Abstract
At micromolar concentrations, many small molecules self-associate into colloidal aggregates that non-specifically inhibit enzymes and other proteins. Here we describe a protocol for identifying aggregate-based inhibitors and distinguishing them from small molecules that inhibit via specific mechanisms. As a convenient proxy for promiscuous, aggregate-based inhibition, we monitor inhibition of β-lactamase in the absence and presence of detergent. Inhibition that is attenuated in the presence of detergent is characteristic of an aggregate-based mechanism. In the 96-well-format assay described here, about 200 molecules can be tested, in duplicate, per hour for detergent-dependent sensitivity. Furthermore, we also describe simple experiments that can offer additional confirmation of aggregate-based inhibition.
INTRODUCTION
Small molecules that specifically inhibit enzymes or modulate protein function are intensely sought. The dominant technique for discovering such ligands is high-throughput screening (HTS). In HTS, large libraries of small molecules are assayed for modulation of a target. Although this technique has had important successes, it is plagued by false-positive ligands. These artifactual ‘hits’ can outnumber the true inhibitors in a HTS ‘hit list’. Several mechanisms have been proposed to explain the prevalence of these artifactual hits, including oxidation potential1, chemical reactivity2 and spectral properties that interfere with assay readout3. One of the more common mechanisms underlying false-positive inhibition is the formation of colloidal aggregates through the self-association of organic molecules in aqueous solutions (Fig. 1)4-9. These aggregates typically form at micromolar concentrations and are often several hundred nanometers in diameter. Once formed, they sequester proteins and non-specifically inhibit their activity. A wide range of molecules behave this way at screening-relevant concentrations, including Lipinski-compliant members of screening libraries, bona fide leads for drug discovery and even drugs6,8,9. If the number of different types of molecules that can behave in this way is surprising, so too is the actual number of molecules that do so. At 30 μM, up to 19% of ‘drug-like’ molecules can form aggregates. At 5 μM, about 1-2% of ‘drug-like’ molecules seem to behave in this way, which is still a large percentage considering that many HTS campaigns aim for a hit rate of less than 1% (ref. 5). Here we describe a counter-screen for aggregation that can be deployed on a library-wide level, as well as a checklist of experiments that can be used to confirm whether individual inhibitors are acting through an aggregation-based mechanism (see ANTICIPATED RESULTS).
Figure 1.
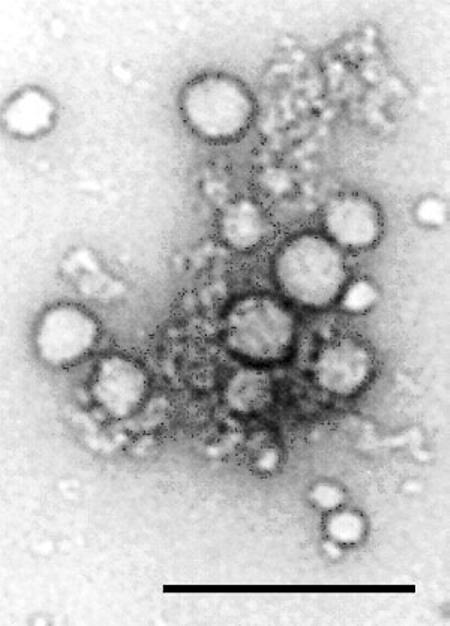
Transmission electron micrograph of aggregates of tetraiodophenolphthalein (dark edged circles) associated with β-galactosidase. This association inhibits the enzyme by sequestering it from substrate, although whether these are adsorption or absorption effects remains uncertain at this time. Bar, 200 nm. Reproduced from ref. 7
Several alternatives to this assay exist, among which are direct physical measurement of aggregation by dynamic light-scattering (DLS) and apparent solubility by nephelometry. Although it is more direct, we found DLS to be both time-consuming and harder to interpret, owing to problems with signal-to-noise in the plate-based prototype instrument we were using. The detergent-sensitive enzyme-inhibition assay, although it is admittedly less direct than physical measurement of particle formation, was found to be more robust, faster and more amenable to miniaturization. This protocol was previously described in ref. 5, and general discussions of the approach are presented in refs. 7 and 10. Note that in ref. 5, the concentration of detergent used was 0.1% vol/vol Triton X-100. Here we recommend a lower percentage, 0.01% vol/vol, which is better tolerated by the enzyme.
