Several self-molecules have been identified as target antigens in autoimmune diseases. Since lack or loss of tolerance to these molecules is one of the key events promoting autoimmunity, researchers are exploring the possibility that the administration of antigens or peptides may stimulate tolerogenic mechanisms and delay or prevent the full phenotypic expression of autoimmune diseases. There is much enthusiasm for such therapies, as these will probably be disease-specific and not associated with the side effects of conventional immunosuppression. Studies have been performed and are ongoing in both rodents and humans, using whole antigens or peptides, and testing diverse administration routes such as intrathymic, intraperitoneal, intravenous, subcutaneous, oral, and intranasal. Despite many studies, robust data demonstrating clinical benefits are not yet available (1).
Antigen and/or peptide-based interventions in diabetes
Type 1 diabetes (T1D) represents one of the most suitable diseases to exemplify such heterogeneous outcomes. Three autoantigens — proinsulin/insulin, glutamic acid decarboxylase (GAD), and tyrosine phosphatase–like protein IA-2 (or ICA512) — have been well characterized in both humans and the NOD mouse model of autoimmune diabetes (2). Although all of these molecules are expressed in pancreatic islets, insulin and its precursor proinsulin are uniquely secreted by pancreatic β-cells. Several studies have suggested an important role for autoimmune responses to epitopes of insulin/proinsulin, such as the peptides B9–23 (the 9–23 amino acid region of the insulin B chain) (3, 4) and B24–C36 (the proinsulin B-chain–C-peptide junction) (5, 6). In NOD mice, both subcutaneous and oral administration of insulin can delay or prevent diabetes; oral insulin induces regulatory CD4+ T cells, while nasorespiratory insulin induces regulatory CD8+γδ-T cells (7–11). However, in a Diabetes Prevention Trial–Type 1 (DPT-1) study that involved the parenteral administration of insulin, no significant effect on progression to overt disease in autoantibody-positive first-degree relatives of T1D patients, who have increased risk of developing diabetes, was demonstrated (12). While the results of the oral insulin arm of DPT-1 are expected in June 2003, a randomized, crossover, pilot trial of intranasal insulin in at-risk first-degree relatives demonstrated changes in immune and metabolic markers that were consistent with an immunoprotective effect (13).
The subcutaneous or intranasal administration of the insulin peptide B9–23 can also prevent diabetes in NOD mice (3), similarly to the neonatal administration of the B10–24 peptide (9). However, the administration of several antigen-derived peptides, in adjuvant, to newborn NOD mice, resulted in the early activation of multiple autoimmune responses (14). Similarly, the intrathymic injection of T1D-associated antigens or peptides resulted in delayed or accelerated diabetogenesis, depending on the peptides used (15). Recent studies in mice have also shown that the repeated administration of insulin or GAD peptides, including the B9–23 peptide, can induce lethal anaphylactic responses (16, 17). While similar occurrences have not been reported in a phase I trial in which a modified insulin B9–23 peptide (altered peptide ligand) was administered subcutaneously to normal individuals, the administration of any proinsulin or insulin peptides has not yet been attempted in the context of T1D prevention trials or in an attempt to halt autoimmunity in new onset patients. Clearly, more studies are needed as a number of factors, including the nature of the antigen or peptide, the dose, the route and/or mode of administration, and the stage of disease at which the subjects enter the trial, can all influence the efficacy and safety of these experimental treatments.
