Abstract
Pituitary tumors cause considerable morbidity due to local invasion, hypopituitarism, or hormone hypersecretion. In many cases, no suitable drug therapies are available, and surgical excision is currently the only effective treatment. We show here abundant expression of nuclear hormone receptor PPAR-γ in all of 39 human pituitary tumors. PPAR-γ activating thiazolidinediones (TZDs) rosiglitazone and troglitazone induced G0-G1 cell-cycle arrest and apoptosis in human, rat somatolactotroph, and murine gonadotroph pituitary tumor cells, and suppressed in vitro hormone secretion. In vivo development and growth of murine somatolactotroph and gonadotroph tumors, generated by subcutaneous injection of prolactin-secreting (PRL-secreting) and growth hormone–secreting (GH-secreting) GH3 cells, luteinizing hormone–secreting (LH-secreting) LβT2 cells, and α-T3 cells, was markedly suppressed in rosiglitazone-treated mice, and serum GH, PRL, and LH levels were attenuated in all treated animals (P < 0.009). These results demonstrate that PPAR-γ is an important molecular target in pituitary adenoma cells and PPAR-γ ligands inhibit tumor cell growth and GH, PRL, and LH secretion in vitro and in vivo. TZDs are proposed as novel oral medications for managing pituitary tumors.
Introduction
PPAR-γ, a member of the nuclear receptor superfamily (1, 2), functions as a transcription factor mediating ligand-dependent transcriptional regulation (3–5). High-affinity PPAR-γ ligands include the insulin-sensitizing thiazolidinedione compounds (TZDs) rosiglitazone and troglitazone (6–8). PPAR-γ activation leads to adipocyte differentiation (6), glucose regulation (9), inhibition of macrophage and monocyte activation (10, 11), and anti-angiogenesis (12). PPAR-γ is expressed in breast, prostate, and colon epithelium, and administration of synthetic PPAR-γ ligands inhibits prostate and colon tumor cell growth (13–16).
Pituitary tumors account for approximately 15% of intracranial tumors and are associated with significant morbidity due to local compressive effects, hormonal hypersecretion, or treatment-associated endocrine deficiency (17, 18). Some pituitary tumors secrete hormones such as prolactin (PRL) and growth hormone (GH), and in most of these PRL- and GH-secreting pituitary tumors, dopamine agonists and/or somatostatin analogues effectively suppress PRL and GH hypersecretion, respectively, and control tumor growth or induce tumor shrinkage (19, 20). Nevertheless, a subset of patients with PRL- and GH-secreting pituitary tumors do not respond to or are intolerant of these drugs. The most commonly encountered pituitary tumors do not secrete hormones efficiently and are termed nonfunctioning adenomas. These are generally macroadenomas, and cause significant morbidity and ultimately mortality due to visual-field loss, headache, and pituitary dysfunction (21). Some nonfunctioning pituitary tumors express dopamine and/or somatostatin receptors, but their response to treatment with dopamine agonists and/or somatostatin is poor, and use of these drugs has largely been discontinued in patients harboring nonfunctioning tumors (22). No effective drug therapies for nonfunctioning pituitary tumors currently exist.
For unresponsive GH- and PRL-secreting, and nonfunctioning pituitary tumors, surgery with or without adjuvant radiation is the treatment mainstay; this treatment has a 50–60% overall control rate in specialized centers (21, 23–24). Although 70% of pituitary microadenomas are successfully resected by transsphenoidal approaches, 25% of PRL-secreting, and 90% of GH-secreting and nonfunctioning tumors are more than 1 cm in diameter at the time of presentation; surgical “cure” rates for these macroadenomas are achieved in about a third of patients in specialized centers (25). Tumor recurrence requires pituitary-directed radiation to suppress tumor growth and hormonal levels, but radiation effects may not be manifest for several years and are ultimately associated with pituitary damage and dysfunction in most patients (26).
Here we report that pituitary PPAR-γ is abundantly expressed in human PRL-, GH-secreting, and nonfunctioning pituitary tumors. PPAR-γ ligands potently inhibit PRL-, GH- and luteinizing hormone–secreting (LH-secreting) pituitary tumor proliferation in vitro and inhibit pituitary tumor growth and PRL, GH, and LH secretion in vivo. These results support a role for PPAR-γ as a novel molecular target for treating patients with nonfunctioning pituitary tumors and PRL- and GH-secreting pituitary tumors that are unresponsive to current dopamine agonists and/or somatostatin receptor antagonists.
Methods
Patients and tissues.
Surgically excised human pituitary tumor tissues were obtained from consecutive unselected patients after surgical resection in accordance with Institutional Review Board guidelines of Cedars-Sinai Medical Center. Tissue aliquots were snap-frozen in liquid nitrogen for later Western blot analysis. Other tissue aliquots were fixed in 4% paraformaldehyde and processed for paraffin embedding. Sections were immunostained using antibodies to human GH, PRL, follicle-stimulating hormone (FSH), and/or LH (1:1,000; DAKO Corp., Carpinteria, California, USA), and PPAR-γ (1:500; Calbiochem-Novabiochem Corp., La Jolla, California, USA) using avidin-biotin-peroxidase, and were counterstained with hematoxylin. Negative controls were performed for each slide using preabsorbed or nonimmune serum.
For primary human pituitary cultures, pituitary tumor tissue freshly obtained at surgery was minced mechanically and digested for 45 minutes at 37°C with 0.35% collagenase and 0.1% hyaluronidase (Sigma-Aldrich, St. Louis, Missouri, USA) in 10 ml DMEM. Cell suspensions were filtered and resuspended for 24 hours in low-glucose DMEM containing 10% FBS, 2 mmol/l glutamine, and antibiotics prior to treatment with vehicle or rosiglitazone.
