Abstract
Insulin is a major target of the autoimmune response associated with destruction of pancreatic β cells in type 1 diabetes. A peptide that spans the junction of the insulin B chain and the connecting (C) peptide in proinsulin has been reported to stimulate T cells from humans at risk for type 1 diabetes and autoimmune diabetes–prone NOD mice. Here we show that proinsulin B24–C36 peptide binds to I-Ag7, the MHC class II molecule of the NOD mouse, and, after intranasal administration, induces regulatory CD4+ T cells that, in the absence of CD8+ T cells, block the adoptive transfer of diabetes. Curiously, however, intranasal B24–C36 did not inhibit development of spontaneous diabetes in treated mice. We then determined that B24–C36, and its core sequence B25–C34, bind to Kd, the NOD mouse MHC class I molecule, and elicit CD8+ CTLs. When the CD8+ T lymphocyte epitope was truncated at the C34 valine anchor residue for binding to Kd, the residual CD4+ T cell epitope, B24–C32/33, significantly inhibited diabetes development after a single intranasal dose. This study identifies a novel CTL epitope in proinsulin and demonstrates that the therapeutic potential of a “tolerogenic” autoantigen peptide can be compromised by the presence of an integral CTL epitope.
Introduction
In type 1 diabetes (T1D), T cells target and destroy insulin-producing β cells in the islets of the pancreas. Several lines of evidence indicate that proinsulin plays a key role as an autoantigen in driving this process. For example, susceptibility of humans to T1D maps to a variable number of tandem repeats 5′ of the insulin gene, the length of which correlates with thymic expression of proinsulin mRNA and inversely with disease risk (1, 2). In the NOD mouse, a model of spontaneous autoimmune diabetes, transgenic expression of proinsulin in APCs prevents mononuclear cell infiltration of the islets (insulitis) and diabetes (3). A peptide in human proinsulin that spans the cleavage site between the B chain of insulin and the connecting (C) peptide in proinsulin, B24–C36, was shown to stimulate peripheral blood T cells from humans at risk for T1D (4). Recently, a peptide spanning the B–C chain junction in mouse proinsulin II was shown to contain a naturally processed epitope for autoreactive T cells in young NOD mice (5).
Peptide autoepitopes are candidate therapeutic agents for preventing autoimmune disease (6). Mucosal administration of a peptide antigen can suppress subsequent systemic immune priming by the antigen (mucosal tolerance) and has been used to inhibit a range of experimental autoimmune diseases (6–8). We therefore administered proinsulin B24–C36 peptide intranasally to NOD mice in the expectation that it would inhibit the development of diabetes. However, although intranasal B24–C36 elicited antidiabetogenic regulatory T cells, it did not protect mice from diabetes. In exploring the reason for this, we determined that proinsulin B24–C36 is a novel “combitope” comprising not only an MHC class II–restricted T cell epitope that elicits regulatory CD4+ T cells but also an overlapping MHC class I–restricted epitope capable of eliciting CTLs that can nullify the therapeutic effect.
Methods
Mice.
NOD mice (Lt/Jax) were bred under specific pathogen-free conditions at The Walter and Eliza Hall Institute of Medical Research. Diabetes was diagnosed if two sequential measurements of retro-orbital venous blood glucose exceeded 11 mM.
Protein and peptides.
Mouse proinsulin II peptides B24–C36 (FFYTPMSRREVED), B24–C32, B24–C33, B25–C33, B26–C34, and B25–C34, hen-egg lysozyme (HEL) 10–23, and Listeria monocytogenes listeriolysin O (LLO) 91–99 (GYKDGNEYI) were synthesized and purified to greater than 95% homogeneity, determined by HPLC-MS, by Mimotopes (Melbourne, Australia). OVA (Sigma-Aldrich, St. Louis, Missouri, USA) and peptides were resuspended in PBS.
Intranasal administration of peptides.
In a series of preliminary experiments, unanesthetized female NOD mice (12 per group) were given either carrier PBS or 0.4, 4, 40, or 80 μg of mouse proinsulin B24–C36 in 10 μl PBS intranasally on three alternating days from 8 weeks of age, and their incidence of diabetes was monitored out to 26 weeks of age. The 0.4 and 4 μg of mouse proinsulin had no effect, whereas both the 40 and 80 μg of mouse proinsulin had a small (11%–24%) effect to decrease diabetes incidence. Subsequently, 40 μg of test or control peptide was given on three alternating days. In diabetes incidence studies, blood glucose was measured every 4 weeks from 100 days of age.
Adoptive transfer of diabetes.
