Abstract
Tissue patterning must be translated into morphogenesis through cell shape changes mediated by remodeling of the actin cytoskeleton. We have found that Capping protein alpha (Cpa) and Capping protein beta (Cpb), which prevent extension of the barbed ends of actin filaments, are specifically required in the wing blade primordium of the Drosophila wing disc. cpa or cpb mutant cells in this region, but not in the remainder of the wing disc, are extruded from the epithelium and undergo apoptosis. Excessive actin filament polymerization is not sufficient to explain this phenotype, as loss of Cofilin or Cyclase-associated protein does not cause cell extrusion or death. Misexpression of Vestigial, the transcription factor that specifies the wing blade, both increases cpa transcription and makes cells dependent on cpa for their maintenance in the epithelium. Our results suggest that Vestigial specifies the cytoskeletal changes that lead to morphogenesis of the adult wing.
Keywords: actin, cytoskeleton, capping protein, wing disc, vestigial, epithelium
Introduction
The specification of organ primordia by intercellular signalling has been well described, but it is not yet clear how such tissue patterning events are translated into organ morphogenesis through cytoskeletal reorganization (Braga and Yap, 2005; Jamora and Fuchs, 2002). The actin cytoskeleton controls cell morphology and polarity, endocytosis, intracellular trafficking, contractility and cell division. Filamentous (F) actin is assembled from monomeric (G) actin subunits. Polymerization occurs predominantly by extension of the fast-growing barbed ends of the filaments, while filaments are disassembled by loss of monomers from the slow-growing pointed ends. Actin filament growth, stability and disassembly are controlled by a plethora of actin-binding proteins. Profilin, formins and Enabled/VASP (Ena/VASP) family proteins promote actin polymerization, while Cofilin, Cyclase-associated protein (CAP) and Capping proteins (CP) restrict actin polymerization by different mechanisms (dos Remedios et al., 2003; Wear and Cooper, 2004; Winder and Ayscough, 2005). Cofilin severs filaments and enhances dissociation of actin monomers from the pointed end (Bamburg, 1999); CAP sequesters actin monomers, preventing their incorporation into filaments (Amberg et al., 1995; Gottwald et al., 1996); and CP restricts accessibility of the barbed end, inhibiting addition or loss of actin monomers (Schafer et al., 1995).
Functional CP is a highly conserved αβ heterodimer which binds the barbed ends of actin filaments through the C-terminal regions of both subunits (Amatruda et al., 1992; Schafer et al., 1992; Wear and Cooper, 2004). CP and the Arp2/3 complex, which promotes filament branching, favor formation of the short highly branched actin filaments required to generate protrusive force at the leading edge of migrating cells. Ena/VASP proteins have the opposite activity, promoting formation of long, unbranched parallel bundles of actin filaments (Bear et al., 2002; Pantaloni et al., 2000; Pollard and Borisy, 2003). In mouse or Dictyostelium cells, depletion of CP can cause extensive formation of filopodia and increase the length and bundling of actin filaments, reducing cell motility (Hug et al., 1995; Mejillano et al., 2004). Another function of CP is to cap a short filament of the actin-related protein Arp1 in the Dynactin complex, which is required for Dynein-mediated transport along microtubules (Schafer et al., 1994).
Drosophila imaginal discs are bilayered epithelial tissues consisting of a columnar monolayer epithelium covered by a squamous peripodial epithelium. The columnar epithelial cells are polarized along the apical-basal axis. Adherens junctions (AJ) composed of E-cadherin and α- and β-catenin link the actin cytoskeleton of neighboring cells, forming an adhesive belt. In Drosophila, two complexes important for polarity, the Bazooka/Par-6/aPKC complex and the Crumbs/Stardust/Pals-associated tight junction (Patj) complex, are present in the subapical region just apical to the AJ. Finally, the septate junction, basal to the AJ and formed by the Discs-large (Dlg)/Scribble/Lethal giant larvae complex, establishes a barrier preventing the diffusion of solutes across the epithelium (Gibson and Perrimon, 2003). Cells lacking these apical complexes are defined as mesenchymal (Fristrom, 1988).
The wing disc has a concentrically organized proximal-distal (PD) axis; the primordium of the wing blade is in the center, surrounded by the wing hinge primordium, with the notum and pleura at the periphery. During the second larval instar, an antagonistic relationship between epidermal growth factor (EGF) and Wingless (Wg) signaling divides the disc into a dorsal region that gives rise to the notum, and a ventral region that forms the wing. The wing is further subdivided by expression of the selector genes vestigial (vg) and scalloped (sd) in the wing blade and homothorax (hth) in the wing hinge (Klein, 2001). Vg and Sd encode subunits of a heterodimeric transcription factor that controls wing identity (de Celis, 1999); vg is required for wing formation (Williams et al., 1991) and its misexpression can induce ectopic wing tissue (Kim et al., 1996).
In a mosaic genetic screen for genes required during early eye differentiation (Janody et al., 2004), we identified loss of function mutations in the genes encoding Capping protein α (Cpa), the CAP homolog Capulet (Capt) and the Cofilin homolog Twinstar (Tsr). Here we show that Cpa, as well as the previously identified Capping protein β (Cpb), prevent extrusion and death of cells in the wing blade epithelium, but are not required for this function in other regions of the wing disc. Although cpa, capt and tsr mutations all increase actin filament polymerization, only cpa is required to maintain vg-expressing cells within the epithelium. Furthermore, Vg enhances transcription of cpa in the wing blade region. These results provide a link between pattern formation controlled by Vg and morphogenesis of the wing blade through cytoskeletal regulation mediated by actin capping proteins.
Materials and Methods
Fly strains and genetics
The mosaic screen for mutations affecting early eye development has been described in detail (Janody et al., 2004). Five lethal alleles of cpa (cpa36P, cpa43D, cpa66A, cpa69E and cpa107E) and 4 lethal alleles of tsr (tsr76E, tsr99E, tsr100A, tsr110M) were recovered from a screen of 45,000 flies on the right arm of the second chromosome. Recombination mapping relative to P(w+) elements localized the cpa gene between 57A8-9 and 57D11-12, 1.5 cM proximal to P element l(2)K02206 and 0.9 cM distal to P element domK08108 (FlyBase). Our alleles failed to complement the lethal P element CG10540KG02261 (FlyBase), which is inserted in the cpa gene. Recombination mapping relative to P(w+) elements localized the tsr alleles to 0.42 cM distal to P element elF6(K13214) (FlyBase) and identified a lethal P element in the tsr gene, l(2)k05633k05633 (FlyBase), which failed to complement our tsr alleles. Other fly stocks used were cpbM143, khck13314, bs2 (FlyBase), captE636, captE593 (Benlali et al., 2000), da-Gal4 (Wodarz et al., 1995), tub-Gal4 (Lee and Luo, 1999), puc-lacZ (Martin-Blanco et al., 1998), UAS-DIAP (Ryoo et al., 2002), UAS-vg23 (Kim et al., 1996), UAS-Nintra (Doherty et al., 1996), UAS-bskDN (Adachi-Yamada et al., 1999b), UAS-Mal-D-ΔN, and UAS-diaCA (Somogyi and Rorth, 2004),. To generate clones marked by the absence of GFP in the wing disc, y, w; FRT42D, cpa or tsr/CyO P(y+) or y, w; FRT40, capt/SM6-TM6B males were crossed to y, w, hsFLP122; FRT42D or 40, ubi-GFP females. To generate clones positively labeled by GFP, y, w; FRT42D, cpa or tsr/CyO P(y+) or y, w; FRT40, capt or cpb CyO P(y+) males were crossed to y, w, hsFLP122, UAS-GFP; FRT42D or FRT40, tub-GAL80; tub-GAL4/TM6B. puc-LacZ, UAS-DIAP1, UAS-vg or UAS-Nintra transgenes were combined with the FRT42D or FRT42D, cpa chromosomes. The offspring were heat-shocked for 1 hr at 37°C at both 24 and 48 hours after a 24 hour egg collection, corresponding to the first and second larval instar. To visualize cpa or tsr mutant clones at different times after induction, larvae were heat-shocked for 1 hr at 37°C at 60h after a 24h egg collection. Wing discs were dissected from crawling late third instar larvae either 36 or 60h after the heat shock; these had clones induced at early third instar or at second instar, respectively. All experiments were performed at 25 °C. To rescue survival of cpa mutants, da-GAL4 or tub-GAL4; cpa/SM6-TM6B flies were crossed to UAS-HA-cpa, cpa/SM6-TM6B. To rescue cpa mutant clones in the wing imaginal disc, y, w, hsFLP122, UAS-GFP; FRT42D, tub-GAL80; tub-GAL4/TM6B flies were crossed to FRT42D, cpa; UAS-HA-cpa/SM6/TM6B.