Cell culture.
Subconfluent rat GH3 (American Type Culture Collection, Rockville, Maryland, USA) and mouse αT3 and LH-secreting gonadotroph tumor cells (LβT2) pituitary cells (gifts from P. Mellon, University of California San Diego, San Diego, California, USA) were cultured in DMEM (Invitrogen Corp., Grand Island, New York, USA) supplemented with antibiotics, 15% FCS, and 2.5% horse serum or 10% FCS, respectively, at 37°C in 5% CO2 for 24 hours prior to treatment with troglitazone or rosiglitazone (TZDs). Medium was replenished with ligand every 2 days, and cells were maintained in serum-containing medium for up to 96 hours.
Animals.
In accordance with Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center guidelines, 4-week-old female Nu/Nu mice were inoculated with rat pituitary GH- and PRL-secreting GH3, mouse gonadotroph αT3, and LH-secreting LβT2 tumor cells (∼200, 000), and animals were randomized to receive rosiglitazone (20–150 mg/kg/d) or vehicle. Mice were euthanized by CO2 inhalation, tumors were weighed, and aliquots were frozen and stored for subsequent analysis.
Cell cycle distribution.
Following treatments, GH3, αT3, LβT2, or human pituitary tumor cells were trypsinized, centrifuged (6225 g for 2 minutes), washed with PBS, and treated with 20 g/ml RNase A (Calbiochem-Novabiochem Corp.). DNA was stained with 100 μg/ml propidium iodide for 30 minutes at 4°C and protected from light prior to analysis with a FACScan (Becton, Dickinson and Co., Franklin Lakes, New Jersey, USA). DNA histogram analysis was performed using ModFit LT software (Verity Software House Inc., Topsham, Maine, USA).
Apoptosis assay: annexin-FITC by flow cytometry.
GH3, αT3, and human pituitary tumor cells were treated, washed in PBS, trypsinized, centrifuged, and washed prior to incubation with an FITC-labeled monoclonal annexin antibody and propidium iodide for 30 minutes at room temperature according to the manufacturer’s instructions (Pharmingen, San Diego, California, USA). Approximately 1 × 106 cells were prepared and analyzed by flow cytometry, propidium iodide–labeled nuclei were gated on light scatter to remove debris, and an FITC-labeled antibody against annexin was used to determine the percentage of annexin-FITC–labeled cells. Annexin-FITC–positive cells were also visualized and photographed with an Olympus BH2 immunofluorescent microscope and AlphaImager (Alpha Innotech Corp., San Leandro, California, USA).
TUNEL.
Apoptosis-induced nuclear DNA fragmentation was detected using the TMR Red In Situ Cell Death Detection Kit (Roche Diagnostics Corp., Indianapolis, Indiana, USA) following the manufacturer’s protocol. Briefly, 48 hours after TZD treatment, GH3, αT3, and human pituitary tumor cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% sodium citrate and 0.6% Tween-20 (pH 7.4) for 2 minutes at 4°C. This was followed by incubation in TUNEL reaction mix (TMR Red/dNTP mix, terminal deoxynucleotidyl transferase, and labeling buffer) for 60 minutes at 37°C. Slides were washed three times (5 minutes for each wash) in PBS/Triton X-100/BSA (0.3%) and visualized on an Olympus BH2 immunofluorescent microscope.
Northern blot analysis.
Total RNA was extracted from cell cultures (∼3 × 107 cells/group) or from excised tissues with TRIzol (Invitrogen Corp.). Rat testis RNA served as a positive control for expression of pituitary tumor transforming gene (PTTG). Electrophoresed RNA was transferred to Hybond N nylon membranes (Amersham International, Buckinghamshire, United Kingdom) and hybridized as previously described (27).
Western blot analysis.
GH3, αT3, and pituitary tumor cells were seeded in 6-well plates for 24 hours prior to treatment with TZD. Cells were then washed with PBS, and protein samples were prepared in RIPA buffer and resolved under reducing conditions on 12% SDS-polyacrylamide gels using standard methods (27). Pituitary tumor tissue protein extracts were prepared in a similar manner. Resolved proteins were transferred to nitrocellulose membranes and probed with antibodies against Bcl-2 (1:500), Bax (1:1,000; Santa Cruz Biotechnology, Santa Cruz, California, USA), pRb (Ser795) (1:1,000), and cleaved and uncleaved caspase-3 (1:500; Cell Signaling Technology Inc., Beverly, Massachusetts, USA) overnight at 4°C. After washing, membranes were incubated with appropriate IgG-HRP conjugates, and immunoreactive protein bands were visualized with ECL (Amersham International). Total protein was normalized by reprobing with anti-actin antibody (1:5,000; Sigma-Aldrich) or Ponceau S staining, and protein bands were quantified by densitometry (Video Densitometer model 620; Bio-Rad Laboratories Inc., Hercules, California, USA).
Statistical analysis.
Assays were performed in triplicate on at least two separate occasions. ANOVA (Kruskal-Wallis) with Dunn’s multiple comparison test or two-tailed nonparametric student t tests were employed as appropriate. P values < 0.05 were considered significant.
Results
PPAR-γ is abundantly expressed in pituitary tumors.
