Abstract
Fibroblast growth factors (FGFs) are signaling molecules of the isthmic organizer, which regulates development of the midbrain and cerebellum. Tissue-specific inactivation of one of the FGF receptor (FGFR) genes, Fgfr1, in the midbrain and rhombomere 1 of the hindbrain of mouse embryos results in deletion of the inferior colliculi in the posterior midbrain and vermis of the cerebellum. Analyses of both midbrain–hindbrain and midbrain-specific Fgfr1 mutants suggest that after establishment of the isthmic organizer, FGFR1 is needed for continued response to the isthmic signals, and that it has direct functions on both sides of the organizer. In addition, FGFR1 appears to modify cell adhesion properties critical for maintaining a coherent organizing center. This may be achieved by regulating expression of specific cell-adhesion molecules at the midbrain–hindbrain border.
Keywords: cerebellum/Cre recombinase/development/FGF/isthmic organizer/midbrain
Introduction
Organizing centers established at the borders between developmental units are commonly used for tissue patterning during embryogenesis. One such organizing center is the isthmic organizer, which forms at the junction between developing midbrain and rhombomere 1 of the hindbrain. Transplantation studies with avian embryos have demonstrated that tissue containing the midbrain– hindbrain junction can induce cells in more anterior and posterior regions of the brain to adopt fates characteristic for midbrain and rhombomere 1 (Nakamura et al., 1988; Martinez et al., 1991, 1995). Under the control of the isthmic organizer the dorsal midbrain develops into superior and inferior colliculi, relaying visual and auditory stimuli, respectively. In turn, dorsal rhombomere 1 forms the cerebellum involved in processes such as motor coordination. Development of some of the ganglia in the ventral brainstem is also thought to be regulated by the isthmic organizer.
The molecular basis for the development and function of the isthmic organizer is beginning to be understood. The border of expression of Otx2 and Gbx2, two homeodomain transcription factors, separates the cells of the future midbrain from the hindbrain and determines the position of the isthmic organizer (Millet et al., 1996, 1999; Broccoli et al., 1999). In addition to the early regionalization of the neurectoderm, signals from the mesoderm are required for the induction of genes, such as En1 and En2, in the mid- and hindbrain region (Hemmati-Brivanlou et al., 1990; Ang and Rossant, 1993). Studies with avian embryos have suggested that fibroblast growth factors (FGFs), potentially FGF4 transiently expressed in the anterior notochord, are important for the induction of mid- and hindbrain-specific gene expression (Shamim et al., 1999). In addition, Fgf8, expressed in the cardiogenic mesoderm, has been suggested to play a role in the induction of the midbrain (Crossley et al., 1996). In addition to the En genes, the paired box transcription factor genes Pax2 and Pax5 are also activated early in the entire midbrain–hindbrain region.
Later in development, patterning and growth of the midbrain and hindbrain rely on the isthmic organizer, which forms in the neurectoderm at the Otx2/Gbx2 border. An important signaling molecule of the isthmic organizer is FGF8. In the mouse embryo, Fgf8 expression is activated after Pax2 and En1 in the entire rhombomere 1 of the hindbrain, and later restricted to a stripe in the most anterior hindbrain (Crossley and Martin, 1995). Both gain- and loss-of-function experiments have suggested that FGF8 is essential for the activity of the isthmic organizer. FGF8 containing beads can mimic the isthmic transplants in induction of midbrain and cerebellum in the diencephalon or cerebellum in the posterior hindbrain (Crossley et al., 1996; Martinez et al., 1999; Irving and Mason, 2000). In addition, zebrafish Fgf8 mutants fail to maintain isthmic gene expression (Reifers et al., 1998), and a hypomorphic mutation in the mouse Fgf8 gene causes midbrain and cerebellar defects (Meyers et al., 1998). Other FGF family members, such as Fgf17 and Fgf18, are also expressed in the isthmic region and may contribute to organizer function (Maruoka et al., 1998; Xu et al., 2000).
Another important signaling molecule of the isthmic organizer is WNT1. Initially, Wnt1 is detected in the entire midbrain, but later Wnt1-positive cells are found as a narrow stripe in the most posterior midbrain next to the Fgf8-expressing cells in the anterior hindbrain. FGF-containing beads can induce Wnt1 expression, implicating Wnt1 as one of the targets of FGF signaling (Crossley et al., 1996). On the other hand, Fgf8 expression is down-regulated in the Wnt1 mutants, suggesting that a midbrain-derived signal in turn maintains Fgf8 (Lee et al., 1997). Other FGF-regulatable genes include Pax2/5 and En1/2, which are expressed around the isthmic organizer in a graded manner. These signaling molecules and transcription factors appear to be involved in a complex regulatory network responsible for the maintenance of their expression and development of the midbrain–hindbrain region (reviewed in Wurst and Bally-Cuif, 2001; Liu and Joyner, 2002).
Cells sense the FGFs by tyrosine kinase-type cell surface receptors. Four FGF receptor (FGFR) genes exist in the mammalian genome (Fgfr1–Fgfr4). All of the FGFRs can bind several FGF family members and the receptor–ligand interaction is affected by the proteoglycan co-receptors expressed on the target cell. Studies with mice carrying null mutations in each of the Fgfr genes have suggested that two of these, Fgfr1 and Fgfr2, carry out the majority of FGF receptor functions during early embryonic development. Embryos homozygous for a Fgfr1-null allele fail in gastrulation (Deng et al., 1994; Yamaguchi et al., 1994), and FGFR1 has been suggested to regulate adhesive and migratory properties of mesodermal cells during their traversal of the primitive streak (Ciruna and Rossant, 2001). In addition to the primitive streak, Fgfr1 is widely expressed in other embryonic tissues, including developing nervous system (Yamaguchi et al., 1992; Walshe and Mason, 2000).
As described above, FGF signaling has been implicated at several stages of development of the mid- and hindbrain. However, the direct target tissues and the receptors of FGF signals are still poorly understood. In the current work we demonstrate by tissue-specific mutagenesis that Fgfr1 is required after establishment of the midbrain–hindbrain region for the response to the signals from the isthmic organizer. Our results further suggest that FGF signaling through FGFR1 is directly involved in regulation of gene-expression in both the mid- and hindbrain. In addition, FGFR1 appears to be important for specific cell-adhesive characteristics at the midbrain–hindbrain boundary.