Immunohistochemistry and In Situ Hybridization
Third instar larval imaginal discs were stained with antibodies as described (Lee and Treisman, 2001). Antibodies used were guinea pig anti-Dlg (1:300; gift from P.J. Bryant), mouse anti-Arm (N2 7A1, 1:10; Developmental Studies Hybridoma Bank (DSHB)), rabbit anti-Caspase 3 (1:500; BD Bioscience), mouse anti-β-galactosidase (1:200; Promega), rabbit anti-Vg (1:20; (Williams et al., 1993), mouse anti HA (1/5000; Covance). Secondary antibodies were from Jackson Immunoresearch, used at 1:200 or Molecular Probes, used at 1:500, conjugated to FITC, Texas Red or Cy5. Rhodamine-conjugated phalloidin (Sigma) was used at a concentration of 0.3 μM. Fluorescence images were obtained on a Leica TCS NT confocal microscope or on a LSM 510 Zeiss confocal microscope. For in situ hybridization, antisense or sense RNA probes, labeled with digoxigenin-UTP (Roche), and encompassing the entire cpa cDNA were used. In situ hybridization was performed as described (Maurel-Zaffran and Treisman, 2000) except that a biotinylated anti-digoxigenin antibody (1:500 Jackson Immunoresearch) was followed by TSA enhancement (TSA-indirect Perkin Elmer Life Science) coupled to Streptavidin Texas Red or Fluorescein (1:100, Perkin Elmer Life Science). Imaginal discs were mounted in Vectashield medium (Vector Laboratories).
Molecular Biology
The cpa cDNA clone (GH10050) was obtained from the Berkeley Drosophila genome project and was amplified by PCR and cloned into HA-pUAST as an EcoRI fragment to generate UAS-HA-cpa. Transgenic flies were generated by standard methods. For each of the five cpa or four tsr alleles recovered in our screen, the coding region was amplified by PCR from DNA obtained from homozygous mutant first instar larvae and from the isogenic FRT42D line. cpa107E, cpa36P and cpa69E contain nonsense mutations at amino acids 162, 174 and 180 respectively, that would truncate the Cpa protein before the actin binding domain. cpa43D contains a nucleotide substitution that changes Asn 190 to Gly. The four tsr alleles contain missense mutations: tsr76E changes Gly 79 to Asp, tsr99E changes Asp 146 to Asn, tsr100A changes Leu 89 to Gln, and tsr110M changes Gly 67 to Glu.
Results
Clones lacking CP, but not Tsr or Capt, are extruded from the wing blade epithelium
In a mosaic genetic screen for genes required during early eye differentiation (Janody et al., 2004), we identified lethal alleles of cpa, tsr and capt. Capping protein, Cofilin and CAP all restrict actin filament polymerization (Bamburg, 1999; Gottwald et al., 1996; Schafer et al., 1995), and loss of any of these genes results in a severe degenerative phenotype in the adult eye (Delalle et al., 2005 and data not shown).
Surprisingly, we found a region-specific requirement for cpa in the wing disc: cpa mutant clones induced at the first or second larval instar could not be recovered in the wing blade epithelium, although mutant clones could develop in the remainder of the wing disc (Fig. 1A). In contrast, clones mutant for tsr or capt survived equally well in all regions of the disc (Fig. 1B,C). Optical cross-sections through the wing disc showed that in the wing blade region, positively labeled cpa mutant cells were found on the basal surface of the disc rather than within the epithelium (Fig. 1D). However, tsr and capt mutant clones were maintained within the epithelium in all regions of the wing disc (Fig. 1F,G). Cpa functions as a heterodimer with its partner Capping protein β (Cpb) (Amatruda et al., 1992; Schafer et al., 1992), and the stability of each subunit depends on its association with the other (Casella and Torres, 1994; Mejillano et al., 2004). As expected, we found that cpb mutant cells were also extruded on the basal surface in the wing blade region, but not in the remainder of the wing disc (Fig. 1E). Thus cpa and cpb are both required to maintain epithelial integrity specifically in the wing blade primordium, suggesting that this subregion of the epithelium has a distinct cellular organization.
Figure 1. cpa or cpb mutant clones are extruded from the wing blade epithelium.
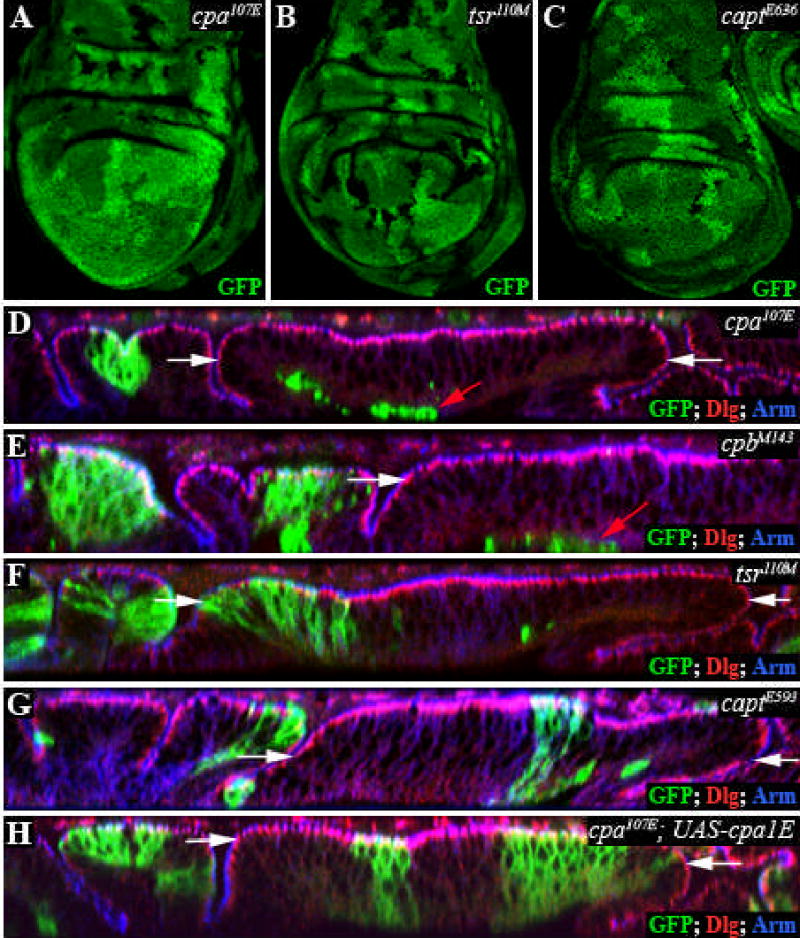
All panels show third instar wing discs. (A–C) Standard confocal sections. cpa107E (A); tsr110M (B) and captE636 (C) mutant clones are marked by the absence of GFP (green). (D–H) Optical cross sections through the wing disc epithelium. Mutant clones are positively labeled with GFP and stained with anti-Dlg (red) and anti-Arm (blue) to outline apical cell membranes. (D) cpa107E; (E) cpbM143; (F) tsr110M; (G) captE593; (H) cpa107E mutant clones overexpressing full-length cpa. cpa or cpb mutant clones are extruded basally in the wing blade primordium (red arrows), but not the notum; this extrusion is rescued by full-length cpa. tsr and capt mutant clones are not extruded. The white arrows define the wing blade region. Dorsal is to the left on optical cross-sections in this and subsequent figures.