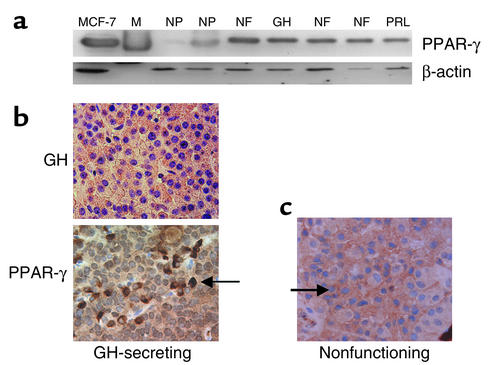
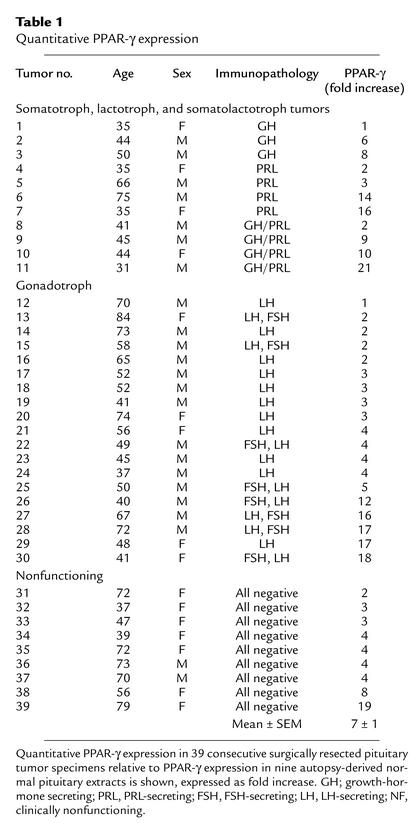
PPAR-γ expression was assessed in 39 consecutive surgically resected pituitary tumor specimens, classified according to tumor immunoreactivity. Three tumors were GH-immunopositive, four were PRL-immunopositive, four were both PRL- and GH-immunopositive, 19 were FSH- and/or LH-immunopositive, and nine were nonfunctioning (lacking hormone immunopositivity). Western blotting of pituitary tumor–derived protein extracts revealed abundant PPAR-γ expression in all 39 pituitary tumors examined, compared with modest PPAR-γ expression in nine normal pituitary–derived extracts (fold increase: 7 ± 1, mean ± SEM) (Figure 1a and Table 1). Immunocytochemical analysis demonstrated abundant heterogeneous PPAR-γ immunoreactivity in pituitary adenoma cells (Figure 1, b and c).
Figure 1.
PPAR-γ is abundantly expressed in human pituitary tumors. Western blot (a) and immunocytochemical (b and c) analysis depicting abundant PPAR-γ expression in surgically resected pituitary tumors, in comparison to PPAR-γ expression in two normal pituitary tissue extracts (NP). β-actin immunoblotting confirmed equivalent total protein loading. MCF-7 breast cancer cells served as a positive control. M, protein marker; NF, nonfunctioning; GH, GH-secreting; PRL, PRL-secreting. Arrow indicates PPAR-γ immunopositive cells.
Table 1.
Quantitative PPAR-γ expression
PPAR-γ ligands inhibit pituitary tumor proliferation.
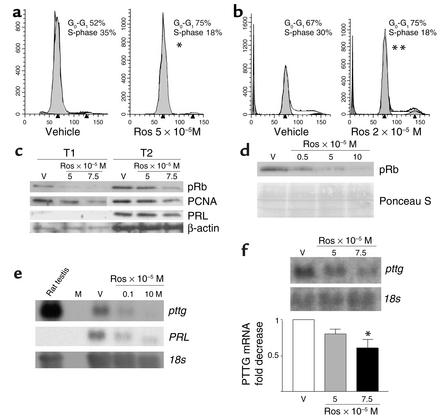
To determine the functional significance of pituitary tumor PPAR-γ expression, effects of PPAR-γ ligands were tested on pituitary tumor cells in vitro. Rosiglitazone treatment (5 × 10–5 M for 96 hours) of human nonfunctioning pituitary tumor cells (n = 3 individual tumors) in vitro, for up to 96 hours in serum-repleted conditions, induced G0-G1 cell-cycle arrest (control G1, 52% ± 4% vs. rosiglitazone-treated G1, 75% ± 6%, P = 0.09) and decreased the number of cells that entered S phase (S, 35% ± 2% vs. rosiglitazone-treated S, 18% ± 4%, P = 0.05) (Figure 2a). Troglitazone or rosiglitazone treatment (10–6 to 10–4 M) of GH3 (PRL- and GH-secreting), αT3 (gonadotroph), and human pituitary tumor cells for up to 48 hours in serum-free conditions, or up to 96 hours for cells maintained in serum-repleted conditions, yielded similar results, as evidenced by a G0/G1 arrest (GH3: control G1, 79 ± 4% vs. rosiglitazone-treated G1, 86 ± 4%, P = 0.0006; αT3: control G1, 67 ± 0.5% vs. rosiglitazone-treated G1, 75 ± 0.5%, P = 0.01). Troglitazone or rosiglitazone treatment also led to decreased numbers of cells in S phase (GH3: control, 16 ± 3.2% vs. rosiglitazone-treated, 12 ± 2.9%, P = 0.006; αT3: control, 30 ± 0.5% vs. rosiglitazone-treated, 18 ± 2.5%, P = 0.03) (Figure 2b). Western blot analysis of protein extracts derived from TZD-treated human nonfunctioning and PRL-secreting (depicted) pituitary tumor cells (Figure 2c) and murine gonadotroph αT3 cells (Figure 2d) showed a dose-dependent decrease of approximately 60% in Ser795 phosphorylated retinoblastoma, decreased proliferating cell nuclear antigen, and decreased PRL expression (Figure 2c), confirming decreased proliferative rates and indicating a checkpoint mechanism for the observed G0/G1 cell-cycle arrest.
