Abstract
To elucidate the role of leptin in regulating neuroendocrine and metabolic function during an acute fast, six to eight healthy, lean men were studied under four separate conditions: a baseline fed state and three 72-hour fasting studies with administration of either placebo, low-dose recombinant-methionyl human leptin (r-metHuLeptin), or replacement-dose r-metHuLeptin designed to maintain serum leptin at levels similar to those in the fed state. Replacement-dose r-metHuLeptin administered during fasting prevents the starvation-induced changes in the hypothalamic-pituitary-gonadal axis and, in part, the hypothalamic-pituitary-thyroid axis and IGF-1 binding capacity in serum. Thus, in normal men, the fall in leptin with fasting may be both necessary and sufficient for the physiologic adaptations of these axes, which require leptin levels above a certain threshold for activation. In contrast to findings in mice, fasting-induced changes in the hypothalamic-pituitary-adrenal, renin-aldosterone, and growth hormone–IGF-1 axes as well as fuel utilization may be independent of leptin in humans. The role of leptin in normalizing several starvation-induced neuroendocrine changes may have important implications for the pathophysiology and treatment of eating disorders and obesity.
Introduction
Leptin is an adipocyte-derived hormone whose absence in mice (1) and humans (2–4) causes abnormal energy homeostasis and profound obesity that is ameliorated by leptin treatment (5, 6). Leptin is secreted into the circulation in a highly organized and pulsatile fashion (7). By activating specific leptin receptors in the hypothalamus, leptin alters the expression of several hypothalamic neuropeptides and thereby regulates energy intake and expenditure (8–10). Although leptin was originally thought to function primarily as an antiobesity hormone in leptin-deficient states, subsequent research has suggested an additional and significant role for leptin in signaling changes in energy balance (especially nutritional deprivation) and in regulating the neuroendocrine and metabolic responses to starvation in rodents (8–10).
Short-term fasting results in a rapid and marked decline in leptin levels out of proportion to the loss of fat mass (11, 12), and it has been proposed that this most likely serves as an adaptive mechanism to promote survival and limit procreation during starvation (8). In mice, the exogenous administration of leptin in physiologic replacement doses prevents the fasting-induced changes of several neuroendocrine axes (8), but this has not yet been directly studied in humans. Understanding the role of leptin in regulating neuroendocrine function during fasting in humans is a matter of profound physiologic interest. Moreover, this may have important therapeutic implications for low-leptin states, such as anorexia nervosa, hypothalamic amenorrhea, and lipoatrophy and may also elucidate the compensatory neuroendocrine mechanisms responsible for the plateauing effect of caloric restriction in the treatment of obesity.
To evaluate the role of leptin in regulating neuroendocrine and metabolic function during an acute fasting period, we studied eight healthy lean men under four separate conditions: a baseline fed state and three separate 72-hour fasting studies with administration of placebo, low-dose recombinant-methionyl human leptin (r-metHuLeptin), and replacement-dose r-metHuLeptin designed to achieve physiologic leptin levels similar to those in the fed state. During each study, we performed detailed characterization of several neuroendocrine axes, body composition, and energy expenditure. Specifically, we analyzed not only end hormone levels but also pulsatility characteristics of neuroendocrine hormones, since pulsatility is required for biological effects in several systems (13).
Methods
Human subjects.
Using an institutional review board–approved clinical protocol, we studied eight lean men (age, 23.3 ± 1.2 years; BMI, 23.7 ± 0.6 kg/m2) in the General Clinical Research Center (GCRC) of the Beth Israel Deaconess Medical Center under an investigator-initiated investigational new drug. Subjects were screened for any medical problems and were admitted to the GCRC under four different conditions: baseline fed state, fasting with placebo administration, fasting with low-dose r-metHuLeptin administration, and fasting with replacement-dose r-metHuLeptin administration designed to achieve physiologic serum leptin levels. The fasting studies with placebo and low-dose r-metHuLeptin were randomized and double-blinded. The interval between admissions was no less than 7 weeks to allow for recovery of hematocrit, to ensure that subjects would return to their baseline weight at the beginning of each admission, and to avoid any potential long-term effects of r-metHuLeptin administration. Although eight subjects were initially enrolled, two withdrew before completing the replacement-dose r-metHuLeptin admission, and thus only data from six evaluable subjects (age, 23.5 ± 1.3 years; BMI, 24.0 ± 0.4 kg/m2) are presented. One subject was not able to complete the fasting/low-dose r-metHuLeptin admission.
During the baseline fed state, subjects were placed on an isocaloric diet to maintain their admission body weight, with four standardized meals per day; 20% of the calories were from breakfast (8:00 am), 35% from lunch (1:00 pm), 35% from dinner (6:00 pm), and 10% from a snack (10:00 pm). Daily sodium intake was 3,768 mg per day. During the fasting studies, subjects received only caffeine-free and calorie-free liquids for 3 days and sodium chloride (NaCl) (500 mg), potassium chloride (KCl) (40 mEq), and a standard multivitamin with minerals each day. They received a snack on the evening before the first study day and then fasted until 10:00 am on the fourth study day, at which time they were given a light standardized breakfast. Ad libitum feeding was allowed starting at 1:00 pm. We recorded the number of calories ingested for breakfast and the first ad libitum meal after fasting. Subjects completed a visual analogue scale to indicate how hungry they felt on day 1 (at the beginning of the fast) and day 4 (just before the first meal at 10:00 am).
During each fed or fasting study, we followed a similar experimental protocol. Subjects were admitted to the GCRC the evening before study day 1 and acclimated to a research bed for 2 days before the frequent sampling day. At 7:00 am on days 1 and 3, resting metabolic rate was directly measured for 20–25 minutes using a DeltaTrac II metabolic monitor (SensorMedics, Yorba Linda, California, USA) while the subject was awake and resting quietly. Blood samples for hormone measurements were obtained at 8:00 am on days 1 and 3 and at 8:30 am on day 4 through an intravenous catheter that was inserted at least 15 minutes before blood sampling. Leptin, testosterone, triiodothyronine (T3), total and free IGF-1, and cortisol were measured on days 1 and 4. Insulin, FFA, sex hormone–binding globulin (SHBG), thyroxine (T4), free thyroxine (FT4), reverse T3 (rT3), thyroxine-binding globulin (TBG), IGF-binding proteins (IGFBPs) 1, 2, and 3, plasma renin activity (PRA), and aldosterone were measured on days 1 and 3. Starting at 8:30 am on day 3, blood samples for leptin, luteinizing hormone (LH), thyrotropin-stimulating hormone (TSH), growth hormone (GH), and cortisol were drawn every 15 minutes through an intravenous line for 24 hours. During the night, blood samples were drawn outside the subject’s room to avoid disturbing his sleep. Subjects were exposed to light from 7:00 am to 11:00 pm and to dark from 11:00 pm to 7:00 am, during which time they slept. At the end of the frequent blood sampling on day 4, 100 μg of gonadotrophin-releasing hormone (GnRH) and 500 μg of thyrotropin-releasing hormone (TRH) were injected intravenously at 8:30 am with blood samples for LH and TSH obtained at 0, 15, 30, 45, and 60 minutes after injection. A 24-hour urine collection was performed on day 3 for catecholamines, creatinine, sodium, and urea nitrogen. Hormone measurements were performed using commercially available immunoassays. At the beginning and end of the fast (7:30 am on day 1 and 9:30 am on day 4), body composition was assessed by bioelectric impedance analysis (Tanita Corporation, Arlington Heights, Illinois, USA) and dual energy X-ray absorptiometry (DEXA) using a Hologic QDR-2000 (coefficient of variation, 1.5% for body fat measurements; Hologic, Waltham, Massachusetts, USA).