The cpa alleles exhibiting this phenotype included likely null alleles that would truncate the protein before the actin-binding domain. To confirm that the loss of cells from the wing pouch epithelium was due to mutations in the cpa gene, we generated transgenic fly lines expressing the full-length cpa transcript under the control of UAS sequences (Brand and Perrimon, 1993). Expression of this transcript rescued the cell extrusion phenotype of cpa mutant clones in the wing blade epithelium (Fig. 1H). Driving expression of this transcript ubiquitously throughout development using daughterless (da)-GAL4 or tubulin (tub)-GAL4 was also sufficient to rescue the lethality of cpa mutants, although some of the rescued adults exhibited wing, eye or bristle defects (Table 1 and data not shown).
Table 1. Rescue of lethality of cpa mutant combinations by full-length Cpa.
For each cross, the percentage rescue to the pupal stage is given, based on comparing the number of non-balancer pupae with the total number of pupae. The SM6.TM6B balancer is marked with Tubby.
| % rescue | ||
|---|---|---|
| cpa rescue | to pupal stage | number |
| cpa43D; tub-GAL4/SM6.TM6B × cpa69E/SM6.TM6B | 0 | 134 |
| cpa43D/SM6.TM6B × cpa107E; UAS-HA-cpa1B/SM6.TM6B | 0 | 215 |
| cpa43D/SM6.TM6B × cpa69E; UAS-HA-cpa1D/SM6.TM6B | 0 | 201 |
| cpa43D/SM6.TM6B × cpa107E; UAS-HA-cpa2A/SM6.TM6B | 0 | 137 |
| cpa43D; tub-GAL4/SM6.TM6B × cpa69E; UAS-HA-cpa1B/SM6.TM6B | 31 | 79 |
| cpa43D; tub-GAL4/SM6.TM6B × cpa69E; UAS-HA-cpa1D/SM6.TM6B | 95 | 238 |
| cpa43D; tub-GAL4/SM6.TM6B × cpa107E; UAS-HA-cpa2A/SM6.TM6B | 29 | 52 |
cpa is required for cell survival in the wing blade epithelium
Our failure to recover cpa mutant clones in the adult wing suggested that extruded mutant cells might be eliminated by apoptosis. Indeed, we found that most cpa mutant cells on the basal surface of the wing blade epithelium contained activated Caspase 3 (Fig. 2A-B”). Extruded cpa mutant clones also expressed puckered-lacZ (puc-lacZ; Fig. 2C), a transcriptional target of the proapoptotic c-Jun N terminal Kinase (JNK) pathway (Adachi-Yamada et al., 1999a), indicating that JNK signaling is activated.
Figure 2. Extrusion of cpa mutant cells is independent of programmed cell death.
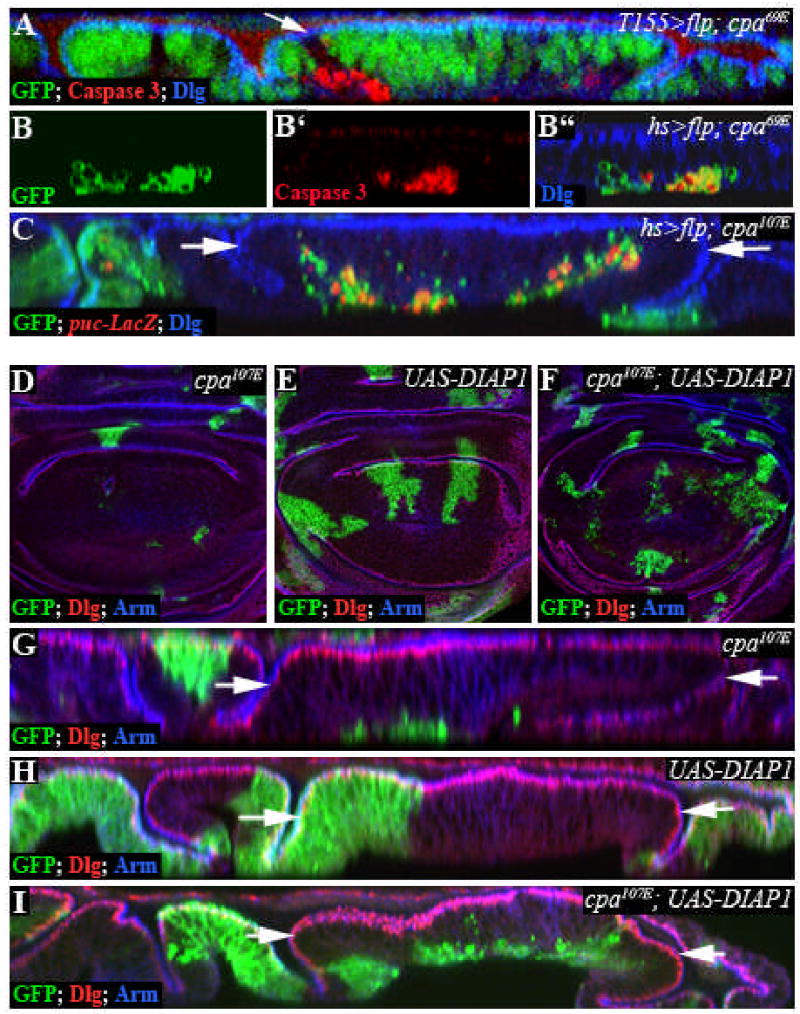
All panels show third instar wing imaginal discs. (A–C) Optical cross sections of discs stained with anti-Dlg (blue in A, B”, C) to outline apical cell membranes and anti-Caspase 3 (red in A, B’, B”) or anti-β-Galactosidase to reveal puc-lacZ expression (red in C). Note that anti-Caspase 3 antibody gives a non-specific background staining seen at the apical surface of the discs. (A) T155-Gal4; UAS-flp induced cpa69E mutant clones marked by the absence of GFP (green). (B–C) hs>flp induced cpa69E (B) or cpa107E (C) mutant clones, positively labelled with GFP (green). (A, B”, C) show the overlay of all three channels. cpa mutant clones express Caspase 3 and puc-lacZ cell autonomously. Cell death is seen when FLP is induced either by heat shock or by the epithelial driver T155-GAL4, and is therefore not due to stressed conditions induced by heat shock, as described for clones mutant for the Dpp receptor thickveins (tkv) (Gibson and Perrimon, 2005). (D–I) Standard confocal sections (D–F) or optical cross sections (G–I) stained with anti-Dlg (red) and anti-Arm (blue). (D, G) cpa107E mutant clones; (E, H) clones overexpressing DIAP1; (F, I) cpa107E mutant clones overexpressing DIAP1. DIAP1 overexpression promotes survival of cpa mutant cells, but fails to prevent their extrusion. The white arrows define the wing blade region.
Vertebrate epithelial cells destined for death are surrounded by a contractile ring of actin and extruded basally (Rosenblatt et al., 2001). To determine whether extrusion of cpa mutant cells from the wing blade epithelium reflects a preliminary stage of apoptosis, we used the MARCM system to express the Drosophila Inhibitor of Apoptosis Protein (DIAP1) to prevent apoptosis of cpa mutant cells. Expression of DIAP1 in wildtype clones had no visible effect (Fig. 2E,H). Survival of cpa mutant cells was greatly enhanced when DIAP1 was overexpressed (Compare Fig. 2D,F); however, mutant cells still lost contact with the apical epithelial surface and were extruded basally (Fig. 2I). The same result was obtained using the caspase inhibitor P35 to block cell death (data not shown). This suggests that extrusion of cpa mutant cells is independent of programmed cell death.
cpa is required to localize adherens junction components in cells in the wing blade
In epithelial cells, the actin cytoskeleton is connected to adherens junctions (AJ) and septate junctions (SJ), which are necessary for stable cell-cell adhesion (Gibson and Perrimon, 2003). Disruption of cortical actin assembly and apical-basal polarity can induce cell extrusion in the wing disc, as in moesin mutants (Speck et al., 2003). In contrast, tkv mutant cells are extruded as cysts that maintain apical junctions with one another (Gibson and Perrimon, 2005; Shen and Dahmann, 2005). Interestingly, we found that an HA-tagged form of Cpa, which could rescue extrusion of cpa mutant cells, accumulated at the apical cell membrane in all regions of the wing disc (Fig. 3A). In the wing blade primordium, HA-Cpa partly co-localized with components of epithelial junctions, including Armadillo (Arm) (Fig. 3B-B”‘). Discs large (Dlg) and Crumbs (data not shown). We therefore examined the apical-basal polarity of cpa mutant cells. Cells that had extruded from the epithelium no longer expressed the AJ components Arm (Drosophila β-catenin) or DE-cadherin (DE-Cad), the SJ component Dlg, or the Crumbs complex component Pals-associated tight junction protein (Patj) (Fig. 3C-C” and data not shown). When we examined discs 36 hours after clone induction, cpa mutant cells in the wing pouch were still present within the epithelium. In these cells, Arm (Fig. 3D-D”) and DE-Cad (data not shown) were mislocalized at basolateral positions, while Dlg localization was unaffected. However, Arm localization was normal in clones induced in the notum region (Fig. 3F-F”). This suggests that, in the wing blade epithelium, Cpa localized at cellular junctions maintains the localization of AJ components. However, Arm was also mislocalized at basolateral positions in tsr mutant clones (Fig. 3E-E”), indicating that mislocalization of AJ components is not sufficient to cause cell extrusion.