The double-edged sword
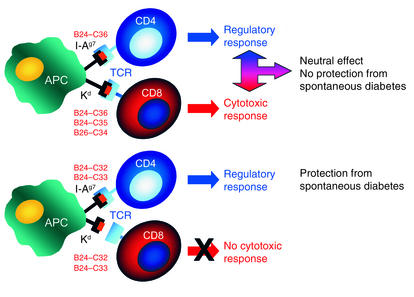
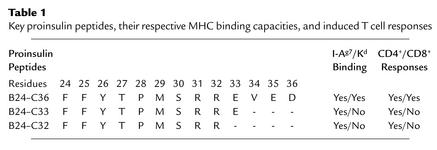
In the current issue of the JCI, Martinez et al. report on a new element that will need to be considered when attempting peptide-based T1D therapy (18). While the goal of peptide administration is to induce regulatory cells and inhibit specific autoimmune responses, the data show that, depending on the peptide used, one may also induce undesired cytotoxic CD8+ T cell responses. In this study, the intranasal administration of the B24–C36 proinsulin peptide to NOD mice induced regulatory cells that could transfer disease protection to another mouse, but the peptide-treated mice were not protected from developing spontaneous disease. The authors then noted that the B24–C36 peptide contains binding motifs not only for I-Ag7, the MHC class II molecule of the NOD mouse, but also for the MHC class I molecule Kd. The B24–C36 peptide contains the B25–C34 and B26–C34 epitopes that bind to Kd, and mice immunized with the latter two peptides mounted specific cytotoxic responses. In contrast, the systemic administration of B25–C34 reduced spontaneous diabetes incidence, confirming a role for CD8+ responses to this epitope in the natural disease process. Thus, intranasal administration of the B24–C36 peptide resulted in both regulatory CD4+ T cell and cytotoxic CD8+ T cell responses recognizing the Kd-restricted B25–C34 epitope contained in the B24–C36 peptide. Such cytotoxic responses blunted the protective effect associated with the induction of the regulatory cells, suggesting that mucosal administration of antigen can sometimes be a double-edged sword. To circumvent this problem, the authors designed a strategy to “disable” the CD8+ epitope contained in the B24–C36 peptide, in other words, to prevent CD8+ T cells from recognizing this epitope, while preserving the ability to induce regulatory CD4+ T cells (Figure 1 and Table 1). This was achieved by treating the mice with truncated peptides, B24–C33 or B24–C32, still capable of binding to IAg7 but not to Kd. Intranasal administration of the truncated peptides resulted indeed in a significant reduction in the incidence of spontaneous diabetes.
Figure 1.
Disabling a CD8+ epitope. Intranasal administration of different proinsulin peptides results in presentation from an antigen-presenting cell (APC) to CD4+ and CD8+ T cells, depending on the peptide administered. Proinsulin peptides B24–C36, B24–C35, and B26–C34 can bind to the MHC class I molecule (Kd), resulting in the activation of cytotoxic T cells. The use of truncated peptides that do not contain the residues critical for binding to Kd, but still bind to the NOD mouse MHC class II molecule (I-Ag7), allows for selective activation of regulatory CD4+ T cells. The same APC is shown presenting simultaneously to both CD4+ and CD8+ T cells for illustration purposes. TCR, T cell receptor.
Table 1.
Key proinsulin peptides, their respective MHC binding capacities, and induced T cell responses
The findings reported by Martinez et al. (18) suggest that the undetected induction of CD8+ T cell responses could explain at least some of the contrasting outcomes reported by several experimental studies and clinical trials. Most importantly, this study has practical implications for the design of clinical trials based on the administration of peptides for preventing autoimmunity. Based on these findings, it would seem helpful to select putative therapeutic peptides for their ability to selectively bind class II but not class I molecules that are used as restriction elements by CD8+ T cells and could potentially antagonize the beneficial effects of class II–restricted, regulatory CD4+ T cells. This strategy can effectively disable the potential of eliciting cytotoxic responses and maximize the protective effects of the regulatory cells. While this study was limited to the NOD mouse MHC molecules, the availability of mouse strains expressing human class II and class I molecules (19–21), in particular those associated with T1D susceptibility, is increasing. This offers researchers the opportunity to test such binding predictions and may provide an in vivo readout of the responses induced by a given peptide also in relation to dose and route of administration. Screening of candidate peptides for clinical trials using such humanized mouse models could guide the choice of peptides for future prevention trials and might maximize our chances of achieving both efficacy and safety.
Footnotes
See the related article beginning on page 1365.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: type 1 diabetes (T1D); glutamic acid decarboxylase (GAD); Diabetes Prevention Trial–Type 1 (DPT-1).