Male 6- to 9-week-old NOD mice (8 or 12 per group) were irradiated (8 Gy) from a cobalt source and 3–6 hours later received 107 pooled diabetogenic splenocytes from recently NOD female mice, together with 107 splenocytes or cells fractionated from this number from either proinsulin peptide– or OVA-treated mice, in 200 μl via the tail vein. The onset of diabetes was monitored every 2 weeks by measuring blood glucose beginning 2 weeks after transfer.
Fractionation of spleen cells.
Splenic CD4+ and CD8+ cells were either selected or depleted by magnetic separation with monoclonal antibodies bound to MACS microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The yield of CD4+ and CD8+ cells was 70%–80% and 20%–30%, respectively, and their depletion or purity by flow cytometry more than 95% and more than 85%, respectively.
I-Ag7 binding assay.
Binding of peptides to purified, soluble I-Ag7 was measured in an ELISA as previously described (9). Competition dose-response binding curves were generated, and peptide binding affinity for I-Ag7 was expressed as an IC50, the molar concentration of peptide that inhibited by 50% binding of the indicator peptide, N-terminal biotinylated HEL 10–23.
Prediction of peptides that bind to MHC class I.
Peptides within mouse proinsulin II B24–C36 that could potentially bind to the NOD mouse MHC class I molecules, Kd and Db, were identified in the Web-based databases SYFPEITHI (10) and BIMAS (11).
Modeling of peptide binding to MHC molecules.
When homology modeling was performed, no crystal structures of Kd or I-Ag7 were available. The two crystal structures of I-Ag7 published subsequently (12, 13) agree in nearly all respects with the modeled structure used here (14). Modeling of I-Ag7 was based on the coordinates of the I-Ak /HEL 50-62 peptide complex (15) and was performed with the program Discover II of Accelrys (San Diego, California, USA). Modeling of Kd was based on the coordinates of HLA-A2 hepatitis B nucleocapsid 18–27 peptide complex (16) (Protein Data Bank access code: 1hhh.pdb [ref. 15]). We found that HLA-A2 is a better base molecule for modeling Kd than the more homologous mouse Kb, giving better agreement with binding and T cell proliferation data for variants of the insulin B15–23 CTL epitope (17). The modeling procedure for Kd was identical to that for I-Ag7, at an ambient pH of 7.0. The models are shown in solid-surface representation with colors according to the surface electrostatic potential (gray, neutral; blue, positive; red, negative). The peptide residues are in van der Waals space-filling form, whereas selected MHC heavy chain residues shown are in stick representation (oxygen, red; nitrogen, blue; carbon, green; hydrogen, white). Secondary structure elements of the MHC molecule are also shown for orientation purposes: α-helix in red, β-pleated sheet in turquoise, and random coil in gray. A transparency function has been included to allow secondary structural elements of the MHC molecule and the peptide residues buried in it to be slightly visible.
Kd-peptide binding stabilization assay.
Kd-transfected RMA-S cells (18), 104 cells per well, were incubated overnight at 27°C in RPMI-1640 medium with 100 μM test peptide, heat-shocked for 2 minutes at 56°C, washed, and incubated with anti-Kd antibody (34-1-2S) for 30 minutes at 4°C, followed by FITC-conjugated sheep anti-mouse immunoglobulin (Silenus Laboratories, Victoria, Australia). LLO 91–99, known to bind Kd with high affinity (19), was used as a reference peptide. Cells were examined with a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, California, USA) that used CellQuest software. Binding of peptide to Kd is observed as a temperature-dependent stabilization of surface Kd expression.
Chromium [51 Cr]-release CTL assay.
Effector cells were generated in female NOD mice by subcutaneous injection of 50 μg peptide in CFA (Difco Laboratories, Detroit, Michigan, USA). After 14 days, splenocytes were harvested and restimulated in vitro for 6 days in HEPES Eagle’s Medium (HEM)/2.5% FCS at 37°C with peptide-coated (10 μg/ml) irradiated (15 Gy) splenocytes (20). Restimulated effector cells (100 μl) were then seeded in duplicate with 104 chromium[51Cr]-labeled RMA-S-Kd target cells (100 μl) prepulsed with 100 μM peptide. RMA-S-Kd targets were prepared by incubating 1 × 106 cells with 100 μM peptide and 100 μCi 51Cr in 200 μl for 2 hours at 27°C. Plates were incubated for 6 hours at 37°C in 5% CO2/air. Percent specific lysis (cpm in 100 μl supernatant) was determined as (experimental release – medium release)/(maximum release – medium release) × 100.
Statistical analysis.
Group differences were analyzed with Fisher exact test (two-tailed) and differences between Kaplan-Meier survival curves were analyzed by the log-rank test, with the use of GraphPad Prism version 3.0a for Macintosh (GraphPad Software Inc., San Diego, California, USA).
Results
Intranasal proinsulin B24–C36 induces CD4+ regulatory T cells but fails to suppress spontaneous diabetes.