Results
Expression of Fgfr1 and Fgfr2 during early development of the mid- and hindbrain
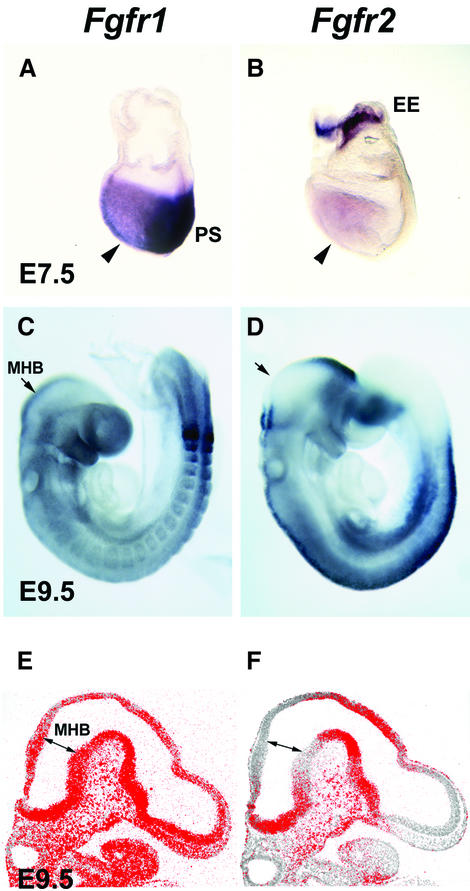
We first analyzed the expression of two potential mediators of isthmic signaling, Fgfr1 and Fgfr2, in mouse embryos by in situ mRNA hybridization. At a late head-fold stage [embryonic day (E) 7.5], around the stage when the isthmic organizer is induced, Fgfr1 expression was detected in the head folds (Figure 1A). Prominent expression was also detected in other regions of the developing embryo, including the primitive streak. These results are consistent with the earlier studies of Fgfr1 expression (Yamaguchi et al., 1992). At E7.5, Fgfr2 was also found to be expressed in the head folds (Figure 1B). Strong Fgfr2 expression was also detected in the extra-embryonic ectoderm. Later, at E8.5–9.5, widespread Fgfr1 expression was observed in the developing central nervous system, including the midbrain–hindbrain region (Figures 1C and E, and 2J and K). Fgfr2 was detected in the diencephalon, hindbrain and spinal cord, especially in the dorsal region (Figure 1D). In contrast to Fgfr1, no Fgfr2 expression was detected at the midbrain–hindbrain boundary in the anterior rhombomere 1 or posterior midbrain (Figure 1F).
Fig. 1. Expression of Fgfr1 and Fgfr2. Whole-mount in situ hybridization analysis of the expression of (A) Fgfr1 and (B) Fgfr2 at E7.5. Expression of both genes is detected in the headfolds (arrowheads). In addition, Fgfr1 is strongly expressed in the primitive streak region (PS) and Fgfr2 in the extra-embryonic ectoderm (EE). At E9.5, Fgfr1 is widely expressed (C), whereas Fgfr2 expression is not detected in the anterior rhombomere 1 and the midbrain (D). In situ hybridization analysis of (E) Fgfr1 and (F) Fgfr2 expression on sagittal sections of E9.5 embryos. Fgfr1 is widely expressed in the neural tube, whereas Fgfr2 appears to be absent from the tissue around the isthmus. Arrows in (C)–(F) mark the midbrain–hindbrain boundary (MHB).
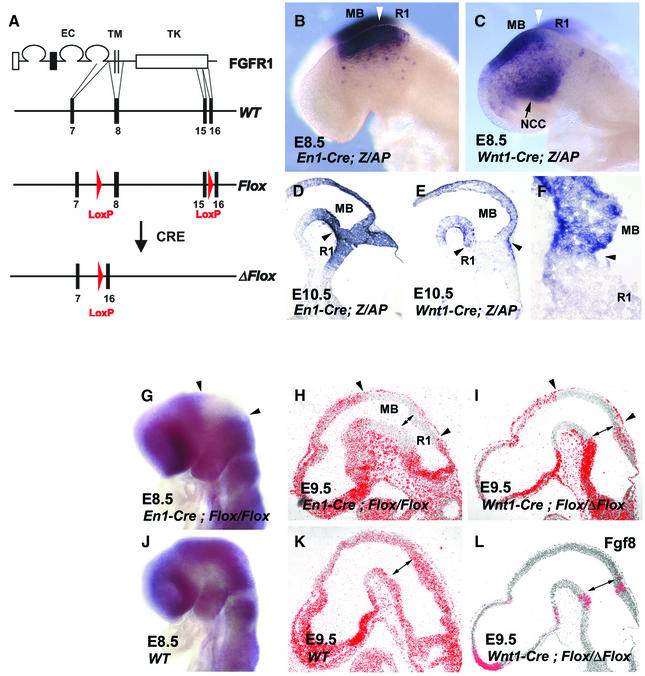
Fig. 2. The conditional Fgfr1 allele, Fgfr1flox, and its inactivation by En1-Cre and Wnt1-Cre. (A) Schematic presentation of the Fgfr1flox allele and its inactivation by the Cre-recombinase. The structures of the FGFR1 protein and the wild-type Fgfr1 locus are shown at the top. Only exons 7, 8, 15 and 16 are depicted. LoxP sites were introduced into introns 7 and 15 by sequential gene targeting to generate the Fgfr1flox allele. Cre-mediated recombination of the Fgfr1flox deletes the transmembrane and most of the intracellular region encoding exons resulting in the inactive Fgfr1Δflox allele. EC, extracellular domain; TM, transmembrane domain; TK, tyrosine kinase domain. To characterize the Cre activity expressed by the En1-Cre and Wnt1-Cre mice, they were crossed with mice carrying a Z/AP reporter allele. (B) Cre-mediated recombination between LoxP sites in the Z/AP allele results in alkaline phosphatase (AP) expression in the midbrain (MB) and rhombomere 1 (R1) of an E8.5 En1-Cre/+; Z/AP/+ embryo. (C) In an E8.5 Wnt1-Cre/+; Z/AP/+ embryo, AP activity is detected in the midbrain and neural crest cells (NCC). Frozen sections of E10.5 (D) En1-Cre/+; Z/AP/+ and (E and F) Wnt1-Cre/+; Z/AP/+ embryos are shown. In the En1-Cre/+; Z/AP/+ embryos, AP was expressed in the midbrain and rhombomere 1. In Wnt1-Cre/+; Z/AP/+ embryos, AP activity was detected in the midbrain and scattered cells of the rhombomere 1. A boundary is observed between the AP-positive midbrain and mostly AP-negative rhombomere 1 (F). Arrowheads in (B)–(F) point to the midbrain–hindbrain boundary. (G–L) Inactivation of Fgfr1 expression by En1-Cre and Wnt1-Cre. Whole-mount in situ hybridization analysis of E8.5 (10 somite stage) En1-Cre/+; Fgfr1flox/flox (G) and wild-type (WT) embryos reveals inactivation of Fgfr1 transcription in the midbrain–hindbrain region by En1-Cre (arrowheads). In situ hybridization analysis of Fgfr1 expression in sagittal sections of E9.5 En1-Cre/+; Fgfr1flox/flox (H), Wnt1-Cre/+; Fgfr1flox/Δflox (I) and wild-type (K) embryos. In En1-Cre/+; Fgfr1flox/flox embryos, inactivation of Fgfr1 expression occurs both in the midbrain and rhombomere 1, whereas in Wnt1-Cre/+; Fgfr1flox/Δflox embryos rhombomere 1 still expresses Fgfr1. Regions of affected Fgfr1 expression are indicated by arrowheads (H and I). A parallel section to the one shown in (I) hybridized with the Fgf8 probe (L). A double-headed arrow in (H)–(L) marks the midbrain–hindbrain boundary.