Experimental design
The counter-screen relies on the detergent-sensitive nature of aggre-gate-based inhibition: molecules that inhibit an enzyme in the absence, but not the presence, of detergent are likely to be inhibiting via the formation of promiscuous aggregates. Thus, the first step of the experiment is to screen molecules for inhibition. AmpC β-lactamase (AmpC) is a convenient choice of enzyme because it has been extensively studied for aggregate-based inhibition, and because it is one of the more sensitive enzymes tested for this effect. In principle, however, any soluble enzyme can be used for this protocol, as compound aggregation is a physical property of small molecules in aqueous buffers. The only limitation to using other enzymes is how well tolerated Triton X-100 is in different assay systems. For systems that do not tolerate non-ionic detergent, 1 mg ml-1 BSA might be considered as a replacement. However, BSA can sequester monomeric small molecules, so this should be used with caution.
The experiments described in this protocol are also amenable to either scale-up or scale-down. There are no problems running these experiments in lower-throughput cuvette-based formats. Higher-throughput formats, such as 1,536-well plates, are conceivable. We recommend that experiments be conducted at least in duplicate, to ensure statistical significance — aggregating molecules can be capricious, and are sensitive to assay conditions and target enzyme concentration, as well as centrifugation, vortexing and other invasive mixing procedures.
If this protocol is applied to a different enzyme system or significantly different assay conditions, it might be necessary to derive new cut-offs for detecting aggregate-based inhibition. To establish what constituted significant inhibition in the development of our technique, we used a panel of known aggregators and non-aggregators. These molecules are listed on our website (http://shoichetlab.compbio.ucsf.edu) and can be screened as positive controls to identify the window of relevant inhibition (see ANTICIPATED RESULTS and ref. 5).
Finally, screening for inhibition in the presence of detergent can be carried out independently from screening for inhibition in the absence of detergent — each experiment can be done separately and the procedure for each is the same. However, when adding detergent individually to each well, add it to buffer before any other components.
MATERIALS
REAGENTS
Reaction buffer: 50 mM potassium phosphate (25 mM KH2PO4 + 25 mM K2HPO4), pH 7
Detergent reaction buffer: 50 mM potassium phosphate + 0.01% Triton X-100 vol/vol, pH 7 ▲ CRITICAL Aqueous Triton X-100 solutions become less effective over time and should be made fresh daily
Protein: 0.00162 mg ml-1 AmpC, containing 0.0006% Triton X-100 vol/vol in KPi; 0.00162 mg ml-1 for 30×; store at 4 °C; the preparation of AmpC has been previously described6,11; exploratory amounts are available from the Shoichet Laboratory ▲ CRITICAL Prepare working enzyme stocks daily from higher concentration stocks (>1 mg ml-1). Enzyme will gradually adsorb to the surface of the container, thus decreasing the concentration of free enzyme. The small amount of detergent added to the enzyme stock will attenuate this effect
Substrate: nitrocefin; 5 mM stock in DMSO; Remel/Oxoid (cat. nos. 651063/BR0063A, respectively) ▲ CRITICAL Store solid stocks at 4 °C and DMSO stocks at -20°C
Compounds: 10 mM stocks in DMSO ▲ CRITICAL DMSO concentration in the reaction should be minimized. AmpC tolerates concentrations <4% vol/vol without serious effect
EQUIPMENT
A UV-visible plate reader
A 96-well-format liquid-handling instrument, such as the Biomek FX (Beckman)
96-well-format tips for use with a liquid-handling robot (e.g., Molecular Bioproducts cat. nos. 919-262-05 and 918-262-05)
96-well plates for reagents and dilutions (e.g., Grenier 96-well plates, cat. no. 65020)
UV-transparent 96-well plates for reactions (e.g., Corning cat. no. 3679)
PROCEDURE
A detergent-based counter-screen for aggregation-based inhibition
Add buffer to each well. For a final reaction volume of 150 μl, pipette 142 μl to × μl, where × is the amount of compound to be added.
Add 5 μl of 30× enzyme solution to the wells.
-
Add × μl of compound (or DMSO for uninhibited positive control) to the wells.