Figure 2.
PPAR-γ function in pituitary tumors. FACS and Western blot analysis of rosiglitazone-treated (10–6 to 10–5 M) human nonfunctioning and PRL-secreting pituitary tumor cells (a) and murine gonadotroph tumor cells (b) revealed increased number of cells in G1 and decreased S phase (*P = 0.001; **P = 0.03) and a dose-dependent decrease in Ser795 phosphorylated retinoblastoma protein (c and d) in addition to decreased proliferating cell nuclear antigen (PCNA) and PRL expression (c). Northern blot analysis following rosiglitazone treatment revealed decreased expression of proliferative marker PTTG mRNA and PRL mRNA expression in rat GH3 cells (e) and decreased PTTG in αT3 gonadotroph cells (f). Rat testis RNA served as a positive control. 18S, β-actin markers, or Ponceau S staining confirmed RNA and protein loading. Ros, rosiglitazone; V, vehicle; M, RNAmarker.
Northern blot analysis of total RNA extracted from TZD-treated GH3 and αT3 cells revealed that the ligand induced a decrease of approximately threefold in expression of the proliferative marker (PTTG) mRNA, confirming decreased proliferative rates of TZD-treated gonadotroph tumor cells (Figure 2, e and f) in addition to decreased PRL mRNA expression (Figure 2e).
PPAR-γ ligands induce pituitary tumor apoptosis in vitro.
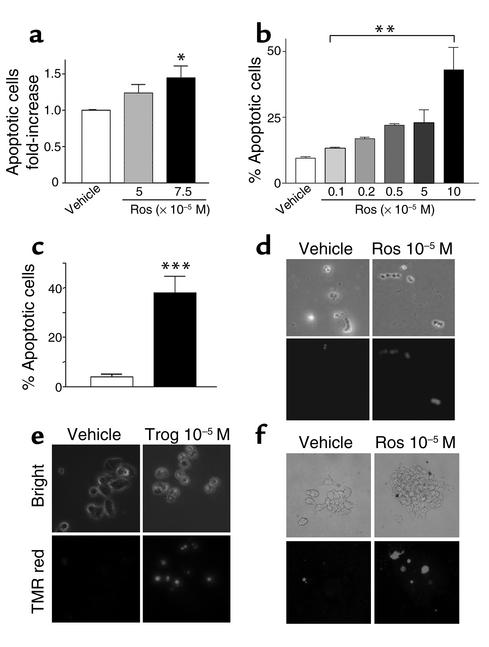
Rosiglitazone treatment (10–6 to 10–4 M) of rat GH3, murine αT3, and human pituitary tumor cells for up to 96 hours demonstrated a dose-dependent increase in apoptosis, as evidenced by increased annexin-FITC–immunoreactive cells in human nonfunctioning pituitary tumors (n = 6) (Figure 3a) (fold change: control, 1 ± 0.005 vs. 1.45 ± 0.15; mean ± SEM, P = 0.045) and in murine gonadotroph LβT2 tumor cells (percentage of apoptotic cells: control, 9 ± 0.5 vs. 0.1 × 10–5 M rosiglitazone–treated, 13 ± 0.3; 0.2 × 10–5 M rosiglitazone–treated, 17 ± 0.5; 0.5 × 10–5 M rosiglitazone–treated, 22 ± 0.7; 5 × 10–5 M rosiglitazone–treated, 23 ± 0.5; 10 × 10–5 M rosiglitazone–treated, 43 ± 9; mean ± SEM, P = 0.003. TUNEL analysis of rosiglitazone-treated rat GH3, murine αT3, and human pituitary tumor cells confirmed that TZD induced cell death and increased apoptosis approximately threefold (Figure 3, b–e) (control, 4 ± 0.9 vs. 10–5 M troglitazone, 38 ± 6.8% apoptosis, P = 0.0002).
Figure 3.
FACS analysis demonstrated a dose-dependent increase in annexin-FITC immunoreactive human nonfunctioning (a) and murine gonadotroph (b) pituitary tumor cells following rosiglitazone treatment in vitro. TUNEL analysis showed increased TMR-red–immunoreactive rat GH3 (c and d), mouse αT3 gonadotroph (e), and human pituitary tumor (f) cells following rosiglitazone and troglitazone (trog) (10–5 M) treatment in vitro, confirming TZD-mediated induction of apoptosis (*P = 0.045; **P = 0.003; ***P = 0.0002). TMR red, tetramethylrhodamine-5 (and -6) red. Results are derived from six individual nonfunctioning pituitary tumor cultures and experiments were performed in triplicate wells.