Generation of a conditional Fgfr1 allele
To study the function of Fgfr1 in the development of the mid- and hindbrain, we wanted to inactivate it in a tissue-specific fashion. To generate a Fgfr1 allele, Fgfr1flox, which can be inactivated by the site-specific recombinase Cre, we employed targeting vectors described earlier (Partanen et al., 1998). Using these vectors and transient Cre expression in embryonic stem cells, we sequentially introduced recognition sites of Cre (loxP sites) into two different introns of the Fgfr1 gene (Figure 2A; and Supplementary figure 1 available at The EMBO Journal Online). In the resulting allele, Fgfr1flox, exons 8–15 encoding the transmembrane domain, juxtamembrane domain and most of the tyrosine kinase domain of FGFR1, are flanked by two loxP sites. Mice hetero- or homozygous for the Fgfr1flox allele are phenotypically indistinguishable from their wild-type littermates. Thus, the introduced loxP sites themselves do not appear to interfere with Fgfr1 expression.
To test the functionality of the Fgfr1flox allele, we crossed the Fgfr1flox/+ mice with mice carrying a Pgk-Cre transgene driving ubiquitous Cre expression (Lallemand et al., 1998). In mice heterozygous for both the Fgfr1flox allele and the Pgk-Cre transgene, recombination between the loxP sites resulted in excision of the genomic DNA flanked by the loxP sites, generating a novel allele, Fgfr1Δflox. The mice heterozygous for the Fgfr1Δflox allele were normal. Thus, the remaining Fgfr1 sequences in the Fgfr1Δflox do not appear to drive expression of dominant-negative gene products, which could significantly interfere with FGF signaling. Embryos homozygous for the Fgfr1Δflox allele have gastrulation defects and die at around E9.5, closely resembling the Fgfr1-null mutants reported previously (Deng et al., 1994; Yamaguchi et al., 1994; data not shown). Therefore, Fgfr1flox behaves as a conditional allele, which can be fully inactivated by the Cre recombinase.
Tissue-specific inactivation of Fgfr1 in the midbrain–hindbrain and midbrain
To study the role of FGFR1 in the isthmic organizer signaling, we wanted to inactivate Fgfr1 in the neuro epithelium of the mid- and hindbrain after their regional specification. For this purpose, we used the En1-Cre mice, which express the Cre recombinase from the En1 locus (Kimmel et al., 2000). We also wanted to inactivate Fgfr1 specifically in the midbrain. For this, we used mice carrying a transgene expressing Cre recombinase under the Wnt1 promoter (Danielian et al., 1998). To analyze the patterns of Cre activity in the En1-Cre and Wnt1-Cre mice, we first crossed them with the Z/AP reporter mouse line (Lobe et al., 1999). The Z/AP reporter allele was observed to be recombined efficiently and specifically in the midbrain–hindbrain region of the En1-Cre/+; Z/AP/+ embryos already at the 8 somite stage (E8.5; Figure 2B). Analyses at E9.5 and E10.5 revealed efficient recombination both in the midbrain and entire rhombomere 1 (Figure 2D; data not shown). Efficient Cre-mediated recombination of the Z/AP allele was also detected in the midbrain of Wnt1-Cre/+; Z/AP/+ mice. In contrast, except for a few scattered cells, cells in the rhombomere 1 carried mostly the unrecombined Z/AP allele. At E9.5–12.5, all the neuroepithelial cells of the midbrain appeared to carry the recombinant allele (Figure 2E and F; data not shown). Although isolated recombinant cells were also observed in the rhombomere 1 (Figure 2E), a boundary between recombinant cells in the midbrain and mostly unrecombinant cells in the hindbrain could be observed (Figure 2E and F).
We then crossed the Fgfr1flox mice with the En1-Cre and Wnt1-Cre mice to inactivate Fgfr1 in a tissue-specific manner. To determine the pattern of Cre-mediated recombination and inactivation of the Fgfr1flox allele, we carried out in situ hybridization analyses of En1-Cre/+; Fgfr1flox/flox and Wnt1-Cre/+; Fgfr1flox/Δflox embryos with a Fgfr1 cDNA probe containing exonic sequences between the loxP sites in the Fgfr1flox allele. In En1-Cre/+; Fgfr1flox/flox embryos at E8.5, reduction in Fgfr1 signal was observed already at the 8 somite stage, and at the 10–11 somite stage the midbrain–hindbrain region appeared negative for Fgfr1 expression (Figure 2G and J; Supplementary figure 3). Radioactive in situ hybridization analysis on tissue sections of En1-Cre/+; Fgfr1flox/flox embryos at E9.5 further demonstrated that midbrain and the entire rhombomere 1 were negative for Fgfr1 expression (Figure 2H and K). In contrast, the Wnt1-Cre/+; Fgfr1flox/Δflox embryos lacked Fgfr1 expression in the midbrain, but still expressed abundant Fgfr1 in the rhombomere 1 (Figure 2I and L). Thus, our experimental approaches allow us to inactivate Fgfr1 by E9.5 in the midbrain–hindbrain and midbrain using the En1-Cre and Wnt1-Cre mice, respectively.
Inactivation of Fgfr1 in the mid- and hindbrain results in ataxia
The majority of En1-Cre/+; Fgfr1flox/flox mice survived until adulthood. However, they were visibly uncoordinated, showing abnormal gait and wide stance. The impaired motor coordination of the En1-Cre/+; Fgfr1flox/flox mice (n = 11) was further demonstrated by behavioral tests, including stationary beam and rotarod assays (Table I).
Table I. Behavioral analysis of Fgfr1 midbrain–hindbrain mutants.
| Wild type (n = 12) | En1-Cre/+; Flox/Flox (n = 11) | P | |
|---|---|---|---|
| Rotarod assay | |||
| t [mean (SD)]a | 176 (59) | 49 (79) | <0.001 |
| Stationary beam assay | |||
| t [mean (SD)]a | 60 (1.4) | 4.3 (5.4) | <0.001 |
| d [mean (SD)]b | 90 (80) | 0.40 (1.0) | <0.005 |
aTime on rotarod/beam (s).
bDistance walked on beam (cm).
Cerebellar and midbrain defects caused by inactivation of Fgfr1 in the midbrain–hindbrain region
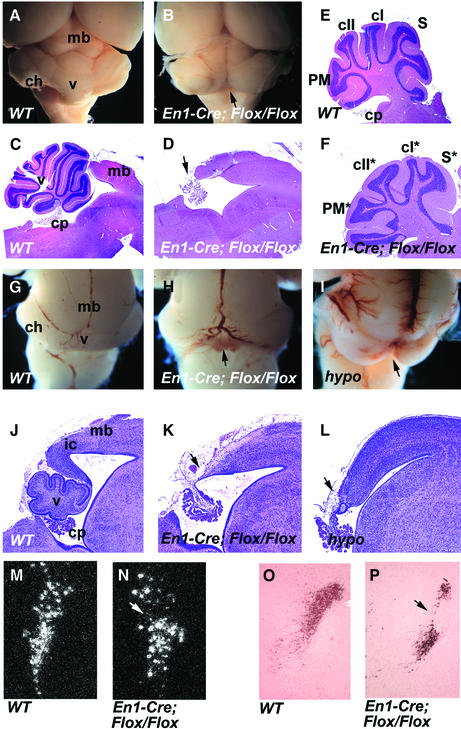
Possibly contributing to the behavioral defects, we observed severe abnormalities in the cerebellum of the En1-Cre/+; Fgfr1flox/flox mice. In adults, the vermis of the cerebellum was completely absent (n = 5; Figure 3A–D). The cerebellar hemispheres were present, although their foliation was abnormal (Figure 3E and F). The defect in the cerebellar vermis was also obvious in the newborn En1-Cre/+; Fgfr1flox/flox mice (n = 3). In addition to the cerebellum, extensive deletions including the inferior colliculi were evident in the posterior midbrain (Figure 3G, H, J and K). A comparable phenotype was observed in newborn En1-Cre/+; Fgfr1flox/Δflox mice (n = 2; data not shown).