Female 8-week-old NOD mice were treated with three intranasal doses of proinsulin B24–C36 peptide. Two weeks later, their unfractionated splenocytes or splenocytes from which CD4+ or CD8+ cells had been depleted or selected, were cotransferred with splenocytes from other recently diabetic NOD mice into young irradiated NOD males. Cotransfer of splenocytes from NOD mice given intranasal proinsulin peptide B24–C36 with diabetogenic splenocytes modestly decreased the incidence of diabetes in recipients (P = 0.05) (Figure 1). Diabetes incidence was significantly reduced by cotransfer of either purified splenic CD4+ cells or splenocytes depleted of CD8+ cells from mice given intranasal proinsulin B24–C36 (P = 0.008 for both compared with splenocytes depleted of CD4+ cells) (Figure 1). Intriguingly, however, intranasal proinsulin peptide given in a single dose, or in three doses either on alternating days from 8 weeks of age or monthly from 4 weeks of age, had only a small effect to suppress spontaneous diabetes development in female NOD mice monitored for 26 weeks or longer (data not shown). These results suggested to us that intranasal B24–C36 could have induced not only regulatory CD4+ but also pathogenic CD8+ T cells, with a net neutral effect of the whole splenocyte population on disease transfer.
Figure 1.
Intranasal proinsulin B24–C36 induces CD4+ T cells that block adoptive transfer of diabetes. Female 8-week-old NOD mice (n = 4 per group) were given 40 μg of proinsulin B24–C36 peptide or OVA protein in 10 μl PBS, intranasally on three alternating days. Two weeks after the last treatment, the mice were killed and 107 pooled splenocytes from mice treated with proinsulin B24–C36 (filled circles) or OVA (open circles), or 107 pooled splenocytes from mice treated with proinsulin B24–C36 either selected for CD4+ cells (filled triangles) or depleted of either CD4+ (filled inverted triangles) or CD8+ (filled squares) cells, were cotransferred with 107 pooled splenocytes from recently NOD female mice into irradiated young NOD male recipients (n = 8 or 12 per group). The development of diabetes in recipients after transfer was monitored by measuring blood glucose every 2 weeks. Results are representative of five similar experiments.
Proinsulin B24–C36 comprises overlapping MHC class II– and class I–restricted epitopes.
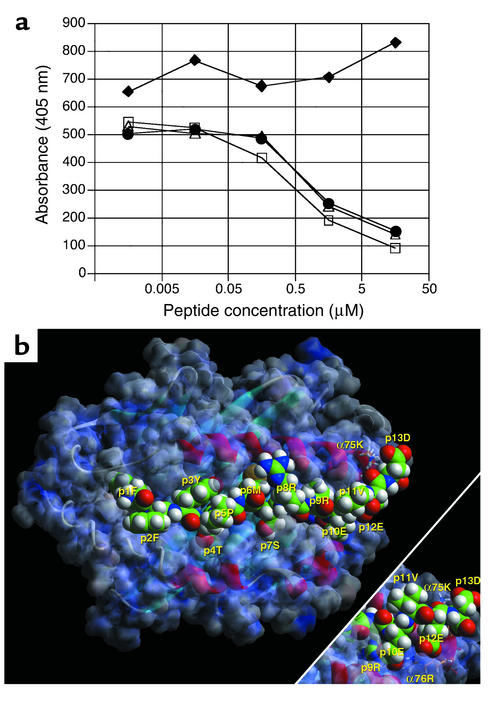
To determine whether proinsulin B24–C36 comprised epitopes for both CD4+ and CD8+ T cells, we first analyzed B24–C36 for MHC class II– and class I–binding peptides. B24–C36 and the shorter peptide B23–C33 bound to I-Ag7, the MHC class II molecule of NOD mice, with relatively high affinity (IC50 = 3–5 μM) compared with the reference HEL 10–23 peptide (Figure 2a). Conversely, proinsulin B22–C31 failed to bind to I-Ag7. Homology modeling (Figure 2b) revealed that the core nonamer sequence accommodated in I-Ag7 was B24–C32 (FFYTPMSRR). However, the three acidic residues beyond position (p) 9 in B24–C36, that is, p10E (C33), p12E (C35), and p13D (C36), have the capacity to greatly enhance binding by forming salt bridges with I-Ag7 α chain 76R (C33, C35) and 75K (C36). There is also an interaction between R (C32) and E (C33), probably accommodated because of the wider opening of the p9 pocket in I-Ag7 compared with other MHC class II alleles (12–14).
Figure 2.