Clinical-quality r-metHuLeptin was administered as four subcutaneous injections per day. For the low-dose r-metHuLeptin admission, based on prior pharmacokinetic studies, doses ranged from 0.001–0.008 mg/kg per day, based on the subject’s baseline leptin level, and were administered every 6 hours starting at 8:00 am on day 1 through day 3 with one last dose at 8:00 am on day 4. For the replacement-dose r-metHuLeptin admission, the daily dose of r-metHuLeptin was 0.04 mg/kg per day on day 1, 0.1 mg/kg per day on days 2 and 3 (to account for the progressive decrease in leptin levels with additional days of fasting), and one dose of 0.025 mg/kg at 8:00 am on the fourth day, with the total daily dose divided into four equal doses given every 6 hours starting at 8:00 am on day 1. The replacement-dose regimen was validated in our previous pharmacokinetics studies in fasting lean men to fully restore the fasting-induced suppression of leptin levels.
Statistical analysis.
The descriptive characteristics of the group variables were expressed as mean values and standard error. Mean values were compared using standard Student’s t tests or Wilcoxon rank-sum tests as appropriate. Data were analyzed initially for the six evaluable subjects and then for all subjects who participated in the study, with similar results found. We report here results from the six evaluable subjects. Values were considered to be significantly different when P was less than or equal to 0.05 (two-tailed).
To analyze the hormone levels measured in both the beginning and the end of each of the four admissions, a separate repeated-measures ANOVA model for each of the hormones under consideration was constructed. The dependent variable — the change in hormone level from day 1 to day 3 or 4 — was modeled on treatment (fed, fasting/placebo, fasting/low-dose r-metHuLeptin, fasting/replacement-dose r-metHuLeptin). However, to gain power for the analysis, the results from the fasting/placebo and fasting/low-dose r-metHuLeptin admissions were combined into a group called “low-leptin states.” To meet the assumptions of the repeated-measures ANOVA, hormone levels that were not normally distributed were transformed using a log transformation. The analysis was conducted in SAS version 8.2 using the MIXED procedure. Using least-squares means, post hoc Bonferroni-corrected t tests of the null hypothesis were conducted to test the hypothesis that the change in hormone level for each treatment is equal to zero. The P values associated with the test of the treatment fixed effect, as well as the P values associated with the tests of the change in hormone levels from the beginning to the final day of each admission, are reported. Since multiple comparisons are made for these hormone levels, a P value of less than 0.0167 is considered statistically significant, and a P value between 0.0167 and 0.05 is considered borderline significant. Hormonal data were also analyzed using nonparametric tests (Friedman’s, 2-related samples, k-related samples), and the results obtained were similar to the ANOVA models presented.
Computerized pulse-analysis programs were used to identify circadian and ultradian rhythms in the hormone time series (leptin, LH, TSH, GH), as previously described (13). Pulsatility parameters include basal levels, pulse frequency (number of significant peaks per 24 hours), interpulse interval (time separating consecutive peak maxima), mass (amount of hormone secreted per burst per unit of distribution volume, corrected for rate of disappearance), pulse amplitude (maximal hormone concentration in a peak), pulsatile production rate (the product of burst mass and frequency), total production rate, percent pulsatility, mean concentration, and integrated area (the trapezoidally reconstructed area under the hormone concentration profile over time). Pulsatility parameters were log transformed before analysis. P values obtained by ANOVA are reported. For parameters in which significant results were obtained (P < 0.05), post hoc tests were performed using the least significant difference method to determine whether there was a significant difference as compared with the fed state.
Results
Prolonged (72-hour) fasting suppresses serum leptin levels out of proportion to the change in fat mass, and r-metHuLeptin administration in replacement doses restores leptin levels.
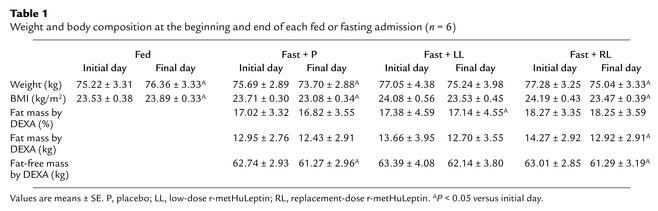
During the fed admission, body weight showed a borderline trend to increase (P < 0.05). During each of the three 72-hour fasting admissions, subjects lost approximately 2 kg of body weight, an effect that achieved only borderline statistical significance (P < 0.05) and that was not significantly altered by r-metHuLeptin (Table 1). This weight loss was due to small decreases in both fat mass and fat-free mass, since the percentage of fat mass by DEXA during each admission remained stable. Despite the small amount of weight loss during each fasting admission, body weight and fat mass on the first day of each subsequent admission did not change significantly, indicating that subjects had returned to their baseline weight at the beginning of each admission and that body composition remained stable over the course of the entire study.
Table 1.
Weight and body composition at the beginning and end of each fed or fasting admission (n = 6)
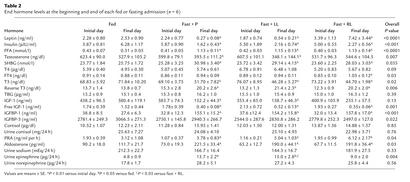
Consistent with the stable body weight and composition over the course of the study, serum leptin levels on the first day of each admission did not change significantly (Table 2). Prolonged fasting for 72 hours decreased serum leptin levels significantly to 10% of baseline — that is, out of proportion to the loss of fat mass (Table 2). In addition, fasting decreased leptin mass, amplitude, pulsatility production rate, mean concentration, and integrated area (Table 3). Low-dose r-metHuLeptin during fasting increased leptin levels to approximately 30% of baseline, whereas replacement-dose r-metHuLeptin fully restored the fasting-induced decline in leptin levels (Tables 2 and 3). The resultant leptin levels were slightly higher than those in the fed state but within the physiologic range for lean men. Pulsatility characteristics for the r-metHuLeptin admissions were not analyzed because of the difficulty of interpreting endogenous leptin pulses in the context of exogenously administered r-metHuLeptin and unknown absorption kinetics of r-metHuLeptin.
Table 2.
End hormone levels at the beginning and end of each fed or fasting admission (n = 6)
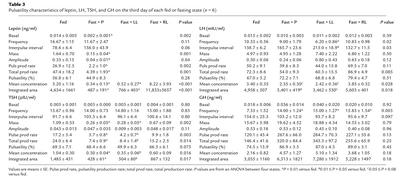
Table 3.
