present in the Cys-less construct may account for the effect on open probability.
FIGURE 6.
[Au(CN)2]− reversibly blocked Cys-less CFTR single-channel currents. (A) Currents recorded from detached, inside out patches (50 units PKA, 40 μM ATP) with symmetric 200 mM [Cl]−, pH = 6.0, at −100 mV and +100 mV in the presence of the indicated concentrations of [Au(CN)2]−. (B) Exposure to [Au(CN)2]− produced a voltage-dependent block of Cys-less CFTR channels indicative of a reversible interaction of the pseudohalide with the channel.
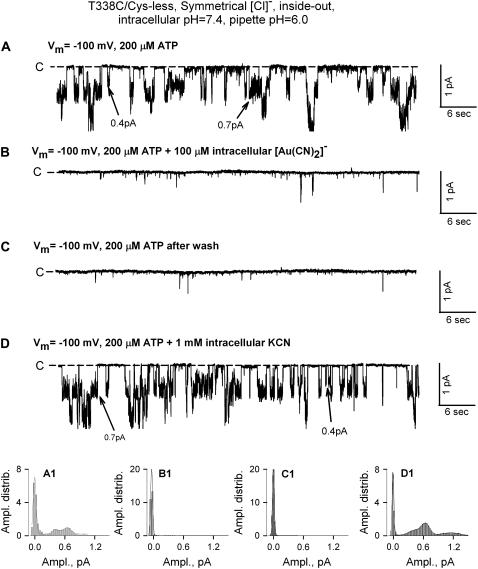
FIGURE 7.
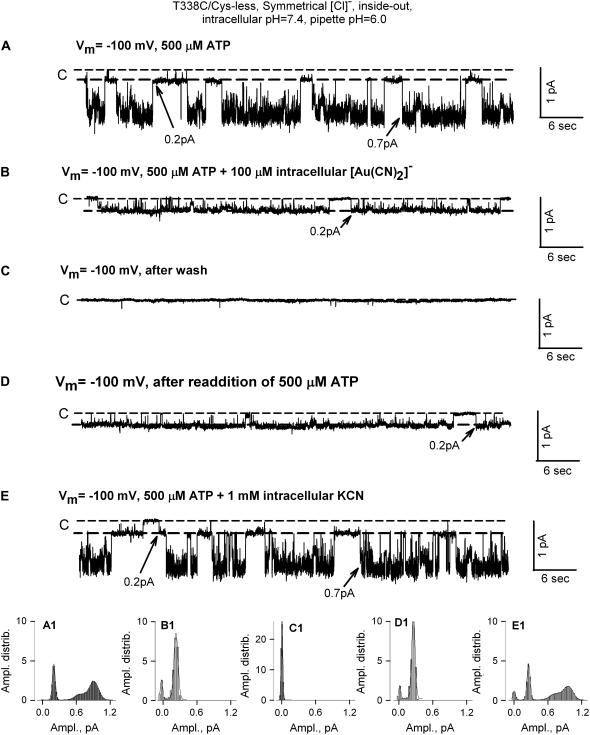
[Au(CN)2]− irreversibly blocked T338C/Cys-less CFTR single-channel currents. (A) Single-channel currents recorded as in Fig. 6 with 200 μM ATP, illustrating multiple current amplitudes commonly seen in constructs containing cysteine at 338. (B) Exposure to 100 μM [Au(CN)2]− abolished single-channel currents. (C) Washing out [Au(CN)2]− did not reverse inhibition. (D) Reversal of inhibition by exposure to KCN (1 mM). The bottom panels (A1–D1) contain amplitude distributions of current levels for the corresponding traces shown in panels A–D.
Exposure to [Au(CN)2]− produced a strikingly different response in T338C/Cys-less CFTR channels (n = 10). The record shown in Fig. 7 A comprised predominantly single-channel currents of ∼0.7 pA that were seen only in the presence of PKA and ATP, although there were occasional openings of smaller amplitude, ∼0.4 pA. This sort of heterogeneity of single-channel current amplitude is commonly seen in constructs containing a cysteine at 338 and appears to be due at least in part to spontaneous reactions of the cysteine at 338 as well as a variable degree of substate behavior (13,22).
Exposure of the patch to 100 μM [Au(CN)2]− essentially abolished channel activity except for some occasional, flickery currents, and this block persisted after the solution bathing the patch was replaced with a solution lacking [Au(CN)2]−. Channel activity was immediately restored, however, when the patch was exposed to a solution containing 1 mM KCN. The fact that all of the single-channel current events were blocked by [Au(CN)2]− as well as the dependence of opening events on PKA/ATP suggest that they represent CFTR channels. The apparent reactivity with [Au(CN)2]− suggests that the more rare, 0.4 pA events could represent a substate of the T338C/Cys-less channel (22).