▲ CRITICAL STEP Only a few controls are necessary to establish the uninhibited rate of the reaction.
Mix by pipetting up and down.
-
Incubate compounds and enzyme for 5 min.
▲ CRITICAL STEP Aggregate-based inhibition is time dependent; therefore, the incubation time is key.
Add 3 μl of 5 mM nitrocefin.
Mix by pipetting up and down.
Monitor reaction at 482 nm for five minutes. This is the emission maximum for nitrocefin—however, off-peak wavelengths can also be used.
• TIMING
Timeline:
Steps 1-4: 5 min
Step 5: 5 min
Step 8: 5 min
? TROUBLESHOOTING
See Table 1.
ANTICIPATED RESULTS
Inhibition
The initial rate of each reaction is the slope of the best-fit line to the early kinetic data. The percentage of inhibited enzyme is defined as:
Here, vi and vc are the inhibited and uninhibited rates of reaction, respectively. We defined statistically significant inhibition as greater than 23.8%, based on the behavior of several known aggregators and non-aggregators at 30 μM (refs. 5,6). Less than 11% inhibition was considered insignificant. Intervening amounts of inhibition were ambiguous, and could indicate a tendency to aggregate at higher concentrations. These cut-offs were statistically obtained from the behavior of compounds in our hands and on our instruments, and thus only represent guidelines. These cut-offs might not be extendable to other enzyme systems, or to significantly different assay conditions or concentrations.
Effect of detergent
We consider a broad range of decrease in inhibition upon the application of detergent to be significant. Most aggregators show a greater than twofold decrease in percentage inhibition upon the application of 0.01% Triton X-100, vol/vol although not all aggregators have the same sensitivity to detergent5,7.
If a molecule exhibits significant inhibition of AmpC, which is diminished by detergent, it is almost certainly acting as an aggregation-based inhibitor. Marginal detergent sensitivity should not be considered a confirmation of true inhibition — some aggregators, such as Congo red, require 0.1% Triton X-100 vol/vol before inhibition is fully reversed. Addressing this widely varying sensitivity definitively might require multiple experiments with different concentrations of detergent or compound. That said, aggregation at one concentration does not rule out a specific mechanism at a lower concentration. If further experimental confirmation of aggregation is required, other hallmarks of this phenomenon can easily be assayed — e.g., the presence of particles detectable by light scattering, the time dependence of inhibition or the dependence of inhibition on enzyme concentration (see below). However, the detergent-dependent enzyme assay is relatively definitive and is the easiest to incorporate into a large-scale screening campaign. It represents a fast and simple way to screen for promiscuous aggregate-based inhibition.
Follow-up experiments for confirmation of aggregate-based inhibition
When testing only a small number of interesting molecules for aggregation-based inhibition, more detailed (and time-consuming) investigation might be called for. The following experiments, in decreasing order of facility, might be useful.
Is inhibition significantly attenuated by small amounts of non-ionic detergent? If so, the compound is likely to be acting through aggregation. We typically use 0.01% Triton-X 100 vol/vol, while others favor Tween-20 or CHAPS12, and we have also used saponin10 and digitonin. Other non-ionic detergents might also work.
In assays that cannot tolerate detergent (e.g., cell-based assays), it might be possible to use high concentrations of serum albumin6. This is currently under investigation — a drawback of this method is that albumin can also sequester well-behaved molecules.
Is inhibition significantly attenuated by increasing enzyme concentration? If so, the compound is likely to be an aggregator. Except when the receptor concentration-to-Ki ratio is high13, increasing receptor concentration should not affect percentage inhibition. Of course, when the receptor is membrane-bound or intracellular, this is difficult to probe.
Is inhibition competitive? If so, the compound is unlikely to be an aggregator. Does the inhibitor retain activity after spinning for several minutes in a microfuge? If not, particle formation is likely (see below).
Can you directly observe particles in the 50-1,000 nm size range? We have typically used DLS for this. Formation of particles does not guarantee promiscuous inhibition, but it is a worrying sign.
Is the dose-response curve unusually steep? There are classical reasons for steep dose-response curves13, but it too is a worrying sign.