Pulsatility characteristics of leptin, LH, TSH, and GH on the third day of each fed or fasting state (n = 6)
R-metHuLeptin administration fully prevents the fasting-induced decrease in testosterone levels and LH pulsatility by acting mainly at the level of the hypothalamus.
In the baseline fed state, testosterone levels remained stable, but fasting for three days significantly decreased serum testosterone levels by approximately 40% of baseline, as expected (14, 15) (Table 2). A similar decline in testosterone levels occurred during fasting with low-dose r-metHuLeptin, whereas replacement-dose r-metHuLeptin fully restored testosterone to baseline levels. The relative changes in testosterone levels were significantly different between admissions by ANOVA (P = 0.007). SHBG remained stable during the fed state but increased significantly and consistently in all three fasting admissions, with no specific effect of replacement-dose r-metHuLeptin. Thus, the net effect of leptin would be to increase free as well as total testosterone levels during fasting.
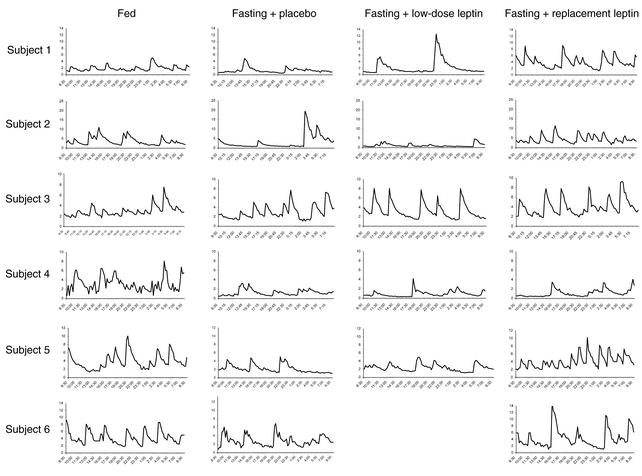
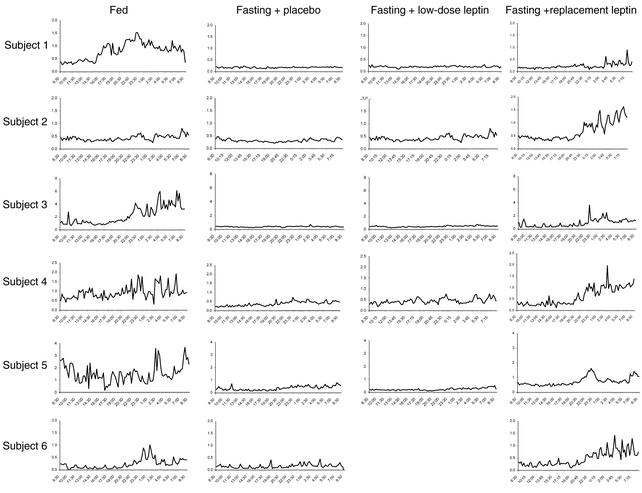
To assess the mechanism underlying these significant changes in testosterone levels, we studied the effect of fasting and/or leptin treatment on LH pulsatility by measuring LH in frequently sampled serum on the third day of each fed or fasting state. Fasting for 3 days decreased the typical LH pulsatility pattern observed in the fed state, as assessed by frequency and interpulse interval (Table 3 and Figure 1). In addition, mean concentration and integrated area decreased by approximately 25%. Although these parameters were not altered by low-dose r-metHuLeptin, replacement-dose r-metHuLeptin fully normalized all these parameters of LH pulsatility. Although total LH production rate tended to be significantly different (P = 0.085), this did not achieve statistical significance, most likely because of the relatively small n of the study. However, correlation analysis of LH pulsatility parameters showed that total production rate correlated highly with mass (r = 0.684, P < 0.001), pulsatility production rate (r = 0.945, P < 0.001), percent pulsatility (r = 0.449, P = 0.032), mean concentration (r = 0.622, P = 0.002), and integrated area (r = 0.674, P < 0.001).
Figure 1.
Twenty-four-hour LH levels (mIU/ml) on the third day of the fed state and fasting studies with administration of placebo, low-dose r-metHuLeptin, or replacement-dose r-metHuLeptin (8:30 am to 8:30 am, n = 6).
We then evaluated whether leptin acts at the hypothalamus to regulate pulsatile GnRH secretion and/or at the pituitary to modify the response of LH secretion to GnRH by performing a standard GnRH stimulation test at the end of the 24-hour frequent sampling during each admission. The integrated LH response to GnRH (area under the curve [AUC]) was significantly different between the admissions (P = 0.028 by ANOVA). Since there was a significant difference overall, we performed post hoc analyses and found that the AUC in the fed state (AUC, 2,395.46 ± 575.83) was similar to that in the fasting/replacement-dose r-metHuLeptin state (AUC, 2,874.84 ± 391.49; P = 0.35 versus fed). However, the AUC was increased during the fasting/low-dose r-metHuLeptin state (AUC, 3,537.83 ± 513.01; P = 0.046 versus fed) and tended to be higher during the fasting/placebo state (AUC, 3,037.90; P = 0.17 versus fed). This raises the possibility that GnRH receptors may be upregulated during fasting (in response to low levels of GnRH/LH), resulting in an exaggerated LH response when exogenous GnRH is administered.
r-metHuLeptin administration prevents the fasting-induced changes in TSH pulsatility but does not significantly affect changes in circulating thyroid hormone levels.
In response to acute fasting, T3 levels decreased by approximately 30% and rT3 levels increased by a similar degree, whereas T4 levels did not change, as expected on the basis of its longer half-life (16, 17) (Table 2). Fasting-induced changes in T3 and rT3 were not altered by r-metHuLeptin, and TBG levels remained stable during all fed or fasting admissions. FT4 levels were stable in the fed and low-leptin states but increased in response to replacement-dose r-metHuLeptin administration (P < 0.01), although the magnitude of the difference was relatively small (Table 2).
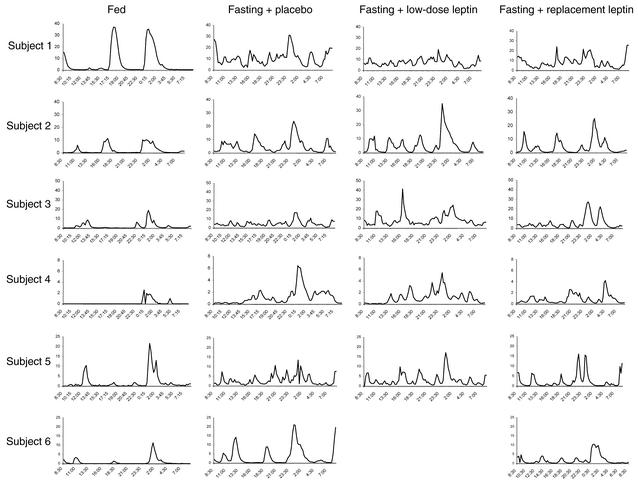
We then assessed whether fasting and/or leptin treatment has an effect on pulsatile TSH secretion. Acute fasting resulted in a marked suppression of TSH secretion with a decrease in integrated area by over 70% and loss of the typical pulsatility characteristics observed in the fed state (Table 3 and Figure 2). Despite the lack of an effect by r-metHuLeptin to reverse the fasting-induced changes in T3 and rT3 levels, replacement-dose r-metHuLeptin significantly blunted the fall in TSH secretion, indicating that leptin regulates the starvation-induced alterations in TSH levels and pulsatility (Table 3).