Fig. 3. Morphology of the midbrain and cerebellum of the midbrain–hindbrain-specific and hypomorphic Fgfr1 mutants. Whole-mount view of (A) wild-type and (B) En1-Cre/+; Fgfr1flox/flox adult brains. The entire vermis is missing in En1-Cre/+; Fgfr1flox/flox mice (arrow). (C and D) Midsagittal and (E and F) parasagittal sections of adult wild-type (C and E) and En1-Cre/+; Fgfr1flox/flox (D and F) brains. Complete aplasia of the vermis is evident in the En1-Cre/+; Fgfr1flox/flox mice [arrow in (D)]. The En1-Cre/+; Fgfr1flox/flox mice have cerebellar hemispheres, but their pattern of foliation is altered (F). Whole-mount views and corresponding midsagittal sections of new-born wild-type (G and J), En1-Cre/+; Fgfr1flox/flox (H and K) and Fgfr1n15YF/n15YF (I and L) mice. Aplasia of the vermis and deletions of the inferior colliculi can be seen in both types of Fgfr1 mutants (arrows). Locus coeruleus, identified by Dopamine-β-hydroxylase mRNA in situ hybridization of adult brain sections (M and N) and anti-tyrosine hydroxylase immuno staining of newborn brain sections (O and P) appears disorganized in the En1-Cre/+; Fgfr1flox/flox mutants (N and P) compared with wild type (M and O). cI, crus I; cII, crusII; ch, cerebellar hemisphere; cp, choroids plexus; ic, inferior colliculus; mb, midbrain; PM, paramedian lobule; S, simplex; v, vermis. The mutant hemisphere lobes are labeled with asterisks.
We also analyzed the development of the midbrain and cerebellum in mice homozygous for the hypomorphic Fgfr1 alleles, Fgfr1n7 and Fgfr1n15YF, in which the Fgfr1 transcript levels are reduced by ∼80 and 90%, respectively (Partanen et al., 1998). Defects in the midline vermis and partial deletions of the inferior colliculi of the midbrain were observed in both Fgfr1n7/n7 (n = 8) and Fgfr1n15YF/n15YF (n = 10) newborn mice (Figure 3I and L; data not shown).
In addition to the dorsal midbrain and cerebellum, we analyzed several ganglia in the alar and basal plates of the midbrain–hindbrain region of En1-Cre/+; Fgfr1flox/flox mutants, including locus coeruleus, substantia nigra, cranial nerves III and IV, pontine nucleus, the nucleus pedunculoponinus tegmentalis and nucleus parabigeminalis. Tyrosine hydroxylase-positive neurons were found in newborn and adult mutants, in both the substantia nigra of the midbrain and the locus coeruleus of the rhombomere 1. Also, Dopamine-β-hydroxylase mRNA in situ hybridization revealed locus coeruleus in the mutants. However, locus coeruleus appeared to be disorganized compared with the wild type (Figure 3M–P). Whole-mount neurofilament staining of E10.5 En1-Cre/+; Fgfr1flox/flox embryos revealed both the oculomotor (III) nerve from the midbrain and trochlear (IV) nerve from the anterior rhombomere 1. Consistently, the oculomotor and trochlear nuclei also appeared unaltered in adult mutants. In addition, pontine nuclei as well as mesopontine nuclei such as nucleus pedunculopontinus tegmentalis and nucleus parabigeminalis were found to be present in the mutants (Supplementary figure 2; data not shown). Thus, in contrast to the dorsal brain structures, no extensive deletions or alterations were observed in the basal plate of the midbrain–hindbrain region.
Inactivation of Fgfr1 in the midbrain–hindbrain region does not affect cellular survival at E9.5
In order to understand the developmental basis for the deletions in the dorsal midbrain and cerebellum, we analyzed the pattern of cell death in En1-Cre/+; Fgfr1flox/flox embryos. TUNEL analysis of apoptosis at E9.5 revealed no statistically significant difference in the number of apoptotic cells in the midbrain–hindbrain region of En1-Cre/+; Fgfr1flox/flox embryos (n = 10) compared with the wild type (n = 8). In addition, analysis of semi-thin sections of E9.5 mutants (n = 3) and whole-mount Nile Blue staining of E10.5 mutants (n = 2) showed no difference in the cellular viability compared with wild-type littermates (data not shown). Therefore, although we cannot completely rule out any effect on the apoptotic index in the midbrain–hindbrain region, cell death at around E9.5 does not appear to be a major component of the phenotype of En1-Cre/+; Fgfr1flox/flox embryos.
Fgfr1 is required for sustained expression of isthmic organizer dependent genes
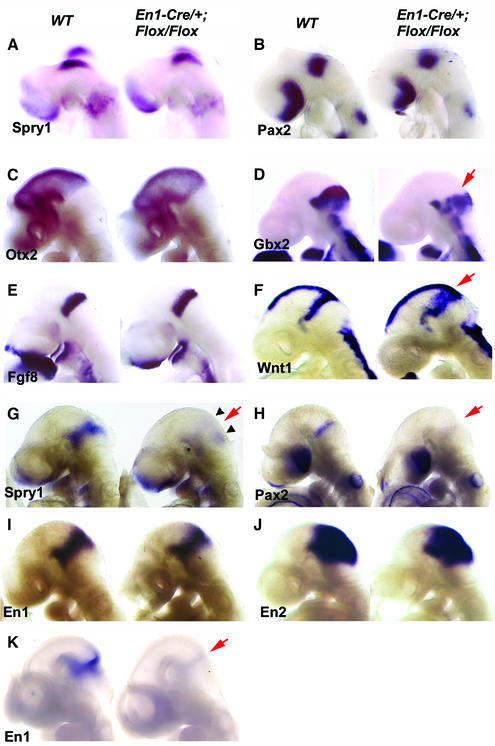
To analyze how the mid- and hindbrain-specific inactivation of Fgfr1 affects the early development of the isthmic region, we analyzed gene expression in E8.5–10.5 En1-Cre/+; Fgfr1flox/flox embryos by whole-mount in situ hybridization. At E8.5 (8–10 somite stage), Pax2, Wnt1 and Sprouty1, a FGF-inducible inhibitor of receptor tyrosine kinase signaling (Minowada et al., 1999), were expressed in En1-Cre/+; Fgfr1flox/flox embryos at a level and pattern comparable to the wild type (Figure 4A and B; data not shown). At E9.5, an anterior marker Otx2 (Figure 4C) and a posterior marker Gbx2 (Figure 4D) were expressed in the correct domains in the En1-Cre/+; Fgfr1flox/flox embryos. In addition, Fgf8 was expressed in the anterior hindbrain of E9.5 En1-Cre/+; Fgfr1flox/flox embryos (Figure 4E), although its expression decreased at later stages especially in the dorsal isthmus (data not shown). Thus, the expression of a specific set of genes, including signaling molecules thought to be important for isthmic organizer activity, was established around the midbrain–hindbrain boundary in the En1-Cre/+; Fgfr1flox/flox embryos at E8.5–9.5.