The record depicted in Fig. 8 appears to contain two channels, one with a current amplitude of ∼0.7 pA and another with a current amplitude of ∼0.2 pA that exhibited a very high open probability. Exposure of the patch to 100 μM [Au(CN)2]− abolished the 0.7-pA-amplitude openings but was without any readily detectable effect on the openings with 0.2-pA amplitude. Those latter events, however, were absent after replacing the bath with a solution lacking PKA and ATP. Adding back PKA and ATP restored the 0.2-pA current but not the 0.7-pA currents. Exposing the patch to 1 mM KCN, however, rapidly restored the latter activity. The behavior of this patch was consistent with the notion that it contained T338C/Cys-less CFTR channels that were blocked by [Au(CN)2]− and a second channel that was unreactive, yet dependent upon PKA and ATP. The smaller conductance channel, as indicated by comparing the two figures, was not seen in all patches (∼10%). Although it is not possible to specify its identity with certainty, the 0.2-pA currents could represent the single-channel counterpart of the smaller fraction of macroscopic CFTR conductance that is rendered unreactive toward both [Au(CN)2]− and MTS reagents by some, as yet unidentified, spontaneous reaction.
FIGURE 8.
[Au(CN)2]− irreversibly blocked one of two channels in a patch. (A) Single-channel currents recorded as in Fig. 6 illustrating two current amplitudes, 0.7 pA and 0.2 pA. (B) Exposure to 100 μM [Au(CN)2]− abolished the 0.7-pA current but was without effect on the 0.2-pA current. (C) Removing PKA and ATP from the bath abolished 0.2-pA channel activity. (D) Addition of PKA and ATP to bath restored 0.2-pA channel activity but not 0.7-pA channel activity. (E) 0.7-pA channel activity was restored by exposing the patch to KCN (1 mM) in presence of PKA and ATP. The bottom panels (A1–E1) contain amplitude distributions of current levels for the corresponding traces shown in panels A–E.
DISCUSSION
High affinity block of T338C CFTR by a permeant anion
[Au(CN)2]− is a very stable coordination compound with a log stability constant (log K) of 37–39 (20,21); where K is given by
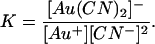 |
(2) |
Yet, in a solution containing excess [CN]−, individual [CN]− undergoes exchange at the Au(I) center at the fast exchange limit detected by 13C-NMR (23). Thus [Au(CN)2]− is typical of Au(I) complexes which, even when thermodyamically quite stable, are nevertheless kinetically labile and susceptible to ligand exchange. Evidence for the formation of a mixed ligand complex of [Au(CN)2]− with a thiol has been obtained for human serum albumin (18) and glutathione (20), but in both cases the equilibrium was thought to lie very much in favor of the reactants, reflecting the large preference for [CN]− as a ligand for Au(I) (20). In the case of the perfused oocyte, however, removal of one of the products of the reaction, [CN]−, by diffusion into an essentially infinite perfusion bath would be expected to drive the reaction toward the mixed ligand product. This conclusion was reinforced by the observation that in the presence of [Au(CN)2]− adding back increasing concentrations of KCN shifted the equilibrium of the reaction away from the mixed ligand product. In addition, if [Au(CN)2]−, a permeant anion, encounters the cysteine thiolate while traversing the pore, the presumed quasicylindrical geometry of the conduction path (7,24) and the linear, cigar-like shape of the anion might combine to bring the metal center in proximity to the thiolate in such a way as to promote ligand-exchange, much the way an enzyme promotes a chemical reaction. The unusually low pKa of the cysteine at 338 (∼7.4) would also be expected to promote ligand exchange (8).