Figure 2.
Twenty-four-hour TSH levels (μIU/ml) on the third day of the fed state and fasting studies with administration of placebo, low-dose r-metHuLeptin, or replacement-dose r-metHuLeptin (8:30 am to 8:30 am, n = 6).
To distinguish whether this effect occurs at the level of the hypothalamus or pituitary, we performed a standard TRH stimulation test simultaneously with the GnRH test. The integrated response of TSH to TRH (as assessed by AUC) was not different between groups (P = 0.361 by ANOVA), most likely because of high interindividual variability. As compared with the fed state (AUC, 686.39 ± 276.77), the TSH response tended to show a decrease during fasting (AUC, 270.20 ± 52.75; P = 0.075), but the TSH responses during fasting with low-dose r-metHuLeptin (AUC, 287.15 ± 38.39; P = 0.16) and with replacement-dose r-metHuLeptin (AUC, 631.75 ± 187.84; P = 0.75) were not significantly different.
r-metHuLeptin administration does not prevent the fasting-induced changes in GH pulsatility or decline in free IGF-1 levels but restores in part total IGF-1 levels.
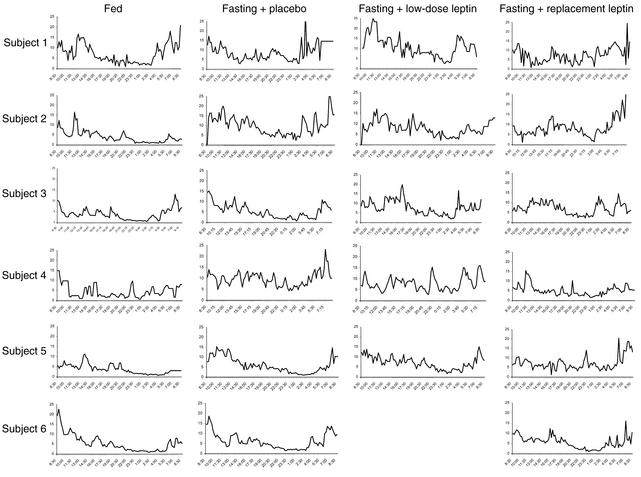
Acute fasting increases GH pulsatility as assessed by frequency, pulsatility production rate, total production rate, mean concentration, and integrated area, although only pulse frequency was statistically significant by ANOVA (P = 0.003). Interpulse interval showed a trend toward significance (P = 0.097). r-metHuLeptin administration during fasting did not significantly alter the fasting-induced changes in GH pulsatility (Table 3 and Figure 3).
Figure 3.
Twenty-four-hour GH levels (ng/ml) on the third day of the fed state and fasting studies with administration of placebo, low-dose r-metHuLeptin, or replacement-dose r-metHuLeptin (8:30 am to 8:30 am, n = 6).
As expected, total IGF-1 levels declined significantly by over 50%, whereas free IGF-1 decreased by approximately 75% with fasting (17, 18) (Table 2). Low-dose r-metHuLeptin administered during fasting resulted in no significant improvement of total and free IGF-1 levels. With replacement-dose r-metHuLeptin, however, total IGF-1 levels tended to increase during fasting and were not significantly different from baseline, in contrast to free IGF-1 levels, which remained suppressed to a similar degree with replacement-dose r-metHuLeptin. IGFBP-1 increased significantly and to a similar magnitude during each of the fasting studies, with no effect of r-metHuLeptin during fasting as compared with fasting alone. Although there was a significant overall difference in the relative changes of IGFBP-3 levels (P = 0.022 by ANOVA), IGFBP-3 did not change from the beginning to the end of each fed or fasting state (Table 2). IGFBP-2 levels were not affected by fasting or r-metHuLeptin (data not shown).
r-metHuLeptin administration does not alter the fasting-induced changes in cortisol secretion and the renin-aldosterone system but blunts the fasting-induced increase in urinary epinephrine.
Baseline cortisol levels at the beginning and end of each admission and 24-hour urine free cortisol on the third day did not change with fasting and/or r-metHuLeptin (Table 2). In addition, there were no significant changes in pulsatility parameters or the integrated area of serum cortisol levels with fasting and/or r-metHuLeptin (data not shown) (Figure 4). However, the 24-hour mean cortisol concentration was significantly increased during all fasting states as compared with the fed state (P = 0.006 by ANOVA; fed, 4.84 ± 0.65; fasting, 7.84 ± 0.49; P = 0.002 versus fed; fasting plus low-dose r-metHuLeptin, 7.97 ± 1.08; P = 0.003 versus fed; fasting plus replacement-dose r-metHuLeptin, 7.19 ± 0.39; P = 0.007 versus fed), indicating mild activation of the hypothalamic-pituitary-adrenal (HPA) axis with fasting. Similarly, fasting accompanied by a low-sodium state (500 mg of NaCl per day) significantly increased PRA and aldosterone levels. Urinary sodium excretion decreased after 3 days of fasting, although the change was not significant, and r-metHuLeptin did not alter the fasting-induced changes in the PRA-aldosterone axis.
Figure 4.
Twenty-four-hour cortisol levels (μg/dl) on the third day of the fed state and fasting studies with administration of placebo, low-dose r-metHuLeptin, or replacement-dose r-metHuLeptin (8:30 am to 8:30 am, n = 6).
We assessed sympathoadrenal activity by measuring 24-hour urine catecholamines on the third day of each admission. Epinephrine is primarily secreted by the adrenal medulla, whereas norepinephrine is the main neurotransmitter of the sympathetic nervous system (SNS). Thus, urinary norepinephrine is an indirect measure of adrenergic activity. With fasting, 24-hour urine epinephrine increased as expected (19) and decreased in part toward baseline with replacement-dose r-metHuLeptin (Table 2). Urine norepinephrine levels showed a nonsignificant tendency to increase during the fasting studies, consistent with previous data (20), and were not affected by r-metHuLeptin.
r-metHuLeptin administration does not significantly alter the fasting-induced metabolic changes or energy expenditure but tends to reduce the increase of food intake during refeeding.