Fig. 4. Analysis of gene expression in the midbrain–hindbrain specific Fgfr1 mutants. Whole-mount in situ hybridization of E8.5 (A and B), E9.5 (C–J) and E10 (K) wild-type and En1-Cre/+; Fgfr1flox/flox embryos with Sprouty1 (A, G), Pax2 (B, H), Otx2 (C), Gbx2 (D), Fgf8 (E), Wnt1 (F), En1 (I, K) and En2 (J) probes. Red arrows indicate altered gene expression. Small arrowheads in (G) indicate remaining Sprouty1 expression in regions distal to the isthmus. See text for details.
In contrast to E8.5, expression of several genes thought to depend on isthmic signals failed to be maintained at later stages. Down-regulation of Sprouty1 was first observed at the 12 somite stage and Sprouty expression was completely abolished from the isthmic domain at E9.5 (Figure 4G; Supplementary figure 3). Interestingly, some Sprouty1 expression was still detected further away from the isthmus in dorsal and ventral regions. Also, expression of Pax2 was virtually absent by E9.5 (Figure 4H). Other isthmic target genes were down-regulated slightly later. En1 and En2 were still expressed at E9.5 (Figure 4I and J). However, their expression rapidly decreased thereafter, and by E10 En1 was barely detectable in either mid- or hindbrain (Figure 4K). In addition, expression of Wnt1 was down-regulated in the posterior midbrain, especially in its dorsal region, after E9.5 (data not shown). Interestingly, before the down-regulation of the Wnt1 signal, Wnt1 expression was observed as a broadened stripe in the posterior midbrain (Figure 4F). In contrast to the wild-type embryos, in which the Wnt1-positive cells formed a tight band next to the Fgf8 expressing anterior hindbrain, the Wnt1-positive and -negative cells were extensively mixed in the En1-Cre/+; Fgfr1flox/flox embryos. Also, Otx2 and especially Gbx2 (Figure 4D) showed heterogeneous expression borders.
Tissue-specific inactivation of Fgfr1 in the midbrain causes deletion of posterior midbrain and cerebellar abnormalities
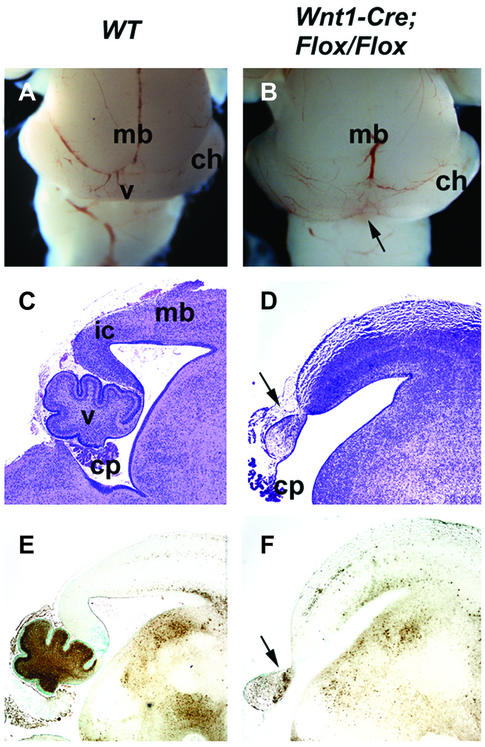
To determine whether Fgfr1 directly regulates development of the midbrain, we crossed the Fgfr1flox mice with mice carrying a Wnt1-Cre transgene. The Wnt1-Cre/+; Fgfr1flox/flox mice die neonatally, possibly due to defects in the craniofacial neural crest (Trokovic et al., 2003). Analysis of the brains of the newborn Wnt1-Cre/+; Fgfr1flox/flox mice (n = 5) revealed deletion of the inferior colliculi of the midbrain, reminiscent of the En1-Cre/+; Fgfr1flox/flox mice (Figure 5A–D). Development of the dorsal cerebellum was also abnormal. However, in contrast to the En1-Cre/+; Fgfr1flox/flox mice, the vermis was not completely missing although it was severely malformed in the Wnt1-Cre/+; Fgfr1flox/flox mutants (Figure 5C–F). Some cellular differentiation was still observed in the mutant vermis by immunohistochemistry with anti-calbindin antibodies (Figure 5F). A comparable phenotype was seen in the Wnt1-Cre/+; Fgfr1flox/Δflox mice (n = 3; data not shown).
Fig. 5. Morphology of the midbrain and cerebellum of the midbrain-specific Fgfr1 mutants. Whole-mount views (A and B) and mid-sagittal sections (C and D) of brains of newborn wild-type (A and C) and Wnt1-Cre/+; Fgfr1flox/flox (B and D) mice. Calbindin expression in wild-type (E) and Wnt1-Cre/+; Fgfr1flox/flox mice (F). Arrows indicate the deletion of the inferior colliculi and malformed vermis. ch, cerebellar hemisphere; cp, choroid plexus; mb, midbrain; v, vermis.
Fgfr1 is autonomously required in the midbrain for the maintenance of expression of isthmus-regulated genes
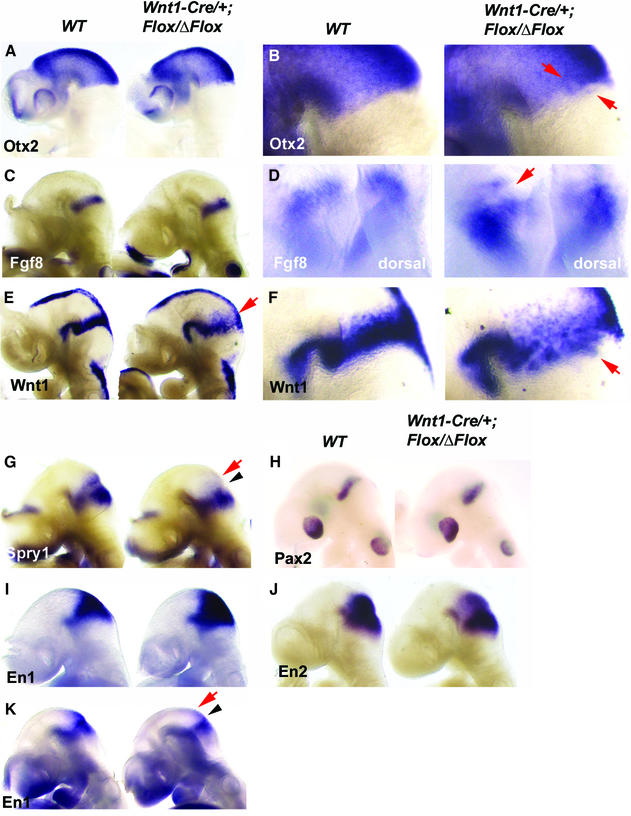
We next analyzed isthmic gene expression in Wnt1-Cre/+; Fgfr1flox/Δflox embryos at E9.5. As judged by the expression of Otx2, Gbx2 and Fgf8 (Figure 6A and C; data not shown), the isthmic organizer forms and is correctly positioned in the Wnt1-Cre/+; Fgfr1flox/Δflox embryos. However, expression of isthmus-regulated genes was abnormal specifically in the midbrain. In contrast to the En1-Cre/+; Fgfr1flox/flox embryos, which showed marked down-regulation of Sprouty1 in both mid- and hindbrain, E9.5 Wnt1-Cre/+; Fgfr1flox/Δflox embryos still expressed Sprouty1 in the hindbrain, but its expression was down-regulated in the midbrain (Figure 6G). Wnt1 showed a similar pattern of expression as in the En1-Cre/+; Fgfr1flox/flox mutants, with early broadened and heterogeneous expression and down-regulation dorsally after E9.5 (Figure 6E and F). The initial broadening of Wnt1 expression was accompanied by slightly expanded expression of En1 and En2 (Figure 6I and J). En1 was thereafter down-regulated specifically in the midbrain by E10 (Figure 6K). In contrast to the En1-Cre/+; Fgfr1flox/flox embryos, expression of Pax2 was still detected as a fuzzy band at the isthmus (Figure 6H). Interestingly, the posterior border of Otx2 expression, as well as the anterior border of Gbx2 and Fgf8 expression, was found to be uneven, showing mixing of positive and negative cell populations in Wnt1-Cre/+; Fgfr1flox/Δflox embryos (Figure 6B and D).