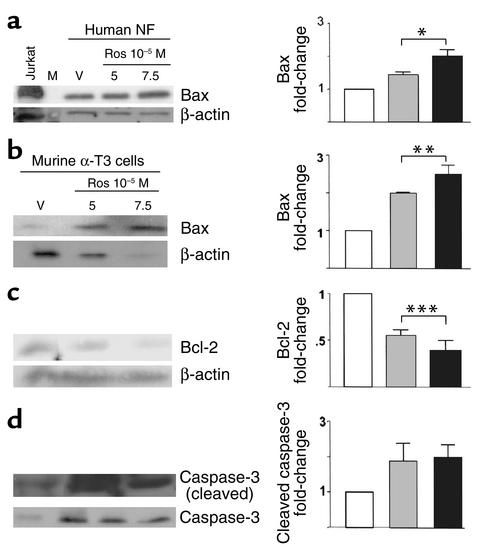
Western blot analysis of protein extracts derived from TZD-treated human nonfunctioning pituitary tumor cells revealed an increase of about twofold in levels of proapoptotic Bax expression (Figure 4a, P < 0.01), and analysis of TZD-treated murine gonadotroph αT3 pituitary tumor cells revealed an increase of about 2.5-fold in Bax expression (Figure 4b, P = 0.03) and decreased expression of the anti-apoptotic protein Bcl-2 (∼90%, Figure 4c, P = 0.02), consistent with the observed increased apoptosis. An increase of about twofold in cleaved caspase-3 and a concomitant decrease in total caspase-3 was observed after TZD treatment, also consistent with enhanced apoptosis (Figure 4d, P = 0.06).
Figure 4.
Mechanism of PPAR-γ–mediated apoptosis. Western blot analysis of rosiglitazone-treated human nonfunctioning and murine αT3 gonadotroph tumor cells demonstrated increased expression of the proapoptotic protein BAX (a and b) and decreased anti-apoptotic Bcl-2 (c). Dose-dependent increase in TZD-mediated cleaved caspase-3 and a concordant decrease in uncleaved caspase-3 supports the proapoptotic effect of PPAR-γ ligands in pituitary tumors (d). Jurkat B cell leukemia cells served as a positive control. αT3, murine αT3 gonadotroph tumor cells. *P < 0.01, **P < 0.03, ***P < 0.02.
PPAR-γ ligands inhibit pituitary tumor growth and GH, PRL, and LH secretion in vivo.
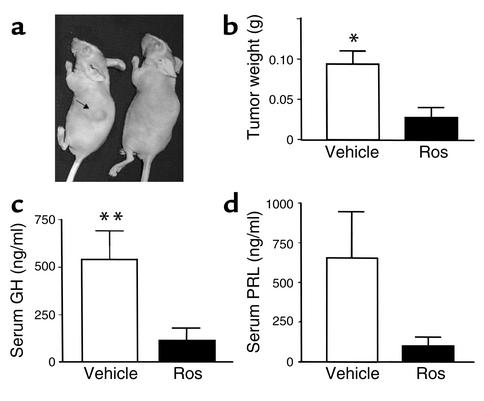
As the TZDs exhibited antiproliferative and proapoptotic effects in vitro, the effects of TZD treatment were assessed on somatolactotroph pituitary tumor growth in vivo. Four-week-old female athymic nude mice were inoculated subcutaneously with GH3 somatolactotroph cells (∼200,000 cells) and animals were randomized to receive either oral rosiglitazone (150 mg/kg/d) or vehicle. After 4 weeks, three of four vehicle-treated mice developed large visible tumors, necessitating their euthanization. In contrast, three of five rosiglitazone-treated mice developed visible subcutaneous tumors (1–2 cm in diameter), but tumor weight was markedly lower in rosiglitazone-treated mice (0.03 ± 0.01 g vs. vehicle, 0.09 ± 0.01 g; P = 0.008) (Figure 5, a and b). Consistent with reduced tumor size, serum GH (vehicle-treated mice, 545 ± 153 ng/ml; rosiglitazone-treated mice, 114 ± 66 ng/ml, P = 0.03) and PRL (vehicle, 656 ± 289; rosiglitazone-treated, 95 ± 58 ng/ml, P = not significant) levels were lower in rosiglitazone-treated mice (Figure 5, c and d).
Figure 5.
Rosiglitazone inhibits somatolactotroph pituitary tumor growth in vivo. Following subcutaneous inoculation of 4-week-old female athymic nude mice with GH3 pituitary tumor cells (∼200,000 cells), animals were randomized to receive either oral rosiglitazone (150 mg/kg/d) or vehicle. Representative photograph (a), tumor weights (b), serum GH (c), and PRL levels (d) after 4 weeks of treatment with vehicle or rosiglitazone. Five animals were in each group. *P = 0.008; **P = 0.03.
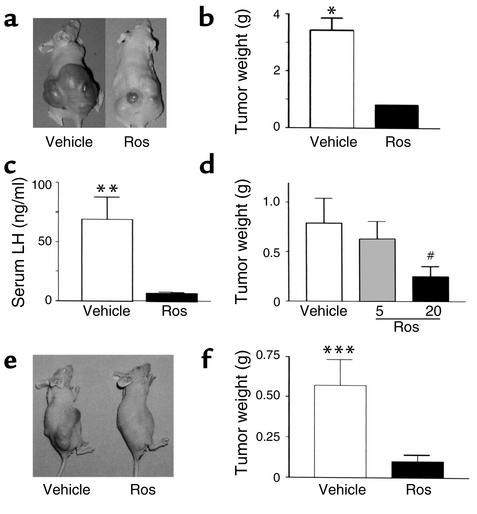
In separate parallel similarly conducted studies, LH-secreting gonadotroph tumor cells (LβT2 cells) were injected into mice as described above, and animals were randomized to receive rosiglitazone (150 mg/kg/d) or vehicle. All animals developed tumors by 6 weeks and were euthanized. Tumor weights (vehicle, 3.44 ± 0.42 g vs. rosiglitazone-treated, 0.81 ± 0.19 g, P < 0.002) (Figure 6, a and b) and plasma LH (vehicle, 69 ± 19 ng/ml vs. rosiglitazone-treated, 6.8 ± 1.0 ng/ml, P = 0.004) levels (Figure 6c) were markedly lower in rosiglitazone-treated mice than in vehicle-treated tumor-bearing animals. Similar tumor growth inhibitory effects were observed following administration of lower rosiglitazone doses of 20 mg/kg/d to animals inoculated with gonadotroph LβT2 cells (vehicle, 0.79 ± 0.25 g vs. rosiglitazone-treated, 0.25 ± 0.1 g, P = 0.04) (Figure 6d) and 50 mg/kg/d to animals inoculated with gonadotroph αT3 cells (vehicle, 0.56 ± 0.16 g vs. rosiglitazone-treated, 0.1 ± 0.04 g, P = 0.002) (Figure 6, e and f). Rosiglitazone (5 mg/kg/d) did not inhibit tumor growth (0.63 ± 0.17 g, P = not significant). These results confirm the potent anti-tumor PPAR-γ ligand effects in vivo and demonstrate the potential role of PPAR-γ ligands for therapy in gonadotroph and nonfunctioning pituitary tumors.