Figure 3. cpa maintains the localization of adherens junction components.
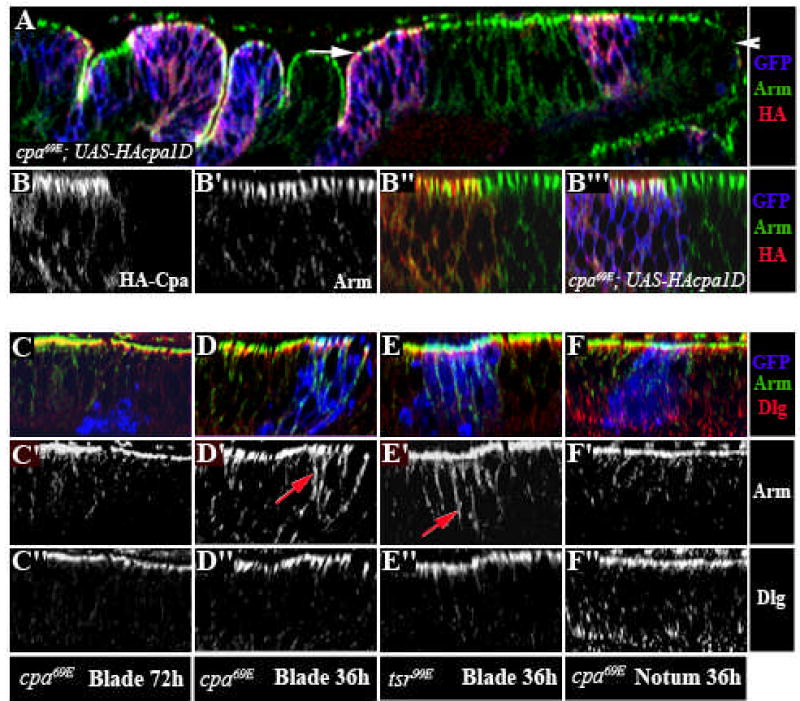
(A–F) Optical cross sections through third instar wing imaginal discs, stained with anti-Arm (red in A, B”,B”‘, C,D,E,F or white in B’,B”,C’,D’,E’,F’) and anti-HA, reflecting UAS-HA-cpa expression (green in A,B”,B”‘ or white in B) or anti-Dlg (green in C,D,E,F or white in C”,D”,E”,F”). (A–B) cpa69E mutant clones overexpressing HA-tagged full-length cpa, positively labelled with GFP (blue in A,B”‘). The white arrow in (A) defines the wing blade region. (B–B”‘) Magnification of the blade primordium. HA-cpa accumulates at the apical membrane, partly co-localizes with Arm in all regions of the wing disc and rescues extrusion of cpa mutant clones in the wing blade primordium. (C–D, F) cpa69E mutant clones positively labeled with GFP (blue in C,D,F) in the wing blade (C,D) or notum (F) primordium, induced both at second or early third instar and dissected at the late third instar stage either 60 hours (C) or 36 hours (D,F) after clone induction. While we could recover mutant clones within the disc epithelium 36 hours after clone induction, all mutant cells were extruded by 60 hours. (E) tsr99E mutant clones in the blade primordium, dissected 36 hours after clone induction and positively labeled with GFP (blue in E). Arm is mislocalized to basolateral regions in extruding cpa mutant cells and in tsr mutant cells that are maintained within the epithelium (red arrows in D’ and E’). Following extrusion of cpa mutant cells, expression of both Arm and Dlg are lost (C).
cpa has cell type-dependent effects on actin filament accumulation
CP tightly caps the fast-growing barbed ends of actin filaments, inhibiting the addition of actin monomers to the filament (Schafer et al., 1995). Decreased expression of cpa or cpb in the Drosophila eye imaginal disc causes actin filament accumulation (Delalle et al., 2005). To confirm that cpa also restricts growth of actin filaments in the wing disc epithelium, we stained wing imaginal discs with phalloidin 36 hours after clone induction, allowing visualization of mutant clones before cell extrusion. As expected, cpa mutant clones accumulated actin filaments in all regions of the wing disc (Fig. 4A-A’). Optical cross sections through the notum showed that, like capt mutant cells (Fig. 4E-E’; and Baum and Perrimon, 2001), cpa mutant cells accumulated actin filaments near the apical cell membrane (Fig. 4C-C”). However, cpa mutant cells in the wing blade accumulated actin filaments around the entire cell cortex (Fig. 4B-B”), resembling tsr mutant cells (Fig. 4D-D’). Since Cpa is concentrated at the apical cell membrane (Fig. 3A-B”‘), the presence of excess actin filaments in basolateral regions may be due to the effect of cpa on apical-basal polarity. Indeed, some of these actin filaments colocalized with Arm displaced from apical adherens junctions (Fig. 4B-B”).
Figure 4. Loss of cpa causes excessive actin polymerization.
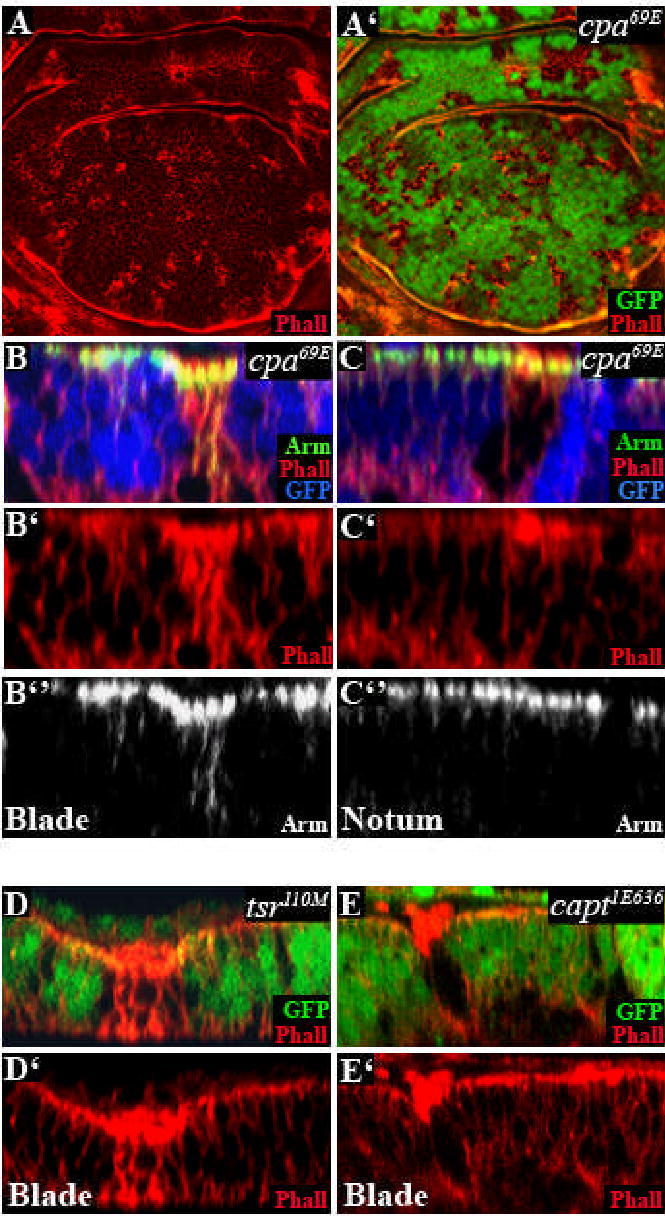
All panels show third instar wing discs in which clones are marked by the absence of GFP (green in A’,D,E or blue in B,C) and stained with TRITC-phalloidin to reveal F-actin (red in A,A’,B,B’,C,C’,D,D’,E,E’)) and anti-Arm (green in B,C or white in B”,C”). (A) standard confocal sections; (B–E) optical cross sections. (A–C) cpa69E mutant clones in the notum (C) or the blade (B) primordium. cpa mutant clones accumulate actin filaments near the apical cell membrane in the notum but throughout the cell in the blade primordium. (D) tsr110M or (E) captE636 mutant clones.