Fig. 6. Analysis of gene expression in the midbrain specific Fgfr1 mutants. Whole-mount in situ hybridization of E9.5 (A–J) and E10.0 (K) wild-type and Wnt1-Cre/+; Fgfr1flox/Δflox embryos with Otx2 (A and B), Fgf8 (C and D), Wnt1 (E and F), Sprouty1 (G), Pax2 (H), En1 (I and K) and En2 (J) probes. Close-up side views of embryos hybridized with Otx2 (B) and Wnt1 (F), as well as a slightly oblique dorsal view of embryos hybridized with Fgf8 probe (D). Arrowheads indicate the isthmus, red arrows indicate altered gene expression. See text for details.
Fgfr1 regulates expression of PB-cadherin, an isthmic cell adhesion molecule
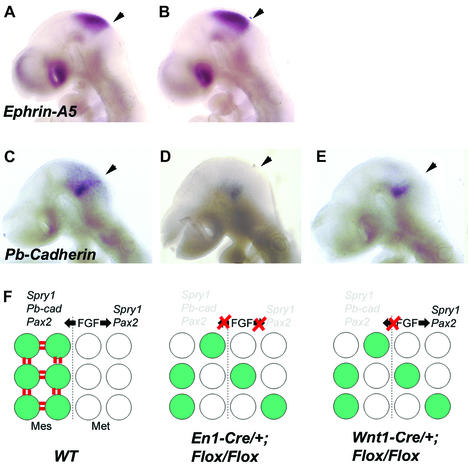
To gain insight into the mechanisms responsible for enhanced cell mixing in the Fgfr1 mutants we next analyzed expression of genes thought to mediate cell adhesion and repulsion. One of these is Ephrin-A5, which is specifically expressed in the midbrain. No change in Ephrin-A5 expression could be observed in E9.5 En1-Cre/+; Fgfr1flox/flox embryos compared with wild type (Figure 7A and B).
Fig. 7. Expression of Ephrin-A5 and PB-cadherin in Fgfr1 mutants and a model for FGFR1 function during the maintenance of the isthmic organizer. Whole-mount in situ hybridization analysis of Ephrin-A5 (A, B) and PB-cadherin (C–E) expression in E9.5 wild-type (A, C), En1-Cre/+; Fgfr1flox/flox (B, D) and Wnt1-Cre/+; Fgfr1flox/Δflox (E) embryos. In both types of Fgfr1 mutants PB-cadherin expression is down-regulated in the midbrain, especially in its dorsal part. Arrowheads in (A)–(E) point to the midbrain–hindbrain boundary. (F) A model for the function of FGF signaling through FGFR1 during maintenance of the isthmic organizer. We suggest that FGFR1 regulates gene expression independently in the midbrain (Mes) and hindbrain (Met). Some of the FGFR1-regulated genes confer specific adhesive characteristics to the cells next to the midbrain–hindbrain border.
Other candidates for regulators of cellular dispersion include Cadherins, a family of mostly homotypic cell-adhesion molecules. One member of the cadherin family, PB-cadherin, has been reported to be expressed in the isthmic region in a pattern overlapping with Wnt1 expression at E10.5 (Kitajima et al., 1999). As the Wnt1-positive cells failed to form a tight band of cells in the En1-Cre/+; Fgfr1flox/flox and Wnt1-Cre/+; Fgfr1flox/Δflox embryos, we wanted to analyze whether the expression of PB-cadherin was altered in the isthmus. We found PB-cadherin expression at the midbrain–hindbrain boundary of wild-type embryos already at E8.5 (data not shown). At E9.5, PB-cadherin was expressed as a stripe throughout the isthmic region (Figure 7C). Weaker expression was detected throughout the midbrain. In contrast, both in En1-Cre/+; Fgfr1flox/flox (n = 5; Figure 7D) and Wnt1-Cre/+; Fgfr1flox/Δflox (n = 4; Figure 7E) embryos, PB-cadherin expression was markedly down-regulated, except for a patch of ventral expression.
Discussion
Genetic loss-of-function studies both in zebrafish (Reifers et al., 1998) and mouse (Meyers et al., 1998; Xu et al., 2000), have demonstrated the importance of FGFs, and FGF8 in particular, in the development of the midbrain– hindbrain region. However, the timing and primary targets of FGF signaling are still unclear. Here we have analyzed the consequences of tissue-specific inactivation of Fgfr1 in the mid- and hindbrain after the establishment of their regional identity. Loss of Fgfr1 results in aplasia of cerebellar vermis and inferior colliculi. Our results suggest that FGFR1 is required both in the mid- and hindbrain for their correct response to signals from the isthmic organizer.
Tissue-specific inactivation of Fgfr1
The tissue-specific inactivation of a conditional Fgfr1 allele, Fgfr1flox, was achieved by crosses with En1-Cre and Wnt1-Cre mice, which allowed apparently complete inactivation of Fgfr1 transcription by E9.5 in the midbrain and anterior hindbrain. Several additional observations suggest efficient recombination by En1-Cre and Wnt1-Cre. First, recombination of the Z/AP reporter allele occurred by E9.5 in virtually all En1- and Wnt1-positive cells in the En1-Cre/+; Z/AP/+ and Wnt1-Cre/+; Z/AP/+ embryos, respectively (Figure 2; data not shown). Secondly, the phenotypes of En1-Cre/+; Fgfr1flox/Δflox and Wnt1-Cre/+; Fgfr1flox/Δflox mice, in which a single recombination event is enough to make a cell null-mutant for Fgfr1, are comparable to the En1-Cre/+; Fgfr1flox/flox and the Wnt1-Cre/+; Fgfr1flox/flox mice, respectively. Thirdly, the phenotype of the hypomorphic Fgfr1 mutants, expressing only ∼10% of the wild-type Fgfr1 mRNA levels, is less severe than the phenotype of the En1-Cre/+; Fgfr1flox/flox mice. Finally, the early changes in the gene expression in midbrain were similar in En1-Cre/+; Fgfr1flox/flox and Wnt1-Cre/+; Fgfr1flox/Δflox embryos.