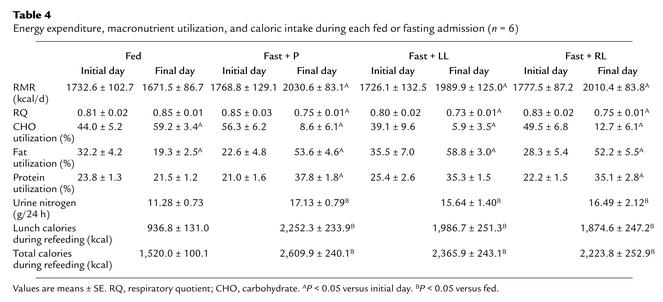
We directly measured resting metabolic rate (RMR) on the first and third day of each admission and found that RMR increased significantly after acute fasting, with no effect from r-metHuLeptin administration (Table 4). The measured respiratory quotient decreased with fasting, indicating a shift of metabolism towards fat utilization, as expected (17). More specifically, carbohydrate utilization decreased by over 75%, and fat utilization increased by 45%. Protein utilization increased by 50%, which was reflected by increased 24-hour urinary nitrogen excretion (P = 0.004 by ANOVA), but these fasting-induced metabolic changes were not altered by r-metHuLeptin administration (Table 4). In addition, serum insulin levels fell by over 70%, and FFA levels increased by nearly 300% with fasting, but r-metHuLeptin administration had no effect on these changes (Table 2).
Table 4.
Energy expenditure, macronutrient utilization, and caloric intake during each fed or fasting admission (n = 6)
Finally, we assessed whether leptin affects spontaneous food intake during refeeding. As expected, the number of ingested calories increased significantly after each of the fasting studies, as compared with the corresponding meal provided in the fed state on an isocaloric diet (Table 4). The final dose of r-metHuLeptin was administered at 8:00 am on the fourth study day, approximately 2 hours before the first meal during refeeding. With replacement-dose r-metHuLeptin, the number of ingested calories tended to decrease but was not significantly different from those in the other fasting studies (P = 0.17 versus fasting/placebo, P = 0.17 versus fasting/low-dose leptin). On the basis of visual analogue scales to indicate subjective sensation of hunger and satiety, subjects felt significantly more hungry on the last day of each fasting admission than on the first day of the admission, with no significant effect of r-metHuLeptin administration (data not shown).
Discussion
Studies in animal models have provided substantial evidence that the starvation-induced fall in leptin levels plays a central role in regulating the neuroendocrine adaptation to starvation (8), suggesting that the suppression of leptin levels with starvation or food restriction may be of more critical importance than the increases in leptin that occur with overfeeding and obesity (9, 21). In humans, as in mice, congenital absence of leptin or functional leptin deficiency due to inactivating mutations of the leptin receptor causes severe obesity accompanied by neuroendocrine abnormalities (2–4, 22). The nature of the neuroendocrine defects in leptin-deficient rodents and in leptin-deficient or leptin-resistant humans differ in some important respects, however, suggesting that the role of leptin in mediating the neuroendocrine response to starvation may be different in humans versus rodents (2–4, 8, 22).
Although observational studies have reported associations of circulating leptin levels with the levels of several neuroendocrine hormones in healthy humans (13, 23, 24), to our knowledge no prior interventional studies have been performed to assess the role of changing leptin levels on neuroendocrine function in lean individuals. Our data suggest that a reduction of leptin levels in lean men regulates the acute fasting-induced changes in the hypothalamic-pituitary-gonadal (HPG) axis and, in part, changes in the hypothalamic-pituitary-thyroid (HPT) axis and IGF-1 binding capacity, but it is not responsible for changes in the HPA, renin-aldosterone, and GH-IGF-1 axes associated with acute fasting. Finally, suppression of leptin does not appear to be responsible for the changes in fuel utilization or energy expenditure observed during short-term fasting and tends to contribute to the increased number of calories ingested in the immediate post-fasting period.
In healthy men, fasting significantly decreases serum testosterone levels through changes in pulsatile LH secretion (14, 15), an effect that may be mediated by decreased hypothalamic GnRH pulses (25). In this study, we found that leptin replacement during fasting had the most significant effect on the HPG axis, with full restoration of LH pulsatility characteristics and testosterone levels. This indicates that the fall in testosterone with decreased leptin is a result of inadequate LH stimulation of testosterone secretion and is consistent with the hypothesis that a threshold leptin level between 0.5 and 2 ng/ml may be necessary for normal LH secretion (26). This effect of low leptin on LH pulsatility is most likely secondary to effects on the hypothalamus to influence pulsatile GnRH release, on the basis of in vitro and animal data (27), although an additional direct effect of leptin on the pituitary cannot be excluded.
Our findings lend further credence to converging lines of evidence from animal models and observational studies in humans indicating an important role of leptin in reproduction (28). Food deprivation decreases testosterone levels in male mice and prolongs the onset of vaginal estrus in female mice, whereas exogenous leptin administration restores the decline in testosterone and LH levels in fasted normal mice (8) and corrects the sterility of leptin-deficient ob/ob mice (29, 30). In healthy women, ultradian fluctuations in leptin levels are synchronous with both LH and estradiol fluctuations (24). Rises in leptin may be associated with the onset of puberty in boys (31), although the literature is divided on this point (32, 33). In addition, reproductive dysfunction occurs in humans with leptin deficiency or resistance due to mutations in the leptin gene or the leptin receptor gene, respectively. These rare cases have included two adult leptin-deficient women with amenorrhea, an adult leptin-deficient man who had never entered puberty (3, 4), and three leptin-resistant adolescent sisters with low gonadotropin levels and no evidence of pubertal development (22). In one case, intervention with leptin replacement therapy for 1 year in a 9-year-old leptin-deficient child resulted not only in marked loss of fat mass but the development of a pulsatile nocturnal pattern of gonadotropin secretion consistent with early puberty (6) that progressed to normal LH and FSH pulsatility with continued leptin replacement (34). Thus, these data are consistent with a role for leptin as a “gate” for normal reproductive function when adequate energy stores are achieved and suggest that leptin may have a role for treating the reproductive dysfunction seen in low leptin states, such as hypothalamic amenorrhea and eating disorders, and may also have therapeutic applications in conditions such as delayed puberty, which is associated with decreased leptin levels (35, 36).
We then examined whether r-metHuLeptin administration regulates the fasting-induced changes in thyroid function. A complex sequence of alterations in serum TSH and thyroid hormone levels has been observed in humans undergoing a short-term fast (37, 38). These include a decrease in TSH pulse amplitude (39), a decrease of serum T3 levels, and an increase of rT3 (the less biologically potent hormone), whereas T4 levels remain unchanged due to its longer half-life. This suggests that fasting shifts T4 conversion from T3 to rT3 (16), and it has been proposed that decreased T3 and thyroid receptor protein levels are of teleological importance in limiting energy expenditure and catabolism during a fast (16).