Proinsulin B24–C36 peptides bind to I-Ag7. (a) Binding of proinsulin B24–C36 peptides to purified, soluble NOD mouse MHC class II, I-Ag7. Competition between biotinylated HEL 10–23 peptide and unlabeled HEL 10–23 (open squares), proinsulin B24–C36 (filled circles), B23–C33 (open triangles), or B22–C31 (filled diamonds) for binding to I-Ag7 measured by ELISA. (b) Model of B24–C36 bound to I-Ag7 at pH 7.0, viewed from the perspective of the T cell receptor (TCR). Anchor residues at position 1F (p1F) (B24) and p9R (C32) point into the groove of I-Ag7 and are only partially visible. The p4T points into the groove at pocket 4 and is partially visible, as is p6M. By contrast, residues at p2F, p3Y, p5P, p7S, and p8R point upward and are accessible by the TCR. Note the interaction of α75K and α76R with the three acidic residues at the C-terminus of the peptide as shown in the main figure and in the bottom right-hand corner inset. The latter is generated by rotating this segment of the main figure by 40 degrees along the x axis (bottom up, top into the paper) and 20 degrees along the y axis (right up, left into the paper). α75K interacts with the side chain carboxylate (and secondarily the terminal carboxylate) of p13D (C36), while α76R interacts with p10E (C33) and p12E (C35).
To identify peptides in B24–C36 that might bind to NOD MHC class I molecules (Kd and Db), we initially scanned the Web-based peptide motif databases SYFPEITHI (10) and BIMAS (11). Three overlapping nonamers, B24–C32, B25–C33, and B26–C34, and one decamer, B25–C34, were identified as potential binders to Kd. Homology modeling (Figure 3a) revealed that the core binding sequence was likely to be the decamer, B25–C34, with anchor residues for Kd binding at p2 (Y) and p10 (V). To confirm this prediction, we performed direct binding studies of synthetic peptides to cell surface Kd, using the TAP-deficient cell line RMA-S stably transfected with Kd. The B25–C34 decamer and, to a lesser extent, B24–C36 itself and the B26–C34 nonamer, but not B24–C32, B24–C33, or B25–C33, were observed to bind to Kd (Figure 3b).
Figure 3.
Proinsulin B24–C36 peptides bind to H2-Kd. (a) Model of proinsulin B25–C34 bound to H-2Kd at pH 7.0, viewed from the perspective of the TCR. Anchor residues at p2Y and p10V point into the groove of Kd and are only partially visible. The p3T points into the groove at pocket D and is not visible. The p7R points into the groove at pocket E and is partly visible. By contrast, residues at p1F, p4P, p5M, p6S, p8R, and p9E point upward and are thus accessible by the TCR. Note the tight packing of p1F into pocket A and against 167W, as well as the interaction of these aromatic residues with 171Y. Residue 152D is below p8R and forms a salt bridge with it (not shown), while 155Y has its aromatic plane nearly parallel to the plane of the paper. (b) Proinsulin B26–C34, B24–C36, and B25–C34 bind to H2-Kd. TAP-deficient RMA-S-Kd cells were incubated in medium alone (no peptide) or in the presence of the indicated proinsulin peptides, or reference LLO peptide, and the level of surface Kd expression was then measured by flow cytometry. Cells incubated in media alone were also stained with an isotype control antibody (dotted line).
The Kd-binding B26–C34 and B25–C34 peptides were then tested to determine whether they could prime Kd-restricted CTLs. Female NOD mice were immunized by subcutaneous injection of 50 μg of peptide in CFA. Spleen cells were recovered after 14 days and restimulated with peptide in culture before assay against 51Cr-labeled RMA-S-Kd cells prepulsed with peptide. In accordance with their ability to bind Kd, B25–C34 and, to a lesser extent, B26–C34 elicited CTLs capable of lysing target RMA-S cells and releasing 51Cr (Figure 4). It is noteworthy that B24–C36 could prime CTLs able to recognize not only B24–C36 itself, but also B25–C34. Priming with the non–Kd-binding peptides, B24–C32, B24–C33, and B25–C33, failed to induce CTLs (data not shown). CTLs were consistently demonstrated after subcutaneous immunization with B24–C36 in adjuvant but could not be reliably detected after intranasal B24–C36.
Figure 4.
Proinsulin B25–C34 elicits Kd-restricted CTLs. Female NOD mice were primed subcutaneously with proinsulin peptide B26–C34 (a), B25–C34 (b), or B24–C36 (c and d) in CFA. Splenocytes from primed mice were then restimulated in culture with B26–C34 (a and c) or B25–C34 (b and d), and CTL activity was assayed. Restimulated spleen cells as effectors (E) were tested for their ability to induce release of radioactivity from 51Cr-labeled RMA-S-Kd cells as targets (T) in the absence of added peptide (open circles) or after loading with specific peptide (filled circles), either B26–C34 (a and c) or B25–C34 (b and d).