Vestigial controls the requirement for cpa in the wing blade epithelium
We observed extrusion of cpa mutant clones only in the wing pouch, indicating that either the position or identity of these cells makes them dependent on cpa. Signaling pathways activated by Wg, Decapentaplegic (Dpp), and Notch (N) can influence growth and/or survival in the wing blade primordium (Klein, 2001). Target genes of these pathways, including Vg (Klein and Arias, 1999) were still expressed in cpa mutant clones (Fig. 5A-A” and data not shown), indicating that cpa is not required for the reception of these signals. Vg is a nuclear protein that confers a wing blade fate on cells in which it is expressed (Williams et al., 1991). We wondered whether transforming hinge or notum cells to wing blade identity by misexpressing Vg would induce extrusion of cpa mutant cells. When we misexpressed vg in cpa mutant clones, we found that clones in all regions of the wing disc were extruded from the epithelium and contained activated Caspase 3 (Compare Fig. 5B,D and E,G). Misexpressing vg in clones that were not mutant for cpa had neither of these effects (Fig. 5C,F). Thus cpa is autonomously required for the survival and maintenance of vg-expressing cells in the wing disc epithelium, independently of the identity of the surrounding cells.
Figure 5. cpa is required in vestigial expressing cells.
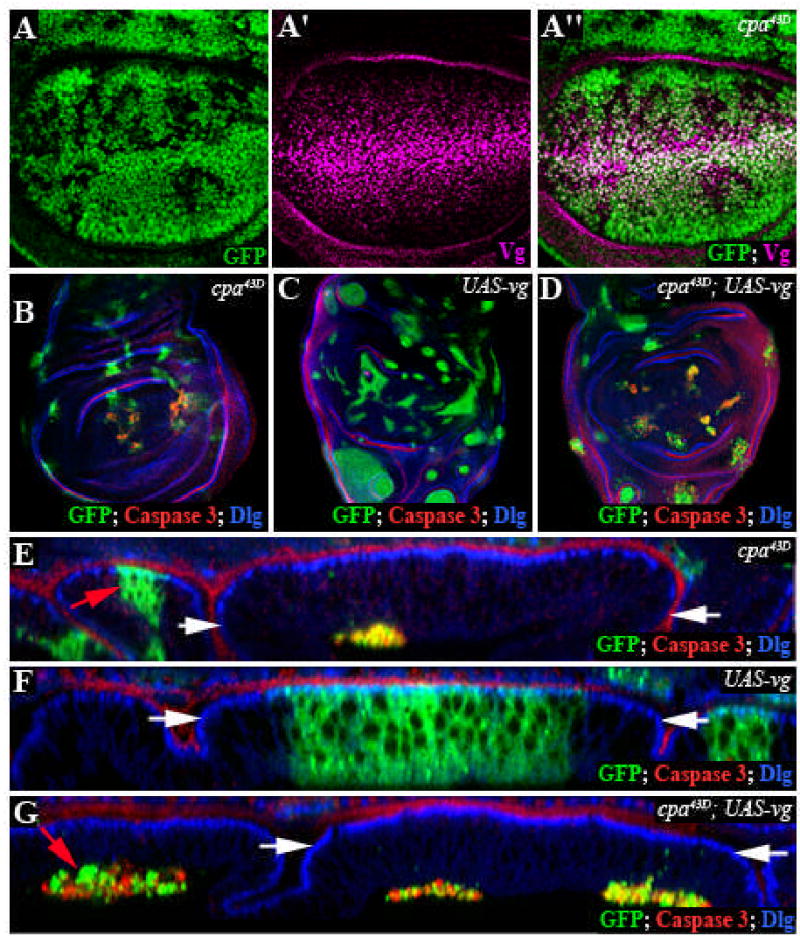
All panels show third instar wing imaginal discs. (A–A”) Standard confocal sections of wing discs with cpa mutant clones marked by the absence of GFP (green in A,A”) and stained with anti-Vg (magenta in A’,A”). Vg is expressed in cpa mutant clones. (B–G) Discs in which clones are positively labeled with GFP (green) and stained with anti-Dlg (blue) and anti-Caspase 3 (red). (B–D) Standard confocal sections; (E–G) optical cross sections. (B, E) cpa43D mutant clones are extruded and die in the wing blade region; (C, F) clones overexpressing vg are not extruded and survive; (D, G) cpa43D mutant clones overexpressing vg are extruded and die in all regions of the disc. Red arrows in (E, G) indicate clones within the notum primordium. The white arrows define the wing blade region.
cpa is a Vg target gene
The requirement for cpa to maintain vg-expressing cells in the wing pouch epithelium suggests that capping actin filaments is crucial for Vg-induced wing blade morphogenesis. We wondered whether cpa expression might itself be induced by Vg. Indeed, in situ hybridization showed that cpa mRNAwas more strongly expressed in the wing pouch than in the hinge primordium or the notum regions, accumulating primarily at the basal surface of the epithelium (Fig. 6A and C; B indicates the background level of the cpa sense probe). As vg is required for the survival of cells in the wing blade, we could only test its effect on cpa transcription in gain of function experiments. Interestingly, misexpressing vg in the notum increased cpa transcript levels (Fig. 6D-D’). The same effect was observed in clones expressing the constitutively active intracellular domain of N (Nintra) (Fig. 6E-E’), which induces misexpression of vg (Fig. 6F-F’). These results suggest that Vg alters the cytoskeletal structure of cells fated to form the wing blade in part by upregulating the expression of actin capping proteins.
Figure 6. Vg and Notch upregulate cpa transcription in the wing blade primordium.
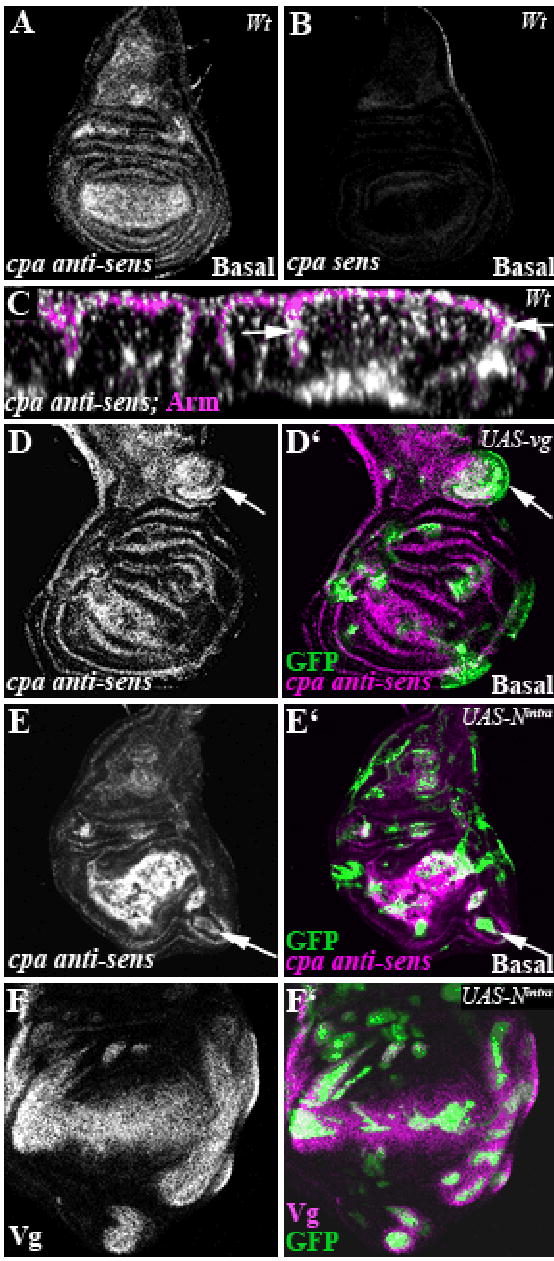
All panels show third instar wing imaginal discs. (A, B, D–F) standard confocal sections. (C) optical cross section, stained with anti-Arm to outline the apical membrane. Clones are positively labeled with GFP (green in D’,E’, F’). (A, C, D, D’; E, E’) cpa anti-sense probe to reveal cpa mRNA expression (white in A,C,D,E and magenta in D’,E’) in wildtype (A, C) or in clones overexpressing vg (white arrow in D, D’) or in clones overexpressing Nintra (white arrow in E, E’). Since cpa mRNA accumulates on the basal surface of the wing disc epithelium, standard confocal sections fail to reveal cpa accumulation in all vg and N-overexpressing clones. (B) cpa sense probe. Note that the background level is very low. (F) clones overexpressing Nintra, stained with anti-Vg (white in F or magenta in F’). Vg is misexpressed.