Our data confirm the expected changes of thyroid hormone levels in response to short-term fasting, are consistent with findings reported in leptin-deficient children (4), and demonstrate for the first time that r-metHuLeptin administration prevents the fasting-induced changes of TSH secretion and results in a slight increase of FT4, as previously described in leptin-deficient subjects (34). Whether leptin regulates hypothalamic TRH release and/or pituitary TSH secretion needs to be studied further. Moreover, since r-metHuLeptin did not alter the fasting-induced changes in T3 and rT3, our findings provide no evidence for an effect of leptin on expression and/or activity of deiodinases, which most likely mediate the short-term starvation-induced changes in T3 and rT3 (16, 17) and may be influenced by changes of other metabolic signals such as FFA. Finally, since TBG levels were not differentially affected by either fasting or r-metHuLeptin administration, one could propose that the observed changes in T3 and T4 also reflect free T3 and free T4 changes. In fact, measured free T4 was unchanged in the fasting state but increased in response to r-metHuLeptin administration, although the magnitude of the change was small.
It has previously been shown that leptin administration in rodents reverses the inhibitory effect of food deprivation on spontaneous pulsatile TSH secretion (40) and prevents the suppression of pro-TRH mRNA in paraventricular nucleus neurons that occurs with fasting (41). In healthy men, we have found that leptin and TSH rhythms exhibit a similar 24-hour pattern of variability with significant pattern synchrony of ultradian fluctuations, a pattern that is impaired in leptin-deficient subjects (23). Interestingly, leptin-resistant subjects with a mutation in the leptin receptor had evidence of hypothalamic hypothyroidism with low T4, normal basal TSH, and sustained TSH response to TRH (22). Although r-metHuLeptin administration has been found to increase FT4 and FT3 levels in leptin-deficient children (34) and to reverse the decreased T3 and T4 levels in four subjects on a long-term hypocaloric diet (42), r-metHuLeptin did not alter the changes in thyroid hormone levels in this acute fasting paradigm with the exception of a slight increase of FT4, despite preventing TSH pulsatility alterations. This was most likely due to the long half-life of thyroid hormones and the short duration of the fast in this study. Thus, further studies are required to study the effect of r-metHuLeptin administration on all components of the HPT axis in response to more prolonged fasting or long-term hypocaloric diets. Findings of these studies will have not only physiologic but also therapeutic importance in the context of the plateauing weight loss seen in response to dieting or use of antiobesity medications.
During early starvation, serum GH levels rise, and pulsatile GH secretion increases with increased pulse frequency and 24-hour integrated GH concentrations (43, 44). Although GH is the main regulator of IGF-1 synthesis in the liver and peripheral tissues, fasting decreases IGF-1 levels despite elevated GH levels, most likely because of changes in other determinants of its secretion, such as hormones (insulin) and nutritional status per se as well as GH resistance in the liver (18, 45). The combination of increased GH and decreased IGF-1 levels may have adaptive value by diminishing the energy expenditure necessary for growth-related processes while enabling GH to promote the mobilization of alternative fuels through lipolysis.
It has previously been shown that the exogenous administration of leptin to fasted mice fully prevents the suppression of both GH and IGF-1 levels and corrects in part the fasting-induced suppression of growth hormone–releasing hormone mRNA expression (46). Leptin-deficient subjects have decreased GH response to insulin-induced hypoglycemia and exercise tests but normal heights (4), whereas leptin-resistant subjects have a mild but significant growth delay during early childhood in addition to decreased GH secretion and low IGF-1 and IGFBP-3 levels (22). We found that replacement-dose r-metHuLeptin does not prevent the fasting-induced changes in GH pulsatility or free IGF-1 levels, but on the basis of partial restoration of total IGF-1 levels and the lack of a specific effect on IGFBP-1, -2, and -3, it may have an effect on one or more of IGFBP-4, -5, or -6. Thus, the role of leptin in regulating insulin-like growth factors and their binding proteins will require further investigation.
Fasting for 5 days has been shown to alter pulsatile and rhythmic cortisol release with a modest elevation of glucocorticoid levels in healthy men (47). In mice, acute starvation increases corticosterone and ACTH levels, whereas exogenous leptin administration reverses the activation of the HPA axis (8). A significant inverse relationship between fluctuations in leptin, ACTH, and cortisol has been demonstrated in humans, independent of glucocorticoid effects on leptin (13), but unlike mice, human subjects with mutations in the leptin or leptin receptor gene appear to have normal adrenal function. Leptin-deficient subjects had elevated basal cortisol and ACTH levels but normal urinary free cortisol and response to dexamethasone suppression (4). Similarly, evaluation of the HPA axis in the leptin-resistant subjects did not reveal any abnormalities (22). In this study, we found evidence for mild activation of the HPA axis with acute fasting but no effect of replacement r-metHuLeptin, suggesting species-specific differences in leptin regulation of the HPA axis as compared with rodents (8). Thus, the synchronous but inverse relationship of cortisol and leptin fluctuations reported previously (13) does not appear to be causal but is probably due to a third factor. In addition, the paradigm used in this study does not exclude the possibility that leptin replacement during fasting needs to be administered in a pulsatile fashion for regulation of the HPA axis. Finally, it remains unknown whether leptin administration may alter ACTH pulsatility or affect β-hydroxysteroid dehydrogenase activity and thus cortisol levels in peripheral tissues.
Fasting with minimal sodium intake is associated with an initial natriuresis phase and negative sodium balance followed by avid sodium retention (48). In this study, subjects received a much smaller dose of NaCl during fasting than in the fed state (500 versus 3,768 mg per day), which may account for the fact that urinary sodium tended to but did not decrease significantly with fasting. Fasting did significantly increase PRA and aldosterone, however, but r-metHuLeptin did not alter these fasting-induced changes in the mineralocorticoid axis. These findings are in contrast to the effect of leptin administration on increase natriuresis (49, 50) and sympathetic activity (51) in normal, nonobese leptin-resistant Zucker rats (51). Our findings are in agreement with a previous study in nonobese men on an isocaloric diet in whom leptin administration (0.3 mg/kg per day) for 6 days had no effect on autonomic activity or urinary catecholamines (52). We found that urine epinephrine levels increased in response to fasting, but replacement-dose r-metHuLeptin had only a moderate effect in partially preventing the rise in urine epinephrine levels and no effect on urine norepinephrine levels. Thus in humans, leptin may regulate epinephrine synthesis in the adrenal medulla but has less effect on peripheral SNS activity as reflected by norepinephrine levels.
Since r-metHuLeptin administration did not alter any fasting-induced metabolic changes (17), we propose that the fall of leptin levels with starvation is not required for the shift in fuel utilization brought about by fasting, which may be due to the fasting-induced suppression of insulin (53). These results are consistent with the finding that leptin replacement in fasting mice had no effect on ketone levels (8). Despite the lack of a significant effect on metabolic parameters and hunger questionnaires (most likely a less sensitive and precise assessment of appetite than caloric measurement), subjects given replacement-dose r-metHuLeptin tended to consume fewer calories in the postfasting state (although this did not reach statistical significance). This raises the possibility that, similar to the results seen in fasted rodents (8), the fall of leptin may contribute to the increased appetite seen after fasting. Interestingly, a recent relatively small, randomized, double-blind clinical trial demonstrated that administration of high, pharmacologic doses of pegylated r-metHuLeptin in addition to a hypocaloric diet produced significant suppression of appetite as measured by eating/hunger questionnaires (54), but this was not seen in response to the physiologic doses used in this study. The potential effect of leptin to regulate appetite and food intake after fasting requires further investigation in larger studies, but if confirmed, these data in association with the role of leptin in normalizing neuroendocrine changes may have implications for the role of leptin in the treatment of obesity.