Isolated recombinant cells were observed also in the rhombomere 1 in the Wnt1-Cre/+; Z/AP/+ embryos both at E8.5 and E10.5. It has been proposed that the midbrain–hindbrain border is not a boundary of cell-lineage restriction (Jungbluth et al., 2001). It is therefore possible that the recombinant cells in rhombomere 1 have their origin in the midbrain. Alternatively, the scattered recombinant cells may result from transient or weak expression of the Wnt1 promoter in rhombomere 1. The presence of a border between recombinant cells of the midbrain and mostly unrecombined rhombomere 1 suggests that mixing of cells between mid- and hindbrain is already restricted to some extent by E10.5. Our in situ hybridization analysis suggested that the majority of the cells in rhombomere 1 still expressed Fgfr1 in the Wnt1-Cre/+; Fgfr1flox/Δflox embryos. We cannot rule out inactivation of Fgfr1 in isolated cells, which could contribute to observed cerebellar defects in these mutants. However, studies of the expression of both Fgfr1 and its target genes are consistent with efficient and specific inactivation of Fgfr1 in midbrain–hindbrain and midbrain by En1-Cre and Wnt1-Cre, respectively.
Regulation of isthmus dependent gene expression by Fgfr1
In the En1-Cre/+; Fgfr1flox/flox embryos, Fgfr1 transcription was inactivated by the 8–10 somite stage. Sprouty1 is a FGF-responsive gene, which is normally expressed next to the FGF source in several regions of the developing embryo and can be induced by ectopic Fgf expression (Minowada et al., 1999). Expression of Sprouty1 in E8.5 En1-Cre/+; Fgfr1flox/flox embryos suggested that FGF signaling was still active in the isthmic region at this stage. This could be due to expression of residual FGFR1 protein or other Fgfrs, perhaps Fgfr2, expression of which was detected in the headfolds at E7.5. Initial expression of residual FGFR1 or other FGFRs may also explain why more extensive deletions of midbrain and cerebellum are observed in Fgf8 mutants (Meyers et al., 1998; Reifers et al., 1998). It is also possible that Fgf8 affects multiple target tissues regulating development of the mid- and hindbrain. This is perhaps a less likely explanation, since tissue-specific inactivation of Fgf8 in the mid- and hindbrain also results in early loss of the entire midbrain and anterior hindbrain (G.Martin, personal communication).
Our results suggest that signaling through Fgfr1 regulates the maintenance of expression of a set of genes near the isthmic organizer at E9.5. As judged based on isthmus-specific gene expression, the organizer itself is established in both midbrain–hindbrain and midbrain-specific Fgfr1 mutants, and expression of Fgf8 and other Fgfs (our unpublished observations) is activated in the anterior hindbrain of the mutants. However, FGF signals fail to elicit normal transcriptional response in the Fgfr1 mutant target cells. In the E9.5 En1-Cre/+; Fgfr1flox/flox mutants, expression of Sprouty1 and Pax2 was not detected in the isthmus. This suggests that very little FGF signaling occurs at the midbrain–hindbrain junction of E9.5 embryos in the absence of FGFR1. Thus, at this stage, FGFR1 appears to be the primary FGF receptor receiving isthmic signals and maintaining isthmus-dependent gene expression.
In Wnt1-Cre/+; Fgfr1flox/Δflox embryos, expression of genes such as Sprouty1 and En1 was specifically altered in the midbrain. This suggests that Fgfr1 is independently involved in the regulation of gene expression in the midbrain. As these genes are down-regulated in both the mid- and hindbrain of En1-Cre/+; Fgfr1flox/flox embryos at the same stage, Fgfr1 must also have a direct role in the regulation of the anterior hindbrain development.
Later, defects in the vermis of the cerebellum, a derivative of rhombomere 1, were also observed in Wnt1-Cre/+; Fgfr1flox/flox mutants. As discussed above, inactivation of Fgfr1 in some cells of the hindbrain might contribute to this phenotype. However, the observed gene expression changes in the midbrain, for example down-regulation of Wnt1, are likely to also have secondary effects on the cerebellar development in Wnt1-Cre/+; Fgfr1flox/flox mice.
Regulation of cell-adhesive properties by Fgfr1?
What could be the molecular and cellular processes regulated by FGFR1? The observed changes in gene expression suggest alterations in cellular identities, i.e. tissue patterning. The behavior of Wnt1-positive cells in both midbrain–hindbrain and midbrain-specific Fgfr1 mutants suggests yet another mechanism. In the wild-type embryos Wnt1 is expressed as a tight band of cells in the posterior midbrain next to Fgf8-expressing cells in the anterior hindbrain. Some mixing of the Wnt1-positive and Wnt1-negative midbrain cells is observed at the anterior margin of the Wnt1 expression domain. In the Fgfr1 mutants, Wnt1-positive cells appear to mix extensively with Wnt1-negative cells. It is possible that signaling through FGFR1 gives Wnt1-positive cells adhesive characteristics, which allow them to sort out of the Wnt1-negative cells. In the absence of Fgfr1, such characteristics are lost and segregation of Wnt1-positive and -negative cells fails. The fact that Otx2, Gbx2 and Fgf8 also showed heterogenous expression borders suggests that loss of Fgfr1 also leads to mixing of midbrain and hindbrain cells.
An alternative hypothesis is suggested by the experiments by Jungbluth et al. (2001), who demonstrated that cells in the avian midbrain and rhombomere 1 were able to mix with each other. If the midbrain–hindbrain border does not represent a true compartment boundary, FGFR1 may regulate readjustment of identities of the cells traversing the midbrain–hindbrain border. However, previous fate-mapping studies (Millet et al., 1996), as well as our analyses of the distribution of genetically marked midbrain cells in Wnt1-Cre/+; Z/AP/+ embryos, do not support extensive cell mixing at the midbrain–hindbrain boundary. In addition, in chimeric mouse embryos consisting of both wild-type and Otx2-null mutant cells, Otx2-negative cells were found to segregate from wild-type cells in the midbrain, perhaps due to change in the expression of cell surface adhesion molecules (Rhinn et al., 1999). Nevertheless, it is still possible that differential cell adhesion is not a general property of the midbrain and rhombomere 1 cells, but is restricted to the cells at the border of these two domains.
A specific cell adhesion molecule, PB-cadherin, is expressed in Wnt1-positive cells at the midbrain– hindbrain junction, arguing for unique adhesive properties of these cells (Figure 7; Kitajima et al., 1999). Interestingly, our results suggest that PB-cadherin is one of the transcriptional targets of FGFR1 signaling, and thus its loss may contribute to the mixing of cells at the midbrain–hindbrain border in the Fgfr1 mutants (see Figure 7F). In addition to the transcriptional response, it is possible that intercellular signaling pathways of FGFR1 more directly regulate cell-adhesive properties. For example, tyrosine phosphorylation has been reported to regulate the activity of catenins, which in turn affect cadherin function (Lilien et al., 2002).