Discussion
Actin capping proteins maintain epithelial integrity
Wing blade cells lacking either cpa or cpb are extruded from the epithelium and subsequently die. A number of possible mechanisms might account for this loss of CP mutant cells. As extrusion of cpa mutant cells still occurs in the presence of the apoptotic inhibitors p35 or DIAP1, apoptosis is likely to be a secondary consequence of extrusion; extruded cells might undergo apoptosis because they are deprived of antiapoptotic signals present in their normal niche. In addition, JNK activity is not essential for extrusion, since cpa mutant clones were not rescued by expression of a dominant negative form of basket (bsk), which encodes JNK (Supplementary Fig. S1E). However, we cannot exclude the possibility that the p35 or DIAP1 inhibitors block apoptosis too late to prevent release of an extrusion signal, as inhibition of caspases with z-VADfmk does not block extrusion of apoptotic MDCK cells (Rosenblatt et al., 2001).
The function of CP in organelle or vesicle transport is unlikely to explain the extrusion phenotype. CP is thought to stabilize the barbed end of the Arp1 microfilament in the Dynactin complex, which is required for transport along microtubules (Schafer et al., 1994). cpa and cpb, like other Dynactin complex subunits, are required to maintain the position of nuclei in Drosophila photoreceptor neurons (Whited et al., 2004) and data not shown). However, removal of kinesin heavy chain (khc), which counteracts Dynein/Dynactin-based transport, failed to rescue extrusion of cpa mutant cells in the wing disc (Supplementary Fig. S1C).
We considered the possibility that the cpa phenotype was due to its effect on monomeric G-actin levels rather than on the filamentous actin cytoskeleton. G-actin has been shown to negatively regulate the nuclear localization and activity of Mal, a transcriptional cofactor for SRF (Miralles et al., 2003), and overexpression of Mal or of its activator diaphanous (Somogyi and Rorth, 2004) can cause extrusion and death of wing epithelial cells (Supplementary Fig. S1F, G). However, overactivity of the MAL/dSRF pathway is unlikely to be responsible for extrusion of cpa mutant cells in the wing blade, as clones mutant for both cpa and blistered (bs), which encodes Drosophila SRF were still extruded from the wing epithelium (Supplementary Fig. S1H).
Extrusion of cpa or cpb mutant cells might be a direct result of defects in the actin cytoskeleton. Consistent with the requirement for CP to inhibit addition of actin monomers to the fast growing end of actin filaments (Schafer et al., 1995), we observed a strong accumulation of actin filaments in cpa mutant clones. However, tsr and capt mutations also induce excessive actin filament polymerization (Fig. 4 and (Baum and Perrimon, 2001) but do not cause cell extrusion. The major function of Tsr (Cofilin) is to promote dissociation of ADP-actin from the pointed end of the filament, while Cpa prevents elongation of the barbed end of each branch and Capt sequesters actin monomers. The phenotypic differences between cpa, tsr and capt might therefore be due to different degrees of branching of the actin network formed in mutant cells (Fig. 7A). Possibly long unbranched filaments do not provide a framework of sufficient strength to withstand forces that place tension on the cell within the epithelium.
Figure 7. Models for the effect of CP and Vg on wing morphogenesis.
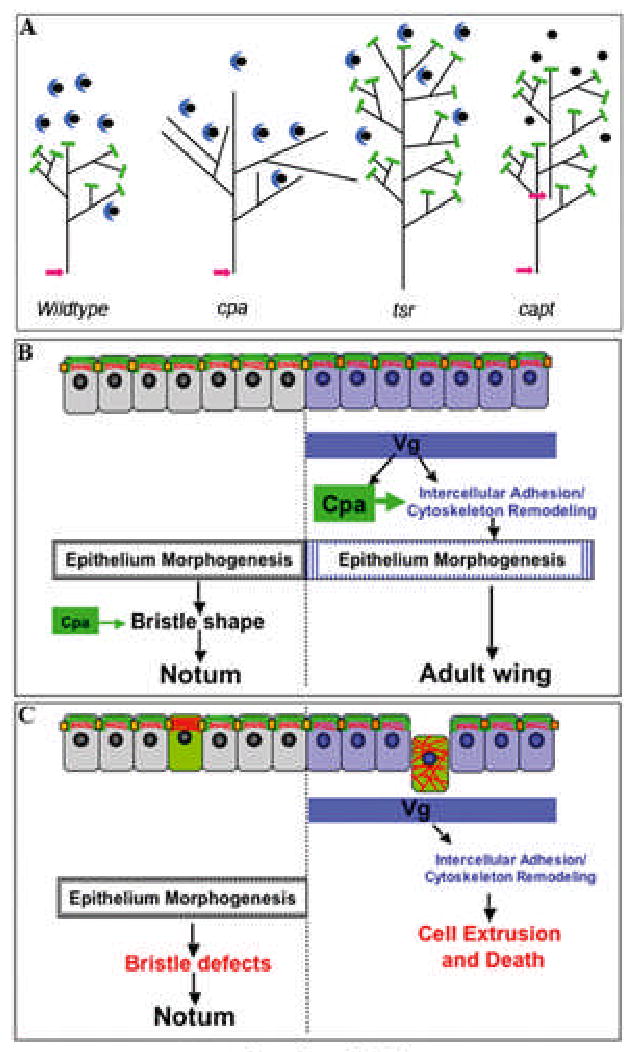
(A) The phenotypic differences between cpa, tsr and capt could be due to different structures of the actin network in mutant cells. CP is represented by green bars, Capt by blue crescents binding to monomeric actin, and Cofilin activity by pink arrows. Loss of cpa would result in extension of each branch of the network. Loss of tsr would make the core filament longer, while the length of branches would be unchanged as they have no free pointed ends. Loss of capt would free more actin monomers for incorporation into networks with the wildtype structure. (B) Cpa (green) is expressed at the apical membrane and co-localized with junctional complexes (orange squares) that link the actin cytoskeleton (in red) of neighboring cells. Vg expression differentiates the wing blade from the notum, enhances cpa expression and also alters the cytoskeleton in such a way as to make the cells dependent on cpa. These changes may contribute to morphogenesis of the adult wing blade. In the notum, Cpa contributes to bristle development. (C) cpa mutant cells (green) accumulate actin filaments near the apical membrane and are maintained in the epithelium in the notum, causing defects in bristle development. However, in the wing primordium, due to other cytoskeletal properties induced by Vg, cpa mutant cells mislocalize junctional components, accumulate actin filaments throughout the cell, are extruded and die.