Mechanism of irreversible inhibition of CFTR conductance
The profound, rapid decrease in the conductance of oocytes expressing T338C CFTR could reflect a decrease in single-channel conductance, a reduction of channel open probability, or some combination of these two effects. Previous studies established that [Au(CN)2]− traverses the CFTR pore (7) and that the single-channel conductance of wt and mutant CFTRs is reduced in the presence of the anion (15,17). In addition, we recently presented extensive evidence consistent with the hypothesis that the side chain of a cysteine at position 338 projects into the pore of CFTR where it is accessible to protons, reducing agents, and the polar thiol reagents, MTSET+ and MTSES− (8). Consistent with a location within the conduction path, pH-induced changes in the partial charge on the cysteine had a profound effect on single-channel and macroscopic conductance. Taken together, these observations suggest that at least a portion of the decrease in single-channel conductance produced by exposure of T338C CFTR to [Au(CN)2]− reflects the deposition of a negative charge within the pore via the mixed ligand complex, Protein-S-[Au-CN]−. In recordings from detached patches, single-channel currents were not detectable in the presence of [Au(CN)2]−. This result is consistent with a virtually complete block of the conduction pathway and with the observation that in some oocytes the macroscopic CFTR conductance could be reduced to near zero. However, because single-channel events were not discernable, we cannot eliminate a change in gating that would derive from the effect of the irreversible modification of the cysteine at 338. Linsdell and Gong reported that cytoplasmic [Au(CN)2]−can alter the gating of wt CFTR currents recorded from detached, inside out patches at concentration below 10 μM (17). Thus the inhibition seen here could, in principle, represent a combination of these two effects. It is important to note, however, that the single-channel studies reported here were conducted using Cys-less CFTR and T338C/Cys-less CFTR to avoid confounding effects resulting from endogenous cysteines that are accessible to reagents applied to the cytoplasmic face of detached patches (10). We were unable to discern any change in the gating of Cys-less CFTR channels in the presence of cytoplasmic concentrations of [Au(CN)2]− ranging from 100 μM to 1 mM.
In any single oocyte expressing T338C CFTR that has been previously exposed to 2-ME or DTT, the CFTR channels appear to comprise a mixed population. In some channels the cysteine at 338 is in the simple thiolate form, whereas in others the thiol has undergone a chemical reaction that renders the thiolate unavailable for ligand exchange or thiol-disulfide exchange reactions. In oocytes in which all, or nearly all, of the channels are in the simple thiolate form, exposure to [Au(CN)2]− reduces the conductance to barely detectable levels, presumably due to a profound effect of the Protein-S-[Au-CN]− species on single-channel conductance or gating or both. In other oocytes, in which only a portion of the channels are in the simple thiolate form, exposure to [Au(CN)2]− produces only a partial inhibition of conductance, presumably because a variable subpopulation of channels cannot participate in the ligand substitution reaction required to render the inhibition irreversible. We recorded a small-amplitude current event illustrated in Fig. 8 B that could represent such unreactive CFTR channels.
These results provide strong evidence for a high-affinity interaction of a permeant, Au-containing anion with a substituted cysteine, suggesting that such compounds will be valuable probes of the conduction pathway of CFTR and perhaps other anion channels.
Acknowledgments
We thank Drs. Martin Mense and David Gadsby for the Cys-less CFTR construct and Dr. Nael McCarty for reading the manuscript.
This work was supported by the National Institute for Diabetes, Digestive and Kidney Diseases (DK45880 to D.C.D. and DK60312 to X.L.) and the Cystic Fibrosis Foundation (DAWSON05X0 to D.C.D. and SERRAN04F0 to J.R.S.).
José R. Serrano and Xuehong Liu contributed equally to this work.
References
- 1.Akabas, M. H., C. Kaufmann, T. A. Cook, and P. Archdeacon. 1994. Amino acid residues lining the chloride channel of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 269:14865–14868. [PubMed] [Google Scholar]
- 2.Akabas, M. H., M. Cheung, and R. Guinamard. 1997. Probing the structural and functional domains of the CFTR chloride channel (Review). J. Bioenerg. Biomembr. 29:453–463. [DOI] [PubMed] [Google Scholar]
- 3.Smith, S. S., X. Liu, Z. R. Zhang, F. Sun, T. E. Kriewall, N. A. McCarty, and D. C. Dawson. 2001. CFTR. Covalent and noncovalent modification suggests a role for fixed charges in anion conduction. J. Gen. Physiol. 118:407–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlin, A., and M. H. Akabas. 1998. Substituted-cysteine accessibility method. Methods Enzymol. 293:123–145. [DOI] [PubMed] [Google Scholar]