Few, if any, of these experiments are definitive by themselves, although the detergent test is fairly reliable. When several of these tests are combined, they are strong indicators of aggregation-based or non-aggregation-based mechanisms of action. Further discussion of characteristic features of aggregation-based inhibition can be found in refs. 6-9.
TABLE 1.
Troubleshooting table.
| PROBLEM | POSSIBLE REASON | SOLUTION |
|---|---|---|
| Reaction rate is low or drops over the course of multiple experiments | Enzyme adsorption to the reagent container | Prepare new enzyme stocks throughout the experiments Reuse the enzyme reagent container |
| High variation in control reaction rates | Poor mixing | Mix more thoroughly |
| Poor reversibility in detergent experiments | Triton X-100 has gone bad | Prepare fresh Triton X-100 |
| Sudden large jumps in absorbance at 482 nm | Popping bubbles from the presence of Triton X-100 | Mix less vigorously and ensure mixing takes place below the liquid level of the well |
Acknowledgments
ACKNOWLEDGMENTS We thank A. McReynolds and K. Coan for reading this manuscript, K.C. for thoughtful discussions and GM71630.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
References
- 1.Hajduk PJ, Huth JR, Fesik SW. Druggability indices for protein targets derived from NMR-based screening data. J. Med. Chem. 2005;48:2518–2525. doi: 10.1021/jm049131r. [DOI] [PubMed] [Google Scholar]
- 2.Rishton GM. Reactive compounds and in vitro false positives in HTS. Drug Discov. Today. 1997;2:382–384. [Google Scholar]
- 3.Walters WP, Namchuk M. Designing screens: how to make your hits a hit. Nat. Rev. Drug Discov. 2003;2:259–266. doi: 10.1038/nrd1063. [DOI] [PubMed] [Google Scholar]
- 4.Feng B, Shoichet BK. Synergy and antagonism of promiscuous inhibition in multiple-compound mixtures. J. Med. Chem. 2006;49:2151–2154. doi: 10.1021/jm060029z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng BY, et al. High-throughput assays for promiscuous inhibitors. Nat. Chem. Biol. 2005;1:146–148. doi: 10.1038/nchembio718. [DOI] [PubMed] [Google Scholar]
- 6.McGovern SL, Caselli E, Grigorieff N, Shoichet BK. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J. Med. Chem. 2002;45:1712–1722. doi: 10.1021/jm010533y. [DOI] [PubMed] [Google Scholar]
- 7.McGovern SL, Helfand BT, Feng BY, Shoichet BK. A specific mechanism of nonspecific inhibition. J. Med. Chem. 2003;46:4265–4272. doi: 10.1021/jm030266r. [DOI] [PubMed] [Google Scholar]
- 8.McGovern SL, Shoichet BK. Kinase inhibitors: not just for kinases anymore. J. Med. Chem. 2003;46:1478–1483. doi: 10.1021/jm020427b. [DOI] [PubMed] [Google Scholar]
- 9.Seidler J, McGovern SL, Doman T, Shoichet BK. Identification and prediction of promiscuous aggregating inhibitors among known drugs. J. Med. Chem. 2003;46:4477–4486. doi: 10.1021/jm030191r. [DOI] [PubMed] [Google Scholar]
- 10.Seidler J, McGovern SL, Doman TN, Shoichet BK. Identification and prediction of promiscuous drugs. J. Med. Chem. 2003;46:4477–4486. doi: 10.1021/jm030191r. [DOI] [PubMed] [Google Scholar]
- 11.Weston GS, Blazquez J, Baquero F, Shoichet BK. Structure-based enhancement of boronic acid-based inhibitors of AmpC β-lactamase. J. Med. Chem. 1998;41:4577–4586. doi: 10.1021/jm980343w. [DOI] [PubMed] [Google Scholar]
- 12.Ryan AJ, Gray NM, Lowe PN, Chung C. Effect of detergent on “promiscuous” inhibitors. J. Med. Chem. 2003;46:3448–3451. doi: 10.1021/jm0340896. [DOI] [PubMed] [Google Scholar]
- 13.Straus OH, Goldstein A. Zone behavior of enzymes: illustrated by the effect of dissociation constant and dilution on the system cholinesterase-physostigmine. J. Gen. Physiol. 1943;26:559–585. doi: 10.1085/jgp.26.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]