Extrusion is associated with dispersion of the adherens junction components Arm and DECad along the lateral membranes. However, this defect is also observed in tsr mutant clones, and mislocalization of adherens junction components caused by overexpression of a dominant form of the polarity gene crumbs (Fan et al., 2003; Grzeschik and Knust, 2005; Izaddoost et al., 2002) or a dominant negative form of Dcdc42 (Eaton et al., 1995) does not lead to cell extrusion. Therefore, mislocalization of AJ components is unlikely to be sufficient to cause extrusion of cpa mutant cells. In contrast, expression of dominant negative Drac1, which prevents actin localization to adherens junctions, induces cell extrusion and death (Eaton et al., 1995). Thus, another possibility is that CP may be critical for linking actin filaments to the membrane, as has been previously proposed in other systems (Hutchings et al., 2003; Schafer et al., 1992; Schafer et al., 1998). Loss of cpb displaces actin bundles from the cell membrane in Drosophila bristles (Hopmann et al., 1996) and CP may specify actin filament position in the sarcomere (Schafer et al., 1995). In the Drosophila wing blade epithelium, loss of CP might disrupt attachment of the actin cytoskeleton to the adherens junctions, breaking the connection between cells and inducing cell extrusion (Fig. 7C). The localization of HA-Cpa to apical junctions and the mislocalization of actin filaments throughout cpa mutant cells in the wing blade are consistent with this possibility. Such a role would be restricted to the wing blade, since cpa mutant cells within the notum epithelium accumulate actin filaments only at the apical cell membrane.
Selector proteins regulate the actin cytoskeleton to pattern the wing imaginal disc
Surprisingly, we found that cpa and cpb are required to prevent cell extrusion and death only in the region of the wing disc giving rise to the wing blade, but not in the primordia of the hinge or notum, or in the eye or leg discs (Delalle et al., 2005) and data not shown). The requirement for cpa depends on the wing blade selector gene Vg, as expression of Vg in notum cells is sufficient to induce their extrusion in the absence of cpa. Vg also enhances the transcription of cpa in the wing blade primordium. Taken together, these results imply that patterning genes regulate cytoskeletal properties in order to achieve distinct morphological outcomes (Fig. 7B,C).
The molecular mechanism that makes Vg-expressing cells dependent on CP for their maintenance in the epithelium is unknown, although our data support a cell-autonomous target of Vg. One possibility is that Vg promotes the expression or recruitment of an actin filament polymerizing factor. The role of CP might be to restrict its activity at barbed ends, preventing the formation of a specific actin-based structure that induces loss of cell-cell contacts and extrusion. For example, Vg activates the expression of the type II transmembrane protein Four jointed (Fj), which regulates the activity of the cadherin Fat (Cho and Irvine, 2004). Mammalian Fat1 can recruit Ena/VASP proteins, which promote actin polymerization at cell-cell contacts by antagonizing CP (Moeller et al., 2004; Tanoue and Takeichi, 2004). However, misexpression of fj does not induce extrusion of either wildtype or cpa mutant cells in the notum (Cho and Irvine, 2004 and data not shown). DE-cadherin levels are also higher in the wing pouch (Jaiswal et al., 2006), but increasing them in the hinge or notum by activating Wg signalling does not cause extrusion of cpa mutant cells (data not shown). Alternatively, Vg might control the expression of factors that promote the remodeling of cell junctions required for morphogenesis of the wing. Cpa could be required to maintain the connection between cells in the epithelium during these morphogenetic movements.
The non-uniform distribution of and requirement for cpa suggests that cytoskeletal organization varies in different regions of the wing disc. Gibson and Perrimon (2005) observed that lateral wing disc cells had moderately reduced levels of basolateral cortical F-actin. Also, filopodial extensions called cytonemes are oriented towards the AP and/or DV boundary within the wing pouch, while hinge cells do not extend cytonemes and notum cells radiate short cytonemes in all directions (Hsiung et al., 2005). Changes in cytoskeletal organization have been shown to establish cell affinity boundaries (Major and Irvine, 2005), control the subcellular localization of transcription factors (Miralles et al., 2003), and modulate the transport of signaling molecules (Benlali et al., 2000; Hsiung et al., 2005). Investigating the control of cpa by Vg may help us to understand how and why patterning genes regulate cell architecture. In addition, identifying additional target genes of Vg may illuminate how actin dynamics and changes in intercellular adhesion control the formation of the wing blade.
Supplementary Figure S1. Dynein/Dynactin-based transport, JNK and blistered are not essential for extrusion of cpa mutant cells.
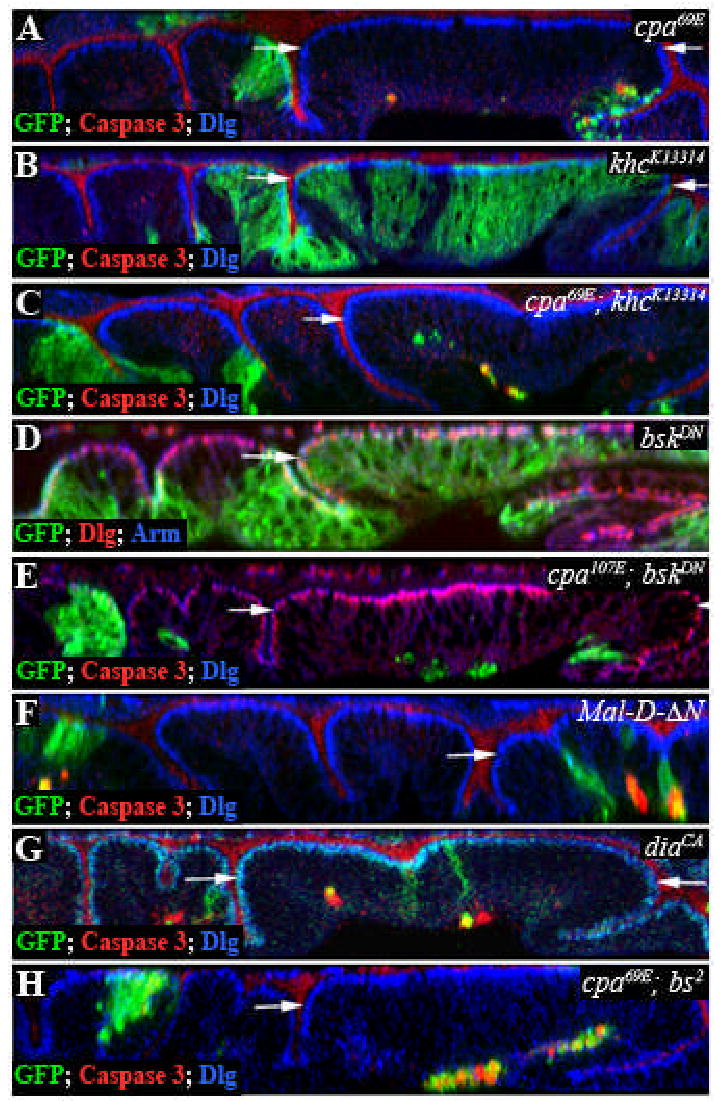
All panels show optical cross sections through the wing disc epithelium of clones positively labeled with GFP (green) and stained with anti-Dlg (blue in A,B,C,F,G,H or red in D,E)) and anti-Caspase 3 (red in A,B,C,G,H) or anti-Arm (red in D,E). (A) cpa69E mutant clones. (B) khcK13314 mutant clones (c) cpa69E; khcK13314 double mutant clones. (D) Clones overexpressing bskDN. (E) cpa69E mutant clones overexpressing bskDN. Removal of bsk or khc fails to rescue extrusion of cpa mutant clones. (F) Clones expressing Mal-D-ΔN. Removal of the N-terminus of Mal-D renders it nuclear and active (Somogyi and Rorth, 2004). (G) Clones expressing diaCA. (H) cpa69E, bs2 double mutant clones. Expression of Mal-D or diaCA causes extrusion and death of epithelial cells, but removing dSRF fails to rescue extrusion of cpa mutant cells. The white arrows define the wing blade region.
Acknowledgments
We thank S. Carroll, B. Mollereau, M. Affolter, P.J. Bryant, K. Hofmeyer, P. Rorth, the Bloomington Drosophila Stock Center, the Drosophila Genomics Resource Center and the Developmental Studies Hybridoma Bank for fly stocks and reagents. We thank Neal Jahren and William Hu for their contributions to the early stages of the project. Special thanks go to Michel Sémériva and Michel Piovant, in whose laboratory many of these experiments were performed. The manuscript was improved by the critical comments of Inés Carrera, Reza Farajian, Kerstin Hofmeyer, Grant Miura, Jean-Yves Roignant, Michel Sémériva, Laurent Perrin, and Josie Steinhauer. This work was supported by the National Institutes of Health (grant EY13777 to J.E.T.). F.J. was the recipient of a fellowship from the Association pour la Recherche sur le Cancer.