Figure 6.
Rosiglitazone treatment inhibits pituitary gonadotroph tumor growth and suppresses LH hormone levels in vivo (n = 5). Mice were inoculated subcutaneously with either LβT2 LH-secreting (a–d) or αT3 (e and f) gonadotroph pituitary tumor cells (∼200,000), and animals were randomized to receive either oral rosiglitazone (LβT2 cells, 5, 20, and 150 mg/kg/d; αT3 cells, 50 mg/kg/d) or vehicle. Representative photograph of tumors (a and e), tumor weights (b, d, and f), and serum LH (c) levels in LβT2 or αT3 gonadotroph tumor–bearing mice after 4 weeks of treatment with vehicle or rosiglitazone. There were five animals in each group. *P = 0.0002, **P = 0.004, ***P = 0.002, #P = 0.04 vs. vehicle.
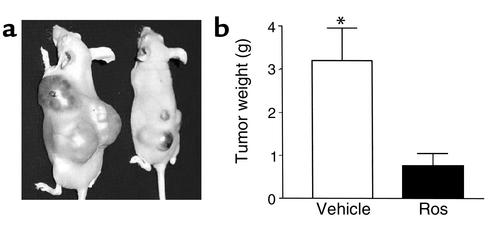
As patients invariably present with already established and actively growing pituitary tumors, the effects of TZD treatment were tested on growth of already established pituitary gonadotroph tumors in vivo. Mouse αT3 gonadotroph pituitary tumor cells were injected subcutaneously into athymic nude mice and tumors were allowed to develop. By 3 weeks after injection, all animals had developed large visible tumors and were then randomized to receive either oral rosiglitazone (150 mg/kg/d) or vehicle. Baseline tumor volumes were not different in the groups subsequently randomized as control or treated groups (data not shown, P = not significant). In both groups, tumor growth continued, but final tumor weights were abrogated in all of four rosiglitazone-treated animals (vehicle, 3.2 ± 0.76 g vs. rosiglitazone-treated, 0.76 ± 0.29 g; P = 0.005) (Figure 7, a and b).
Figure 7.
Rosiglitazone treatment retards growth of established pituitary gonadotroph tumors in vivo (n = 5). Mice were inoculated subcutaneously with αT3 gonadotroph pituitary tumor cells (∼200,000) and tumors were allowed to develop. By 3 weeks after inoculation, all animals had developed large visible tumors and were then randomized to receive either oral rosiglitazone (150 mg/kg/d) (n = 5) or vehicle (n = 5). Initial tumor volumes were comparable in the vehicle- and rosiglitazone-treated groups. After 4 more weeks, vehicle-treated control animals had become debilitated, necessitating euthanization. Tumor weights were abrogated in the rosiglitazone-treated animals. *P = 0.005.
Discussion
About 80% of nonfunctioning and GH-secreting and 25% of PRL-secreting pituitary tumors are macroadenomas (≥ 1 cm diameter) at the time of diagnosis, and cavernous sinus invasion or optic chiasm compression is commonly encountered (17, 21). No medical therapies are currently available for nonfunctioning pituitary tumors. Some PRL- and GH-secreting pituitary tumors fail to respond to dopamine agonists and/or somatostatin analogues, and few safe, effective alternative therapies exist for this subset of patients (21, 28). In experienced specialized centers, surgical resection of pituitary microadenomas (<1 cm diameter) offers an overall cure rate of about 70–80%, but for macroadenomas (>1 cm diameter), control rates approximate only 30%, and postoperative radiation is often administered to prevent tumor recurrence. The extensive surgical resection required also portends significant risk to surrounding normal pituitary tissue, leading to partial or total hypopituitarism in approximately 50% of cases (21, 25, 26).
PPAR-γ was abundantly expressed in all 39 pituitary tumors examined, compared with PPAR-γ expression in normal pituitary tissue. PPAR-γ protein immunostaining is heterogeneously distributed within the pituitary tumor cells themselves. As autopsy-derived normal pituitary tissues were harvested and frozen within 8 hours, it is unlikely that the observed low-level PPAR-γ expression is due to protein degradation, particularly as pituitary hormone immunostaining was intact.
TZD treatment of rat somatolactotroph, murine gonadotroph, and human pituitary tumor cells in vitro induced G0/G1 cell arrest, decreased expression of the critical cell-cycle regulatory protein Rb, and increased apoptosis, indicating the functional significance of the observed pituitary tumor PPAR-γ expression. Furthermore, TZD treatment led to marked abrogation of somatolactotroph and gonadotroph pituitary tumor growth, including regression of already established and actively growing pituitary gonadotroph tumors and suppression of GH, PRL, and LH secretion, respectively, although it is likely that the observed hormone suppression results from the reduction in tumor cell number caused by TZD treatment.