References
- 1.Nepom GT. Therapy of autoimmune diseases: clinical trials and new biologics. Curr. Opin. Immunol. 2002;14:812–815. doi: 10.1016/s0952-7915(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 2.Liu E, Eisenbarth GS. Type 1A diabetes mellitus-associated autoimmunity. Endocrinol. Metab. Clin. North Am. 2002;31:391–410. doi: 10.1016/s0889-8529(01)00017-2. [DOI] [PubMed] [Google Scholar]
- 3.Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9–23) Proc. Natl. Acad. Sci. U. S. A. 1996;93:956–960. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong FS, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, et al. Evidence that a peptide spanning the B-C junction of proinsulin is an early autoantigen epitope in the pathogenesis of type 1 diabetes. J. Immunol. 2001;167:4926–4935. doi: 10.4049/jimmunol.167.9.4926. [DOI] [PubMed] [Google Scholar]
- 6.Rudy G, et al. Similar peptides from two beta cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Mol. Med. 1995;1:625–633. [PMC free article] [PubMed] [Google Scholar]
- 7.Muir A, Schatz D, Maclaren N. Antigen-specific immunotherapy: oral tolerance and subcutaneous immunization in the treatment of insulin-dependent diabetes. Diabetes Metab. Rev. 1993;9:279–287. doi: 10.1002/dmr.5610090408. [DOI] [PubMed] [Google Scholar]
- 8.Bergerot I, Fabien N, Maguer V, Thivolet C. Oral administration of human insulin to NOD mice generates CD4+ T cells that suppress adoptive transfer of diabetes. J. Autoimmun. 1994;7:655–663. doi: 10.1006/jaut.1994.1050. [DOI] [PubMed] [Google Scholar]
- 9.Maron R, Guerau-de-Arellano M, Zhang X, Weiner HL. Oral administration of insulin to neonates suppresses spontaneous and cyclophosphamide induced diabetes in the NOD mouse. J. Autoimmun. 2001;16:21–28. doi: 10.1006/jaut.2000.0471. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson M, Maclaren N, Luchetta R, Burr I. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes. 1990;39:933–937. doi: 10.2337/diab.39.8.933. [DOI] [PubMed] [Google Scholar]
- 11.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J. Exp. Med. 1996;184:2167–2174. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Prevention Trial–Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 13.Harrison LC, et al. Intranasal insulin trial (INIT) in preclinical type 1 diabetes. Diabetes. 1999;48:A206. (Abstr.) [Google Scholar]
- 14.Tian J, Olcott AP, Kaufman DL. Antigen-based immunotherapy drives the precocious development of autoimmunity. J. Immunol. 2002;169:6564–6569. doi: 10.4049/jimmunol.169.11.6564. [DOI] [PubMed] [Google Scholar]
- 15.Cetkovic-Cvrlje M, et al. Retardation or acceleration of diabetes in NOD/Lt mice mediated by intrathymic administration of candidate beta-cell antigens. Diabetes. 1997;46:1975–1982. doi: 10.2337/diab.46.12.1975. [DOI] [PubMed] [Google Scholar]
- 16.Liu E, et al. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9-23 and B:13-23. J. Clin. Invest. 2002;110:1021–1027. doi:10.1172/JCI200215488. doi: 10.1172/JCI15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedotti R, et al. Severe anaphylactic reactions to glutamic acid decarboxylase (GAD) self peptides in NOD mice that spontaneously develop autoimmune type 1 diabetes mellitus. BMC Immunol. 2003;4:2. doi: 10.1186/1471-2172-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez NR, et al. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J. Clin. Invest. 2003;111:1365–1371. doi:10.1172/JCI200317166. doi: 10.1172/JCI17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das P, Abraham R, David C. HLA transgenic mice as models of human autoimmune diseases. Rev. Immunogenet. 2000;2:105–114. [PubMed] [Google Scholar]
- 20.Wong FS, Moustakas AK, Wen L, Papadopoulos GK, Janeway CA., Jr Analysis of structure and function relationships of an autoantigenic peptide of insulin bound to H-2K(d) that stimulates CD8 T cells in insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5551–5556. doi: 10.1073/pnas.072037299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong FS, Wen L. The study of HLA class II and autoimmune diabetes. Curr. Mol. Med. 2003;3:1–15. doi: 10.2174/1566524033361591. [DOI] [PubMed] [Google Scholar]