Treatment with the Kd-restricted CTL epitope, proinsulin B25–C34, reduces diabetes incidence.
Systemic administration of autoepitope peptides with or without incomplete Freund’s adjuvant (IFA) has been reported to trigger antigen-induced cell death and delete autoantigen-specific T cells (21–24). To obtain evidence that B25–C34–specific CTLs may be involved in β cell destruction, 50 μg of B25–C34 in IFA was administered intraperitoneally to female NOD mice (n = 10) at 18 days of age, before the onset of insulitis. This treatment delayed diabetes onset and reduced the incidence of diabetes to 30% at 280 days, compared with 70% in mice similarly treated with either B24–C32 or HEL 10–23 (P = 0.03).
Disabling the B25–C34 CTL epitope permits diabetes prevention by intranasal peptide.
To determine whether the failure of intranasal B24–C36 to prevent spontaneous diabetes, despite induction of regulatory CD4+ T cells, was due to the B25–C34 CTL epitope, B24–C36 was truncated at the C-terminus to eliminate the C34 valine anchor residue for binding to Kd. To confirm that intranasal administration of the truncated B24–C33 peptide could induce regulatory CD4+ T cells, 40 μg of the peptide was administered intranasally to NOD mice on three alternating days from 8 weeks of age. Two weeks later, 107 splenocytes, 106 purified splenic CD4+ cells, or 107 splenocytes depleted of CD4+ cells, from treated mice, were cotransferred intravenously with 107 splenocytes from recently NOD females into irradiated 6-week-old NOD males (n = 12 per group). Splenic CD4+ cells from mice that had received intranasal B24–C33 prevented transfer of diabetes: 6 weeks after cotransfer, only one of eight recipients of whole splenocytes and none of the eight recipients of purified splenic CD4+ cells from B24–C33–treated mice were diabetic, in contrast to seven of the eight recipients of splenocytes depleted of CD4+ T cells (P = 0.001).
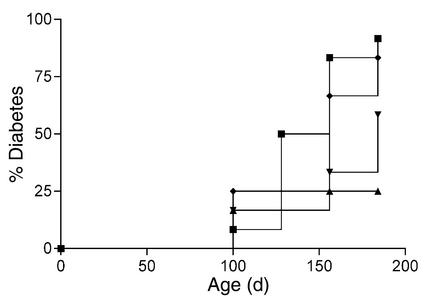
To determine the effect of intranasal B24–C33 and the core I-Ag7 –binding nonamer sequence, B24–C32, on spontaneous diabetes incidence, female NOD mice (n = 12 per group) were given a single 40-μg dose of each peptide, the parent B24–C36 peptide, or PBS carrier only at 8 weeks of age. B24–C36 had no effect on diabetes incidence: at 182 days of age 83% of mice had become diabetic compared with 92% of PBS controls. On the other hand, after treatment with B24–C33 or B24–C32, the incidence of diabetes (log-rank survival analysis) was significantly reduced, being 33% (P = 0.02) and 25% (P = 0.01), respectively, at 154 days and 58% (P = 0.03) and 25% (P = 0.004), respectively, at 184 days (Figure 5).
Figure 5.
Disabling the B25–C34 CTL epitope permits diabetes prevention by proinsulin peptide. A single intranasal dose of mouse proinsulin B24–C36 (diamonds), B24–C33 (inverted triangles), or B24–C32 (triangles) peptide, or PBS carrier only (squares), was given to 8-week-old female NOD mice (n = 12 per group). The experiment was terminated at 26 weeks of age, when the majority of control mice had become diabetic.
Discussion
A sequence in proinsulin, spanning the cleavage site between the B chain of insulin and the C-peptide of proinsulin, is recognized by T cells from humans at risk for T1D (4) and NOD mice (5). Here we show in the NOD mouse that proinsulin B24–C36 comprises overlapping CD4+ and CD8+ T cell epitopes. B25–C34 is a novel MHC class I–restricted (Kd-restricted) CTL epitope that overlaps the core MHC class II–binding (I-Ag7–binding) nonamer, B24–C32, and limits the ability of the latter to prevent diabetes after intranasal administration. Although the B24–C36 peptide induced regulatory CD4+ T cells after intranasal administration, it did not alter the incidence of spontaneous diabetes. The CTL epitope was disabled by truncation of B24–C36 at its C-terminus to eliminate the C34 valine, an anchor residue for binding into the p9 pocket of Kd. Without the C34 valine, the peptide cannot bind Kd, as demonstrated. However, it can still bind to I-Ag7 and, when administered intranasally, not only induced regulatory T cells but suppressed the development of diabetes. Interestingly, CTLs could be elicited not only by B25–C34 but also by the B24–C36 peptide, which also bound to Kd. Although MHC class I molecules are generally considered to accommodate shorter nonamer or decamer peptides, the C-terminal binding pocket of some MHC class I molecules can allow C-terminal peptide extensions out of the binding cleft (25). The only other CTL epitope reported in the NOD mouse, insulin B chain 15–23 (26), also overlaps a CD4+ T cell epitope within the B chain 9–23 sequence. Contiguous or overlapping CD4+ and CD8+ T cell epitopes have been found as well in the two other islet autoantigens, tyrosine phosphatase–like insulinoma antigen-2 (27, 28) and glutamic acid decarboxylase (29). These findings suggest that a single combitope sequence may be processed by an APC and serve to efficiently elicit both helper CD4+ T cell and associated CD8+ CTL responses.