The lower doses of PPAR-γ ligands shown here to be effective for pituitary tumors in vitro (10–6 to 10–4 M) and in vivo (20–50 mg/kg/d) are similar to those previously shown to be effective in inhibiting development of atherosclerosis and prostate and thyroid cancer (15, 29, 30) but are higher than those found to modify insulin action (31). As we did not observe murine gonadotroph tumor growth inhibition using lower rosiglitazone doses (5 mg/kg/d), higher doses of PPAR-γ ligands than those currently used in diabetes may be required to inhibit pituitary tumor growth.
We recently reported selective PPAR-γ expression in normal human pituitary corticotrophs and showed that PPAR-γ ligands effectively suppress adrenocorticotrophic hormone in models of Cushing disease (32). However, following rosiglitazone treatment (150 mg/kg/d), normal mice exhibited an intact pituitary-adrenal axis in both unstressed and stressed conditions.
TZD’s have been shown to inhibit prostate, breast, and colorectal tumor growth in vitro (13–15). Several mechanisms have been proposed for TZD-induced cell-cycle arrest. These include p21 (CiP) (33), and p27 (Kip) (34) expression — mediated prevention of Rb-phosphorylation by inhibiting cyclin D1-kinase action (35), increased. Candidates that mediate TZD-induced apoptosis include reduced Bcl-2 and increased Bax and TRAIL (13, 36). Western blot analysis of TZD-treated pituitary tumor protein extracts revealed reduced phosphorylated Rb and Bcl-2 and increased Bax expression, providing a potential mechanism for the observed TZD-mediated cell-cycle arrest and apoptosis.
Much of the comorbidity encountered in patients harboring pituitary macroadenomas is due to hormonal deficiency caused by pituitary destruction or following surgical or radiotherapeutic pituitary ablation (18, 21, 25). In this regard, a potential role for TZDs in the management of pituitary tumors is compelling, because in addition to exerting inhibitory effects on pituitary tumor growth, they inhibit tumor GH, PRL, and LH secretion, as evidenced by reduced plasma GH, PRL, and LH in mice during 6 weeks of rosiglitazone treatment, although TZD-mediated hormonal inhibition may indeed be indirect.
As the sensitivity of some cancer cell lines to the growth-inhibitory effect of TZDs may not correlate closely with levels of PPAR-γ expression (37), some have suggested that TZD-mediated cell proliferation is independent of PPAR-γ expression (38). However, based on observed antiproliferative effects, high-affinity PPAR-γ ligands at higher concentrations than previously identified therapeutic ranges for these compounds represents a potential pituitary tumor therapy, especially given the current lack of medical treatment options for these tumor types.
Rosiglitazone, a potent TZD oral antidiabetic agent recently approved for use in the USA, differs structurally from pioglitazone and troglitazone, with greater PPAR-γ binding affinity and antihyperglycemic potency. Clinical surveillance of more than 4,500 patients has shown that rosiglitazone is a safe, effective mono- or combination therapy for patients with type 2 diabetes (39). Unlike troglitazone, which has been associated with idiosyncratic hepatotoxicity, rosiglitazone is associated with a low incidence of liver abnormalities, as documented in 3,500 patient-years of exposure. Given abundant PPAR-γ expression in pituitary tumors compared with normal pituitary tissue, a role for the use of PPAR-γ receptor ligands, such as rosiglitazone, is proposed for managing patients harboring GH- and PRL-secreting pituitary tumors, which are unresponsive to dopamine agonists and somatostatin receptor analogues. Evidence is also presented that PPAR-γ ligands are useful candidates for the management of nonfunctioning pituitary tumors, for which no medical therapies currently exist.
Acknowledgments
This work was supported by the Doris Factor Molecular Endocrinology Laboratory, the Annenberg Foundation, and NIH (grant CA-75979). We thank William Yong for neuropathological assistance.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: thiazolidinedione (TZD); prolactin (PRL); growth hormone (GH); luteinizing hormone (LH); follicle-stimulating hormone (FSH); LH-secreting gonadotroph tumor cells (LβT2); pituitary tumor transforming gene (PTTG).
References
- 1.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–660. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 2.Schoonjans K, Martin G, Staels B, Auwerx J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr. Opin. Lipidol. 1997;8:159–166. doi: 10.1097/00041433-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signaling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer CAN, Hsu MH, Griffin KJ, Johnson EF. Interaction of the peroxisome proliferator-activated receptor alpha with the retinoid X receptor alpha unmasks a cryptic peroxisome proliferator response element that overlaps an ARP-1-binding site in the CYP4A6 promoter. J. Biol. Chem. 1995;270:16114–16121. [PubMed] [Google Scholar]
- 5.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 6.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 7.Kliewer SA, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 8.Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J1 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 9.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 10.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 11.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 12.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem. 1999;274:9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 13.Staels B, et al. Activation of human aortic smooth-muscle cells is inhibited by PPAR-alpha but not PPAR-gamma activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 14.Elstner E, et al. Ligands for peroxisome proliferator-activated receptor-γ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota T, et al. Ligand for peroxisome proliferator-activated receptor-γ (troglitazone) has potent anti-tumor effects against prostate cancer both in vitro and in vivo. Cancer. Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 16.Sarraf P, et al. Differentiation and reversal of malignant changes in colon cancer through PPAR gamma. Nat. Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 17.Heaney AP, Melmed S. Molecular pathogenesis of pituitary tumors. Oxford textbook of endocrinology. JAH Wass and SM Shalet, editors Oxford University Press Oxford, United Kingdom. 2002;2:109–120. [Google Scholar]
- 18.Katznelson L, et al. Hypogonadism in patients with acromegaly: data from the multi-centre acromegaly registry pilot study. Clin. Endocrinol. 2001;54:183–188. doi: 10.1046/j.1365-2265.2001.01214.x. [DOI] [PubMed] [Google Scholar]
- 19.Molitch, M.E. 2001. Prolactinomas. In Prolactin. N.D. Horseman, editor. Kluwer Academic Publishers. Boston, Massachusetts, USA. 82–99.