- 5.Holmgren, M., Y. Liu, Y. Xu, and G. Yellen. 1996. On the use of thiol-modifying agents to determine channel topology. Neuropharmacology. 35:797–804. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, H., and A. Karlin. 1997. Identification of acetylcholine receptor channel-lining residues in the M1 segment of the beta-subunit. Biochemistry. 36:15856–15864. [DOI] [PubMed] [Google Scholar]
- 7.Smith, S. S., E. D. Steinle, M. E. Meyerhoff, and D. C. Dawson. 1999. Cystic fibrosis transmembrane conductance regulator. Physical basis for lyotropic anion selectivity patterns. J. Gen. Physiol. 114:799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu, X., Z. R. Zhang, M. D. Fuller, J. Billingsley, N. A. McCarty, and D. C. Dawson. 2004. CFTR: a cysteine at position 338 in TM6 senses a positive electrostatic potential in the pore. Biophys. J. 87:3826–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, K. W., L. Csanady, D. Seto-Young, A. C. Nairn, and D. C. Gadsby. 2000. Severed molecules functionally define the boundaries of the cystic fibrosis transmembrane conductance regulator's NH(2)-terminal nucleotide binding domain. J. Gen. Physiol. 116:163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotten, J. F., and M. J. Welsh. 1997. Covalent modification of the regulatory domain irreversibly stimulates cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 272:25617–25622. [DOI] [PubMed] [Google Scholar]
- 11.Smit, L. S., D. J. Wilkinson, M. K. Mansoura, F. S. Collins, and D. C. Dawson. 1993. Functional roles of the nucleotide-binding folds in the activation of the cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA. 90:9963–9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson, D. J., M. K. Mansoura, P. Y. Watson, L. S. Smit, F. S. Collins, and D. C. Dawson. 1996. CFTR: the nucleotide binding folds regulate the accessibility and stability of the activated state. J. Gen. Physiol. 107:103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, X., C. Alexander, J. Serrano, E. Borg, and D. C. Dawson. 2006. Variable reactivity of an engineered cysteine at position 338 in cystic fibrosis transmembrane conductance regulator reflects different chemical states of the thiol. J. Biol. Chem. 281:8275–8285. [DOI] [PubMed] [Google Scholar]
- 14.Mansoura, M. K., S. S. Smith, A. D. Choi, N. W. Richards, T. V. Strong, M. L. Drumm, F. S. Collins, and D. C. Dawson. 1998. CFTR: anion binding as a probe of the pore. Biophys. J. 74:1320–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong, X., S. M. Burbridge, E. A. Cowley, and P. Linsdell. 2002. Molecular determinants of Au(CN)(2)(−) binding and permeability within the cystic fibrosis transmembrane conductance regulator Cl(−) channel pore. J. Physiol. 540:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong, X., and P. Linsdell. 2003. Coupled movement of permeant and blocking ions in the CFTR chloride channel pore. J. Physiol. 549:375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linsdell, P., and X. Gong. 2002. Multiple inhibitory effects of Au(CN)(2−) ions on cystic fibrosis transmembrane conductance regulator Cl(−) channel currents. J. Physiol. 540:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canumalla, A. J., S. Schraa, A. A. Isab, C. F. Shaw 3rd, E. Gleichmann, L. Dunemann, and M. Turfield. 1998. Equilibrium binding constants and facile dissociation of novel serum albumin-dicyanoaureate(I) complexes. J. Biol. Inorg. Chem. 3:9–17. [Google Scholar]
- 19.Lewis, G., and C. F. Shaw 3rd. 1986. Competition of thiols and cyanide for gold (I). Inorg. Chem. 25:58–62. [Google Scholar]
- 20.Shaw 3rd, C. F. 1999. The biochemistry of gold. In Gold: Chemistry, Biochemistry and Technology. H. Schmidbauer, editor. Wiley & Sons, Chichester, UK. 259–308.
- 21.Dickson, P. N., A. Wehrli, and G. Geier. 1988. Coordination chemistry of gold (I) with cyanide and 1-methylpyridine-2 thione. Kinetics and thermodynamics of ligand exchange at gold (I) in aqueous solution. Inorg. Chem. 27:2921–2925. [Google Scholar]
- 22.Zhang, Z., G. Cui, X. Liu, B. Song, D. Dawson, and N. McCarty. 2005. Determination of the functional unit of the cystic fibrosis transmembrane conductance regulator chloride channel. One polypeptide forms one pore. J. Biol. Chem. 280:458–468. [DOI] [PubMed] [Google Scholar]
- 23.Pesek, J. J., and W. R. Mason. 1979. Carbon-13 magnetic resonance spectra of diamagnetic cyano complexes. Inorg. Chem. 18:924–928. [Google Scholar]
- 24.Dawson, D. C., X. Liu, Z.-R. Zhang, and N. A. McCarty. 2003. Anion conduction by CFTR: mechanisms and models. In CFTR Chloride Channel. K. Kirk and D. Dawson, editors. Landes Bioscience, Georgetown, TX. 1–34.