Although we detected B25–C34–specific CTLs in the NOD mouse spleen after subcutaneous priming, we were unable to detect their priming by intranasal B24–C36 administration. This may be due to the limited sensitivity of the 51Cr-release assay. We have used this assay to detect OVA-specific CTLs after low-dose intranasal OVA (30), but because OVA is a non–self-protein, it would be recognized by a higher frequency of CTL precursors. Even so, when CTLs are primed by mucosal OVA, they exhibit lower lytic activity than when they are primed subcutaneously in adjuvant (20). It is also relevant that the CTLs generated by subcutaneous priming with B24–C36 in adjuvant exhibited lower lytic activity than CTLs primed to non–self-OVA or LLO peptides. Thus, a relatively low frequency of CTLs or a relatively low avidity of CTLs for B25–C34 self-peptide is likely to militate against the detection of these CTLs ex vivo after intranasal treatment.
Two findings implicate the B25–C34 CTL epitope in the pathogenesis of NOD mouse diabetes. First, disabling the CTL epitope by truncation of the C-terminal anchor residue at C34 uncovered the ability of the N-terminal CD4+ T cell epitope, B24–C32/33, to prevent diabetes after intranasal administration. Second, when B25–C34 was administered systemically to young mice, before the onset of insulitis, diabetes development was suppressed. Immunoprotection after systemic high-dose administration of a CTL epitope has previously been reported in a transgenic T cell receptor (TCR) model of autoimmune diabetes (24). This effect of B25–C34 administration just before the appearance of insulitis is consistent with evidence that direct β cell recognition by MHC class I–restricted CD8+ T cells is a requirement for the onset of insulitis (31).
Mucosal administration of autoantigen often confers only partial protection from experimental autoimmune disease and in some cases has been shown to exacerbate disease (32–34). In addition, trials in humans of oral myelin basic protein for multiple sclerosis (35), oral collagen type II for rheumatoid arthritis (36), and oral insulin for T1D (37, 38) did not demonstrate clinical benefit. One explanation for these findings could be the simultaneous induction of protective immunity (mucosal tolerance), shown here to be mediated by regulatory CD4+ T cells, and pathogenic immunity mediated by CTL. Previously, in mice bearing OVA-specific TCRs and expressing OVA transgenically in pancreatic β cells, a single oral dose of OVA was shown to activate CTLs, leading to autoimmune β cell destruction and diabetes (20). Furthermore, pathogenic OVA-specific CTLs could be induced in mice by a variety of doses and schedules of oral, aerosol, or intranasal OVA that had been used by others to induce mucosal tolerance (30). These models reveal that mucosal administration of autoantigen may be a double-edged sword with the potential to induce not only protective but also pathogenic immunity. The outcome of mucosal antigen administration may therefore reflect a balance between protective and pathogenic immunity. In the context of autoimmune disease prevention, induction of CTL immunity could nullify the benefit of mucosal tolerance mediated by regulatory T cells and, in a worst-case scenario, exacerbate disease. Avoidance of CTL immunity should improve the efficacy and safety of mucosal autoantigen administration for preventing autoimmune disease. This may be achieved by mutating or deleting CTL epitopes, as shown here, or by blocking costimulation-dependent CTL activation as shown previously in a transgenic OVA model (39).
Acknowledgments
We thank Arno Mullbacher for the RMA-S-Kd cell line, Michelle Latimer for care of mice, Demetrios Kyrkas for technical assistance, Andrew Lew for comments on the manuscript, and Catherine O’Shea for secretarial assistance. This work was supported by the National Health and Medical Research Council of Australia, a Program Grant from the Juvenile Diabetes Research Foundation (to L.C. Harrison), and a grant from the Research Committee of the Technological Educational Institute of Epirus (to G.K. Papadopoulos).
Footnotes
See the related Commentary beginning on page 1280.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: type 1 diabetes (T1D); hen-egg lysozyme (HEL); Listeria monocytogenes listeriolysin O (LLO); T cell receptor (TCR).