- 20.Giustina A, et al. Criteria for cure of acromegaly: a consensus statement. J. Clin. Endocrinol. Metab. 2000;85:526–529. doi: 10.1210/jcem.85.2.6363. [DOI] [PubMed] [Google Scholar]
- 21.Nobels FR, et al. Long-term treatment with the dopamine agonist quinagolide of patients with clinically nonfunctioning pituitary adenoma. Eur. J. Endocrinol. 2000;143:615–621. doi: 10.1530/eje.0.1430615. [DOI] [PubMed] [Google Scholar]
- 22.Frohman LA. Acromegaly: what constitutes optimal therapy? J. Clin. Endocrinol. Metab. 1996;81:443–445. doi: 10.1210/jcem.81.2.8636245. [DOI] [PubMed] [Google Scholar]
- 23.Bevan JS, Burke CW. Nonfunctioning pituitary adenomas do not regress during bromocriptine therapy but possess membrane-bound dopamine receptors which bind bromocriptine. Clin. Endocrinol. 1986;25:561–572. doi: 10.1111/j.1365-2265.1986.tb03610.x. [DOI] [PubMed] [Google Scholar]
- 24.Freda PU, Wardlaw SL. Diagnosis and treatment of pituitary tumors. J. Clin. Endocrinol. Metab. 1999;84:3859–3866. doi: 10.1210/jcem.84.11.6202. [DOI] [PubMed] [Google Scholar]
- 25.Kreutzer J, Vance ML, Lopes MB, Laws ER., Jr Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J. Clin. Endocrinol. Metab. 2001;86:4072–4077. doi: 10.1210/jcem.86.9.7819. [DOI] [PubMed] [Google Scholar]
- 26.Brada M, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin. Endocrinol. 1993;38:571–578. doi: 10.1111/j.1365-2265.1993.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 27.Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. Early involvement of estrogen-induced pituitary tumor transforming gene (PTTG) and bFGF in pituitary tumor pathogenesis. Nat. Med. 1999;5:1317–1321. doi: 10.1038/15275. [DOI] [PubMed] [Google Scholar]
- 28.Lamberts SW, de Quijada M, Klijn JG. The effect of tamoxifen on GH and PRL secretion by human pituitary tumors. J. Endocrinol. Invest. 1980;3:343–347. doi: 10.1007/BF03349368. [DOI] [PubMed] [Google Scholar]
- 29.Li AC, et al. Peroxisome proliferator-activated receptor γ ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T. Ligands for peroxisome proliferator-activated receptor gamma inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2001;86:2170–2177. doi: 10.1210/jcem.86.5.7493. [DOI] [PubMed] [Google Scholar]
- 31.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with rosiglitazone. N. Engl. J. Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 32.Heaney AP, Fernando M, Yong W, Melmed S. Functional PPAR-γ receptor represents a novel therapeutic target in Cushing’s disease. Nat. Med. 2002;11:1281–1287. doi: 10.1038/nm784. [DOI] [PubMed] [Google Scholar]
- 33.Sugimura A, et al. Troglitazone suppresses cell growth of myeloid leukemia cell lines by induction of p21WAF1/CIP1 cyclin-dependent kinase inhibitor. Biochem. Biophys. Res. Commun. 1999;261:833–837. doi: 10.1006/bbrc.1999.1049. [DOI] [PubMed] [Google Scholar]
- 34.Motomura W, Okumura T, Takahashi N, Obara T, Kohgo Y. Activation of peroxisome proliferator-activated receptor gamma by troglitazone inhibits cell growth through the increase of p27KiP1 in human pancreatic carcinoma cells. Cancer Res. 2000;60:5558–5564. [PubMed] [Google Scholar]
- 35.Wakino S, et al. Peroxisome proliferator-activated receptor γ ligands inhibit retinoblastoma phosphorylation and G1 → S transition in vascular smooth cells. J. Biol. Chem. 2000;275:22435–22441. doi: 10.1074/jbc.M910452199. [DOI] [PubMed] [Google Scholar]
- 36.Goke R, Goke A, Goke B, Chen Y. Regulation of TRAIL-induced apoptosis by transcription factors. Cell. Immunol. 2000;201:77–81. doi: 10.1006/cimm.2000.1650. [DOI] [PubMed] [Google Scholar]
- 37.Mueller E, et al. Terminal differentiation of human breast cancer through PPAR-γ. Mol. Cell. 1998;1:465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 38.Aktas H, et al. Depletion of intracellular Ca2+ stores, phosphorylation of eIF2α, and sustained inhibition of translation initiation mediate the anticancer effects of clotrimazole. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8280–8285. doi: 10.1073/pnas.95.14.8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstein BJ. Rosiglitazone. Int. J. Clin. Pract. 2000;54:333–337. [PubMed] [Google Scholar]