Leptin binds to the leptin receptor (ObR) in the hypothalamus, activating the signal transducer and activator of transcription-3 (STAT3) signaling pathway, which mediates the metabolic effects of leptin through changes in melanocortin production and energy homeostasis. Recent evidence from transgenic animal models suggests that, in addition to the STAT3 signaling system, a distinct and parallel signal transduction pathway exists that regulates hypothalamic neuropeptide Y (NPY) and controls fertility, most likely through activation of extracellular signal-related kinase (ERK) and PI3K kinase (55). Although the thyroid axis has not been studied in detail in these transgenic animal models, it is well known that NPY also regulates the HPT axis (56). Thus, our data in lean men are consistent with a clear effect of exogenously administered leptin to regulate the ObR–ERK/PI-3 kinase–NPY pathway but not the ObR-STAT3-melanocortin pathway for signal transduction in the hypothalamus of lean men. Elucidation of the factors that inactivate the ObR–STAT3 system in humans is of major physiologic and therapeutic importance.
In summary this study represents, to our knowledge, the first interventional study to assess the role of leptin in the physiology of acute fasting in humans and demonstrates that its role differs in several respects from that in rodents. We found that replacement-dose r-metHuLeptin administered during an acute fasting state prevents key changes in the HPG and HPT axes and, in part, changes of total IGF-1, demonstrating that the fall in leptin with fasting may be both necessary and sufficient for these physiologic adaptations in normal men. In contrast to findings in rodents, fasting-induced changes in the HPA, renin-aldosterone, and GH/IGF-1 axes as well as fuel utilization are independent of leptin in the setting of acute leptin deficiency in humans. These findings suggest that decreased leptin levels may be responsible for several neuroendocrine abnormalities seen in low leptin states, such as anorexia nervosa, hypothalamic amenorrhea, and lipoatrophy. Thus, interventional studies involving leptin administration are required to fully clarify the physiologic and potentially therapeutic role of leptin in these specific disease states. These data may also explain the development of compensatory neuroendocrine changes underlying the plateauing effect of hypocaloric diets prescribed for weight loss in overweight patients. In this regard, further studies are needed to establish whether leptin also regulates neuroendocrine function in overweight, leptin-resistant subjects and in women, who have higher endogenous leptin levels. In addition, more prolonged fasting studies are required to evaluate the effect of chronic leptin administration on neuroendocrine axes, particularly those axes in which a longer time frame may be required for fasting-induced changes to be evident. Finally, although the HPG and HPT axes appear to only require leptin levels above a certain threshold for activation, it remains possible that regulation of other neuroendocrine axes, such as the HPA axis, may require leptin pulsatility and thus administration of leptin in a pulsatile fashion in order to be evident. Ongoing and future studies will fully elucidate these important issues in human leptin physiology.
Acknowledgments
This study was supported by NIH NIDDK grant RO1- 58785, NIH grant MO1-RR01032, NIH grant K30-HL04095, and a grant from Amgen Inc. to C. S. Mantzoros. We are deeply indebted to and gratefully thank Jeffrey S. Flier for invaluable intellectual input and discussion of ideas; the GCRC nurses at Beth Israel Deaconess Medical Center for collecting the samples for this research, especially Julie Carpenter for assistance with study coordination; Kandace Bradford and Lisa Miller for help with the statistical analysis; Lizabeth Martin for performing DEXA scans; Linda Ungsunan, John Bullen, and Aspasia Karalis for assistance with the hormone assays; and the GCRC nutritionists for their help with the nutritional analyses and the isocaloric and fasting diets.
Footnotes
Conflict of interest: Christos S. Mantzoros has received research support and consulting fees from Amgen Inc.
Nonstandard abbreviations used: recombinant-methionyl human leptin (r-metHuLeptin); General Clinical Research Center (GCRC); triiodothyronine (T3); sex hormone–binding globulin (SHBG); thyroxine (T4); free thyroxine (FT4); reverse T3 (rT3); thyroxine-binding globulin (TBG); IGF-binding proteins (IGFBPs); plasma renin activity (PRA); luteinizing hormone (LH); thyrotropin-stimulating hormone (TSH); gonadotrophin-releasing hormone (GnRH); thyrotropin-releasing hormone (TRH); dual energy X-ray absorptiometry (DEXA); area under the curve (AUC); hypothalamic-pituitary-adrenal (HPA); sympathetic nervous system (SNS); resting metabolic rate (RMR); hypothalamic-pituitary-gonadal (HPG); hypothalamic-pituitary-thyroid (HPT); leptin receptor (ObR); signal transducer and activator of transcription-3 (STAT3); extracellular signal-regulated kinase (ERK); neuropeptide Y (NPY).
References
- 1.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 3.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 4.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 5.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 6.Farooqi IS, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 7.Sinha MK, et al. Ultradian oscillations of leptin secretion in humans. Biochem. Biophys. Res. Commun. 1996;228:733–738. doi: 10.1006/bbrc.1996.1724. [DOI] [PubMed] [Google Scholar]
- 8.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 9.Flier JS. Clinical review 94: what’s in a name? In search of leptin’s physiologic role. J. Clin. Endocrinol. Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 10.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann. Intern. Med. 1999;130:871–881. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 11.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J. Clin. Endocrinol. Metab. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 12.Weigle DS, et al. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J. Clin. Endocrinol. Metab. 1997;82:561–565. doi: 10.1210/jcem.82.2.3757. [DOI] [PubMed] [Google Scholar]
- 13.Licinio J, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat. Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 14.Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye WH. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J. Clin. Endocrinol. Metab. 1991;73:35–41. doi: 10.1210/jcem-73-1-35. [DOI] [PubMed] [Google Scholar]
- 15.Veldhuis JD, et al. Amplitude suppression of the pulsatile mode of immunoradiometric luteinizing hormone release in fasting-induced hypoandrogenemia in normal men. J. Clin. Endocrinol. Metab. 1993;76:587–592. doi: 10.1210/jcem.76.3.8445014. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DF, Kaplan MM, Stanley CA, Utiger RD. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N. Engl. J. Med. 1979;300:579–584. doi: 10.1056/NEJM197903153001102. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald, R.S., and Smith, R.J. 2001. Starvation. In Principles and practice of endocrinology and metabolism. K.L. Becker, editor. Lippincott, Williams, and Wilkins. Philadelphia, Pennsylvania, USA. 1247–1251.