References
- 1.Pugliese A, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 2.Vafiadis P, et al. Class III alleles of the variable number of tandem repeat insulin polymorphism associated with silencing of thymic insulin predispose to type 1 diabetes. J. Clin. Endocrinol. Metab. 2001;86:3705–3710. doi: 10.1210/jcem.86.8.7733. [DOI] [PubMed] [Google Scholar]
- 3.French MB, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice [erratum 1997, 46:924] Diabetes. 1997;46:34–39. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 4.Rudy G, et al. Similar peptides from two beta cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Mol. Med. 1995;1:625–633. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, et al. Evidence that a peptide spanning the B-C junction of proinsulin is an early autoantigen epitope in the pathogenesis of type 1 diabetes. J. Immunol. 2001;167:4926–4935. doi: 10.4049/jimmunol.167.9.4926. [DOI] [PubMed] [Google Scholar]
- 6.Harrison LC, Hafler DA. Antigen-specific therapy for autoimmune disease. Curr. Opin. Immunol. 2000;12:704–711. doi: 10.1016/s0952-7915(00)00166-7. [DOI] [PubMed] [Google Scholar]
- 7.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv. Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 8.Krause I, Blank M, Shoenfeld Y. Immunomodulation of experimental autoimmune diseases via oral tolerance. Crit. Rev. Immunol. 2000;20:1–16. [PubMed] [Google Scholar]
- 9.Harrison LC, et al. A peptide-binding motif for I-A(g7), the class II major histocompatibility complex (MHC) molecule of NOD and Biozzi AB/H mice. J. Exp. Med. 1997;185:1013–1021. doi: 10.1084/jem.185.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rammensee, H.G., Bachmann, J., Emmerich, N., and Stevanovich, S. An Internet Database for MHC Ligands and Peptide Motifs. http://134.2.96.221/scripts/MHCserver.dll/home.htm. [DOI] [PubMed]
- 11.Taylor, R., and Parker, K. An Internet Database for HLA Peptide Motifs. http://bimas.dcrt.nih.gov/.
- 12.Corper AL, et al. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science. 2000;288:505–511. doi: 10.1126/science.288.5465.505. [DOI] [PubMed] [Google Scholar]
- 13.Latek RR, et al. Structural basis of peptide binding and presentation by the type I diabetes-associated MHC class II molecule of NOD mice. Immunity. 2000;12:699–710. doi: 10.1016/s1074-7613(00)80220-4. [DOI] [PubMed] [Google Scholar]
- 14.Moustakas AK, Routsias J, Papadopoulos GK. Modeling of the MHC II allele I-A(g7) of NOD mouse: pH-dependent changes in specificity at pockets 9 and 6 explain several of the unique properties of this molecule. Diabetologia. 2000;43:609–624. doi: 10.1007/s001250051350. [DOI] [PubMed] [Google Scholar]
- 15.Fremont DH, Monnaie D, Nelson CA, Hendrickson WA, Unanue ER. Crystal structure of I-Ak in complex with a dominant epitope of lysozyme. Immunity. 1998;8:305–317. doi: 10.1016/s1074-7613(00)80536-1. [DOI] [PubMed] [Google Scholar]
- 16.Madden DR, Garboczi DN, Wiley DC. The antigenic identity of peptide-MHC complexes: a comparison of the conformations of five viral peptides presented by HLA-A2. Cell. 1993;75:693–708. doi: 10.1016/0092-8674(93)90490-h. [DOI] [PubMed] [Google Scholar]
- 17.Wong FS, Moustakas AK, Wen L, Papadopoulos GK, Janeway CA. Analysis of structure and function relationships of an autoantigenic peptide of insulin bound to H-2K(d) that stimulates CD8 T cells in insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5551–5556. doi: 10.1073/pnas.072037299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullbacher A, Lobigs M. Up-regulation of MHC class I by flavivirus-induced peptide translocation into the endoplasmic reticulum. Immunity. 1995;3:207–214. doi: 10.1016/1074-7613(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 19.Sijts AJ, Palmer EG. Enhanced intracellular dissociation of major histocompatibility complex class I-associated peptides: a mechanism for optimizing the spectrum of cell surface-presented cytotoxic T lymphocyte epitopes. J. Exp. Med. 1997;185:1403–1411. doi: 10.1084/jem.185.8.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanas E, Carbone FR, Allison J, Miller JF, Heath WR. Induction of autoimmune diabetes by oral administration of autoantigen. Science. 1996;274:1707–1709. doi: 10.1126/science.274.5293.1707. [DOI] [PubMed] [Google Scholar]