References
- Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature. 1999a;400:166–9. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999b;19:2322–9. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda JF, Gattermeir DJ, Karpova TS, Cooper JA. Effects of null mutations and overexpression of capping protein on morphogenesis, actin distribution and polarized secretion in yeast. J Cell Biol. 1992;119:1151–62. doi: 10.1083/jcb.119.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg DC, Basart E, Botstein D. Defining protein interactions with yeast actin in vivo. Nat Struct Biol. 1995;2:28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Baum B, Perrimon N. Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat Cell Biol. 2001;3:883–90. doi: 10.1038/ncb1001-883. [DOI] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–21. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Benlali A, Draskovic I, Hazelett DJ, Treisman JE. act up controls actin polymerization to alter cell shape and restrict Hedgehog signaling in the Drosophila eye disc. Cell. 2000;101:271–81. doi: 10.1016/s0092-8674(00)80837-5. [DOI] [PubMed] [Google Scholar]
- Braga VM, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr Opin Cell Biol. 2005;17:466–74. doi: 10.1016/j.ceb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Casella JF, Torres MA. Interaction of Cap Z with actin. The NH2-terminal domains of the alpha 1 and beta subunits are not required for actin capping, and alpha 1 beta and alpha 2 beta heterodimers bind differentially to actin. J Biol Chem. 1994;269:6992–8. [PubMed] [Google Scholar]
- Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- de Celis JF. The function of vestigial in Drosophila wing development: how are tissue-specific responses to signalling pathways specified? Bioessays. 1999;21:542–5. doi: 10.1002/(SICI)1521-1878(199907)21:7<542::AID-BIES2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Delalle I, Pfleger CM, Buff E, Lueras P, Hariharan IK. Mutations in the Drosophila orthologs of the F-actin capping protein alpha- and beta-subunits cause actin accumulation and subsequent retinal degeneration. Genetics. 2005;171:1757–65. doi: 10.1534/genetics.105.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN. Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 1996;10:421–434. doi: 10.1101/gad.10.4.421. [DOI] [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–73. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Eaton S, Auvinen P, Luo L, Jan YN, Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J Cell Biol. 1995;131:151–64. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SS, Chen MS, Lin JF, Chao WT, Yang VC. Use of gain-of-function study to delineate the roles of crumbs in Drosophila eye development. J Biomed Sci. 2003;10:766–73. doi: 10.1159/000073964. [DOI] [PubMed] [Google Scholar]
- Fristrom D. The cellular basis of epithelial morphogenesis. A review. Tissue Cell. 1988;20:645–90. doi: 10.1016/0040-8166(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Apicobasal polarization: epithelial form and function. Curr Opin Cell Biol. 2003;15:747–52. doi: 10.1016/j.ceb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–9. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- Gottwald U, Brokamp R, Karakesisoglou I, Schleicher M, Noegel AA. Identification of a cyclase-associated protein (CAP) homologue in Dictyostelium discoideum and characterization of its interaction with actin. Mol Biol Cell. 1996;7:261–72. doi: 10.1091/mbc.7.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik NA, Knust E. IrreC/rst-mediated cell sorting during Drosophila pupal eye development depends on proper localisation of DE-cadherin. Development. 2005;132:2035–45. doi: 10.1242/dev.01800. [DOI] [PubMed] [Google Scholar]
- Hopmann R, Cooper JA, Miller KG. Actin organization, bristle morphology, and viability are affected by actin capping protein mutations in Drosophila. J Cell Biol. 1996;133:1293–305. doi: 10.1083/jcb.133.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–3. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- Hug C, Jay PY, Reddy I, McNally JG, Bridgman PC, Elson EL, Cooper JA. Capping protein levels influence actin assembly and cell motility in dictyostelium. Cell. 1995;81:591–600. doi: 10.1016/0092-8674(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Hutchings NJ, Clarkson N, Chalkley R, Barclay AN, Brown MH. Linking the T cell surface protein CD2 to the actin-capping protein CAPZ via CMS and CIN85. J Biol Chem. 2003;278:22396–403. doi: 10.1074/jbc.M302540200. [DOI] [PubMed] [Google Scholar]
- Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–83. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Agrawal N, Sinha P. Fat and Wingless signaling oppositely regulate epithelial cell-cell adhesion and distal wing development in Drosophila. Development. 2006;133:925–35. doi: 10.1242/dev.02243. [DOI] [PubMed] [Google Scholar]
- Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–8. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Janody F, Lee JD, Jahren N, Hazelett DJ, Benlali A, Miura GI, Draskovic I, Treisman JE. A mosaic genetic screen reveals distinct roles for trithorax and polycomb group genes in Drosophila eye development. Genetics. 2004;166:187–200. doi: 10.1534/genetics.166.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382:133–8. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- Klein T. Wing disc development in the fly: the early stages. Curr Opin Genet Dev. 2001;11:470–5. doi: 10.1016/s0959-437x(00)00219-7. [DOI] [PubMed] [Google Scholar]
- Klein T, Arias AM. The vestigial gene product provides a molecular context for the interpretation of signals during the development of the wing in Drosophila. Development. 1999;126:913–25. doi: 10.1242/dev.126.5.913. [DOI] [PubMed] [Google Scholar]
- Lee JD, Treisman JE. Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr Biol. 2001;11:1147–52. doi: 10.1016/s0960-9822(01)00323-2. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–61. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132:3823–33. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–70. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel-Zaffran C, Treisman JE. pannier acts upstream of wingless to direct dorsal eye disc development in Drosophila. Development. 2000;127:1007–1016. doi: 10.1242/dev.127.5.1007. [DOI] [PubMed] [Google Scholar]
- Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–73. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–42. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Moeller MJ, Soofi A, Braun GS, Li X, Watzl C, Kriz W, Holzman LB. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. Embo J. 2004;23:3769–79. doi: 10.1038/sj.emboj.7600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni D, Boujemaa R, Didry D, Gounon P, Carlier MF. The Arp2/3 complex branches filament barbed ends: functional antagonism with capping proteins. Nat Cell Biol. 2000;2:385–91. doi: 10.1038/35017011. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–57. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Bergmann A, Gonen H, Ciechanover A, Steller H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat Cell Biol. 2002;4:432–8. doi: 10.1038/ncb795. [DOI] [PubMed] [Google Scholar]
- Schafer DA, Gill SR, Cooper JA, Heuser JE, Schroer TA. Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J Cell Biol. 1994;126:403–12. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Hug C, Cooper JA. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J Cell Biol. 1995;128:61–70. doi: 10.1083/jcb.128.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Mooseker MS, Cooper JA. Localization of capping protein in chicken epithelial cells by immunofluorescence and biochemical fractionation. J Cell Biol. 1992;118:335–46. doi: 10.1083/jcb.118.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Welch MD, Machesky LM, Bridgman PC, Meyer SM, Cooper JA. Visualization and molecular analysis of actin assembly in living cells. J Cell Biol. 1998;143:1919–30. doi: 10.1083/jcb.143.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–90. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Somogyi K, Rorth P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev Cell. 2004;7:85–93. doi: 10.1016/j.devcel.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–7. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Takeichi M. Mammalian Fat1 cadherin regulates actin dynamics and cell-cell contact. J Cell Biol. 2004;165:517–28. doi: 10.1083/jcb.200403006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear MA, Cooper JA. Capping protein: new insights into mechanism and regulation. Trends Biochem Sci. 2004;29:418–28. doi: 10.1016/j.tibs.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Whited JL, Cassell A, Brouillette M, Garrity PA. Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons. Development. 2004;131:4677–86. doi: 10.1242/dev.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Bell JB, Carroll SB. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes Dev. 1991;5:2481–95. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Carroll SB. Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development. 1993;117:571–584. doi: 10.1242/dev.117.2.571. [DOI] [PubMed] [Google Scholar]
- Winder SJ, Ayscough KR. Actin-binding proteins. J Cell Sci. 2005;118:651–4. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]