- 18.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 19.Christensen NJ, Mathias CJ, Frankel HL. Plasma and urinary dopamine: studies during fasting and exercise and in tetraplegic man. Eur. J. Clin. Invest. 1976;6:403–409. doi: 10.1111/j.1365-2362.1976.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 20.Andersson B, Wallin G, Hedner T, Ahlberg A, Andersson OK. Acute effects of short-term fasting on blood pressure, circulating noradrenaline and efferent sympathetic nerve activity. Acta Med. Scand. 1988;223:485–490. doi: 10.1111/j.0954-6820.1988.tb17685.x. [DOI] [PubMed] [Google Scholar]
- 21.Kolaczynski JW, Ohannesian J, Considine RV, Marco C, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J. Clin. Endocrinol. Metab. 1996;81:4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 22.Clement K, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 23.Mantzoros CS, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J. Clin. Endocrinol. Metab. 2001;86:3284–3291. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- 24.Licinio J, et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2541–2546. doi: 10.1073/pnas.95.5.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aloi JA, Bergendahl M, Iranmanesh A, Veldhuis JD. Pulsatile intravenous gonadotropin-releasing hormone administration averts fasting-induced hypogonadotropism and hypoandrogenemia in healthy, normal-weight men. J. Clin. Endocrinol. Metab. 1997;82:1543–1548. doi: 10.1210/jcem.82.5.3947. [DOI] [PubMed] [Google Scholar]
- 26.Ballauff A, et al. Serum leptin and gonadotropin levels in patients with anorexia nervosa during weight gain. Mol. Psychiatry. 1999;4:71–75. doi: 10.1038/sj.mp.4000478. [DOI] [PubMed] [Google Scholar]
- 27.Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary. 2001;4:87–92. doi: 10.1023/a:1012947113197. [DOI] [PubMed] [Google Scholar]
- 28.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil. Steril. 2002;77:433–444. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 29.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 30.Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- 31.Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys: rising leptin levels may signal the onset of puberty. J. Clin. Endocrinol. Metab. 1997;82:1066–1070. doi: 10.1210/jcem.82.4.3878. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham MJ, Clifton DK, Steiner RA. Leptin’s actions on the reproductive axis: perspectives and mechanisms. Biol. Reprod. 1999;60:216–222. doi: 10.1095/biolreprod60.2.216. [DOI] [PubMed] [Google Scholar]
- 33.Foster DL, Nagatani S. Physiological perspectives on leptin as a regulator of reproduction: role in timing puberty. Biol. Reprod. 1999;60:205–215. doi: 10.1095/biolreprod60.2.205. [DOI] [PubMed] [Google Scholar]
- 34.Farooqi IS, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 2002;110:1093–1103. doi:10.1172/JCI200215693. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill MS, Hall CM, Tillmann V, Clayton PE. Constitutional delay in growth and puberty (CDGP) is associated with hypoleptinaemia. Clin. Endocrinol. 1999;60:721–726. doi: 10.1046/j.1365-2265.1999.00736.x. [DOI] [PubMed] [Google Scholar]
- 36.Matkovic V, et al. Leptin is inversely related to age at menarche in human females. J. Clin. Endocrinol. Metab. 1997;82:3239–3245. doi: 10.1210/jcem.82.10.4280. [DOI] [PubMed] [Google Scholar]
- 37.Spencer CA, Lum SM, Wilber JF, Kaptein EM, Nicoloff JT. Dynamics of serum thyrotropin and thyroid hormone changes in fasting. J. Clin. Endocrinol. Metab. 1983;56:883–888. doi: 10.1210/jcem-56-5-883. [DOI] [PubMed] [Google Scholar]
- 38.Lopresti JS, Gray D, Nicoloff JT. Influence of fasting and refeeding on 3,3′,5′-triiodothyronine metabolism in man. J. Clin. Endcrinol. Metab. 1991;72:130–136. doi: 10.1210/jcem-72-1-130. [DOI] [PubMed] [Google Scholar]
- 39.Romjin JA, et al. Pulsatile secretion of thyrotropin during fasting: a decrease of thyrotropin pulse amplitude. J. Clin. Endocrinol. Metab. 1990;70:1631–1636. doi: 10.1210/jcem-70-6-1631. [DOI] [PubMed] [Google Scholar]
- 40.Seoane LM, Carro E, Tovar S, Casanueva FF, Dieguez C. Regulation of in vivo TSH secretion by leptin. Regul. Pept. 2000;92:25–29. doi: 10.1016/s0167-0115(00)00145-2. [DOI] [PubMed] [Google Scholar]
- 41.Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight reduction on energy expenditure and circulating concentrations of thyroid hormones. J. Clin. Endocrinol. Metab. 2002;85:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 43.Ho KY, et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J. Clin. Invest. 1988;81:968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartman ML, et al. Augmented growth hormone (GH) secretory burst frequency and amplitude mediate enhanced GH secretion during a two-day fast in normal men. J. Clin. Endocrinol. Metab. 1992;74:757–765. doi: 10.1210/jcem.74.4.1548337. [DOI] [PubMed] [Google Scholar]
- 45.Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors in the fed and fasted states. J. Clin. Endocrinol. Metab. 1982;55:999–1002. doi: 10.1210/jcem-55-5-999. [DOI] [PubMed] [Google Scholar]
- 46.LaPaglia N, Steiner J, Kirsteins L, Emanuele M, Emanuele N. Leptin alters the response of the growth hormone releasing factor–growth hormone–insulin-like growth factor-I axis to fasting. J. Endocrinol. 1998;159:79–83. doi: 10.1677/joe.0.1590079. [DOI] [PubMed] [Google Scholar]
- 47.Vance ML, Thorner MO. Fasting alters pulsatile and rhythmic cortisol release in normal man. J. Clin. Endocrinol. Metab. 1989;68:1013–1018. doi: 10.1210/jcem-68-6-1013. [DOI] [PubMed] [Google Scholar]
- 48.Kamel KS, Lin S, Cheema-Dhadli S, Marliss EB, Halperin ML. Prolonged total fasting: a feast for the integrative physiologist. Kidney Int. 1998;35:531–539. doi: 10.1046/j.1523-1755.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 49.Villarreal D, Reams G, Freeman RH, Taraben A. Renal effects of leptin in normotensive, hypertensive, and obese rats. Am. J. Physiol. 1998;275:R2056–R2060. doi: 10.1152/ajpregu.1998.275.6.R2056. [DOI] [PubMed] [Google Scholar]
- 50.Jackson EK, Li P. Human leptin has natriuretic activity in the rat. Am. J. Physiol. 1997;272:F333–F338. doi: 10.1152/ajprenal.1997.272.3.F333. [DOI] [PubMed] [Google Scholar]
- 51.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackintosh RM, Hirsch J. The effects of leptin administration in non-obese human subjects. Obes. Res. 2001;9:462–469. doi: 10.1038/oby.2001.60. [DOI] [PubMed] [Google Scholar]
- 53.Cahill GF, et al. Hormone-fuel interrelationships during fasting. J. Clin. Invest. 1966;45:1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hukshorn CJ, et al. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J. Clin. Endocrinol. Metab. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 55.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 56.Fekete C, et al. Neuropeptide Y1 and Y5 receptors mediate the effects of neuropeptide Y on the hypothalamic-pituitary-thyroid axis. Endocrinology. 2002;143:4513–4519. doi: 10.1210/en.2002-220574. [DOI] [PubMed] [Google Scholar]