- 21.Clayton JP, et al. Peptide-specific prevention of experimental allergic encephalomyelitis. Neonatal tolerance induced to the dominant T cell determinant of myelin basic protein. J. Exp. Med. 1989;169:1681–1691. doi: 10.1084/jem.169.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liblau R, Tisch R, Bercovici N, McDevitt HO. Systemic antigen in the treatment of T-cell-mediated autoimmune diseases. Immunol. Today. 1997;18:599–604. doi: 10.1016/s0167-5699(97)01171-7. [DOI] [PubMed] [Google Scholar]
- 23.Heeger PS, et al. Revisiting tolerance induced by autoantigen in incomplete Freund’s adjuvant. J. Immunol. 2000;164:5771–5781. doi: 10.4049/jimmunol.164.11.5771. [DOI] [PubMed] [Google Scholar]
- 24.Bercovici N, et al. Systemic administration of agonist peptide blocks the progression of spontaneous CD8-mediated autoimmune diabetes in transgenic mice without bystander damage. J. Immunol. 2000;165:202–210. doi: 10.4049/jimmunol.165.1.202. [DOI] [PubMed] [Google Scholar]
- 25.Horig H, Young AC, Papadopoulos NJ, DiLorenzo TP, Nathenson SG. Binding of longer peptides to the H-2Kb heterodimer is restricted to peptides extended at their C terminus: refinement of the inherent MHC class I peptide binding criteria. J. Immunol. 1999;163:4434–4441. [PubMed] [Google Scholar]
- 26.Wong FS, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 27.Honeyman MC, Stone NL, Harrison LC. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA- 2: potential for mimicry with rotavirus and other environmental agents. Mol. Med. 1998;4:231–239. [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K, Honeyman MC, Harrison LC. Cytotoxic T cells to an epitope in the islet autoantigen IA-2 are not disease-specific. Clin. Immunol. 2001;99:360–364. doi: 10.1006/clim.2001.5031. [DOI] [PubMed] [Google Scholar]
- 29.Quinn A, McInerney MF, Sercarz EE. MHC class I-restricted determinants on the glutamic acid decarboxylase 65 molecule induce spontaneous CTL activity. J. Immunol. 2001;167:1748–1757. doi: 10.4049/jimmunol.167.3.1748. [DOI] [PubMed] [Google Scholar]
- 30.Hanninen A, Braakhuis A, Heath WR, Harrison LC. Mucosal antigen primes diabetogenic cytotoxic T-lymphocytes regardless of dose or delivery route. Diabetes. 2001;50:771–775. doi: 10.2337/diabetes.50.4.771. [DOI] [PubMed] [Google Scholar]
- 31.Kay TWH, Parker JL, Stephens LA, Thomas HE, Allison J. RIP-beta 2-microglobulin transgene expression restores insulitis, but not diabetes, in beta 2-microglobulin null nonobese diabetic mice. J. Immunol. 1996;57:3688–3693. [PubMed] [Google Scholar]
- 32.Miller A, Lider O, Abramsky O, Weiner HL. Orally administered myelin basic protein in neonates primes for immune responses and enhances experimental autoimmune encephalomyelitis in adult animals. Eur. J. Immunol. 1994;24:1026–1032. doi: 10.1002/eji.1830240503. [DOI] [PubMed] [Google Scholar]
- 33.Terato K, Ye XJ, Miyahara H, Cremer MA, Griffiths MM. Induction by chronic autoimmune arthritis in DBA/1 mice by oral administration of type II collagen and Escherichia coli lipopolysaccharide. Br. J. Rheumatol. 1996;35:828–838. doi: 10.1093/rheumatology/35.9.828. [DOI] [PubMed] [Google Scholar]
- 34.Bellmann K, Kolb H, Rastegar S, Jee P, Scott FW. Potential risk of oral insulin with adjuvant for the prevention of Type I diabetes: a protocol effective in NOD mice may exacerbate disease in BB rats. Diabetologia. 1998;41:844–847. doi: 10.1007/s001250050997. [DOI] [PubMed] [Google Scholar]
- 35.Weiner HL, et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 36.McKown KM, et al. Lack of efficacy of oral bovine type II collagen added to existing therapy in rheumatoid arthritis. Arthritis Rheum. 1999;42:1204–1208. doi: 10.1002/1529-0131(199906)42:6<1204::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 37.Pozzilli P, et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia. 2000;43:1000–10004. doi: 10.1007/s001250051482. [DOI] [PubMed] [Google Scholar]
- 38.Chaillous L, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet. 2000;356:545–549. doi: 10.1016/s0140-6736(00)02579-4. [DOI] [PubMed] [Google Scholar]
- 39.Hanninen A, Martinez NR, Davey GM, Heath WR, Harrison LC. Transient blockade of CD40 ligand dissociates pathogenic from protective mucosal immunity. J. Clin. Invest. 2002;109:261–267. doi:10.1172/JCI200213720. doi: 10.1172/JCI13720. [DOI] [PMC free article] [PubMed] [Google Scholar]