A failure in the segregation between mid- and hindbrain cells has been reported in hypomorphic Wnt1Sw mutants (Bally-Cuif et al., 1995). Our results suggest that the behavior of the midbrain cells is affected prior to loss of Wnt1 expression in En1-Cre/+; Fgfr1flox/flox and Wnt1-Cre/+; Fgfr1flox/Δflox embryos. It is therefore possible that the cell-sorting defect in Wnt1Sw/Sw mice results from down-regulation of isthmic FGF signals. The broadening of the Wnt1 expression domain may also explain the initial slight expansion of the midbrain in Wnt1-Cre/+; Fgfr1flox/Δflox embryos, as WNT1 has been suggested to stimulate cellular proliferation in the midbrain–hindbrain region (W.Wurst, unpublished data).
An interesting parallel in organizer regulation can perhaps be found in the wing imaginal disc of Drosophila. In the wing disc the signaling molecule Hedgehog, expressed by the cells in the posterior compartment, not only induces expression of the morphogen Decapentaplegic but also affects adhesive properties in the adjacent cells of the anterior compartment (Blair and Ralston, 1997; Rodriguez and Basler, 1997). Regulation of adhesive properties by FGF signaling could explain in part how the isthmic organizer is kept as a straight and coherent signaling center, and how the distinct mid- and hindbrain domains are maintained.
Materials and methods
Homologous and site-specific recombination in embryonic stem cells
See Supplementary figure 1 for detailed description of generation of the Fgfr1flox allele by gene targeting.
Generation and genotyping of mutant mice
Fgfr1flox/+ embryonic stem cells were aggregated with morula stage embryos of the ICR strain to produce chimeric mice, which transmitted the Fgfr1flox allele through the germ line. All the experiments were performed in an outbred (129sv/ICR) background. The Fgfr1flox allele was detected by PCR with primers LoxP-1 (5′-AATAGGTCCCTCGA CGGTATC-3′) and Fgfr1 i73 (5′-CTGGGTCAGTGTGGACAGTGT-3′). The wild-type Fgfr1 allele was detected with the primers Fgfr1 wt5′ (5′-CCCCATCCCATTTCCTTACCT-3′) and Fgfr1 wt3′ (5′-TTCTGGT GTGTCTGAAAACAGCT-3′). The different Cre alleles were detected with the primers Cre5′ (5′-AATCTCCCACCGTCAGTACG-3′) and Cre3′ (5′-CGTTTTCTGAGCATACCTGGA-3′). The Z/AP allele was detected by β-galactosidase staining.
β-galactosidase and alkaline phosphatase staining
β-galactosidase and alkaline phosphatase staining of whole-mount E8.5–10.5 embryos as well as frozen sections was performed as described previously (Lobe et al., 1999).
Behavioral analyses
In the rotarod assay, the mice were placed on a rotating rod (diameter 15 cm, speed 15 r.p.m.). The time the mice were able to stay on top of the rod was measured. The experiment was terminated after 5 min. Each mouse was assayed nine times (Table I).
In the stationary beam assay the mice were placed in the middle of a horizontal beam (diameter 2 cm, length 150 cm). The time the mice stayed on the beam and the distance they walked on the beam were measured. The experiment was terminated after 1 min. Each mouse was assayed nine times (Table I).
Histology and immunohistochemistry
Adult tissues were fixed by intracardial perfusion with 4% paraformaldehyde. Brain tissues of newborn mice were dissected in phosphate-buffered saline (PBS) and fixed overnight in 4% paraformaldehyde. The tissues were dehydrated, embedded in paraffin and sectioned in 5 µm slices. Hematoxylin–eosin was used for counterstaining. Immuno histochemistry on paraffin sections with anti-calbindin (Swant cat. CB38) and anti-tyrosine hydroxylase (Chemicon AB152) antibodies was performed according to standard procedures. For semi-thin sections, embryos were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2, overnight at 4°C, post-fixed in 1% osmium tetroxide, and embedded in Epon. One micrometer plastic sagittal sections were cut and stained with toluidine blue.
Analysis of cell death
TUNEL assays. To detect apoptotic cells, TUNEL assays were performed on paraffin sections of E9.5 embryos using the in situ cell death detection kit (Roche cat. 1684 795).
Nile Blue sulfate (NBS) staining. Following dissection, embryos (E10.5) were washed in PBS, and incubated for 30 min at 37°C in filtered NBS (Sigma N-5632) saturated water diluted 1:1000 in PBT (PBS containing 0.1% Tween-20). Embryos were then washed several times in PBT at room temperature and photographed immediately.
mRNA in situ hybridization analyses
Whole-mount mRNA in situ hybridization of E8.5–10.5 embryos with En1, En2 (Davis and Joyner, 1988), EphrinA5 (a gift from David Wilkinson), Fgfr1 (bp 1152–1724 of NM010206), Fgfr2 (a gift from Alka Mansukhani), Fgf8 (Crossley and Martin, 1995), Gbx2, Otx2 (Acampora et al., 1997), Pax2 (a gift from Gregory Dressler), PB-cadherin (clone ID: UI-M-BH1-akr-h-03-0-UI), Sprouty1 (a gift from Seppo Vainio) and Wnt1 (McMahon et al., 1992) riboprobes was performed as described previously (Henrique et al., 1995). At least four mutants and four littermate controls were hybridized with each of the probes. Hybridization and subsequent treatments of the mutants and the littermate controls were carried out simultaneously in the same vial. Radioactive in situ hybridizations on paraffin sections with Fgf8, Fgfr1, Fgfr2 and Dopamine-β-hydroxylase (bp 620–1018 of S50200) probes were carried out as described by Wilkinson and Green (1990).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Lois Schwartz, Eija Koivunen, Outi Koljonen, Mona Augustin, Päivi Hannuksela, Maria von Numers, Stefanie Pirrung and Nadine Trepesch for expert technical assistance. We also wish to thank Andras Nagy for the Z/AP mice, Peter Lonai for the Pgk-Cre mice, Henri Huttunen for help with immunohistochemistry and Vootele Vojkar for help in behavioral analyses. This work was supported by the Academy of Finland, the Sigrid Juselius foundation and Biocentrum Helsinki (J.P.), the Viikki Graduate School in Biosciences (R.T., N.T.), the Bundesministerium für Bildung und Forschung and the Deutsche Forschungsgemeinschaft (W.W.).
References
- Acampora D., Avantaggiato,V., Tuorto,F. and Simeone,A. (1997) Genetic control of brain morphogenesis through Otx gene dosage requirement. Development, 124, 3639–3650. [DOI] [PubMed] [Google Scholar]
- Ang S.L. and Rossant,J. (1993) Anterior mesendoderm induces mouse Engrailed genes in explant cultures. Development, 118, 139–149. [DOI] [PubMed] [Google Scholar]
- Bally-Cuif L., Cholley,B. and Wassef,M. (1995) Involvement of Wnt-1 in the formation of the mes/metencephalic boundary. Mech. Dev., 53, 23–34. [DOI] [PubMed] [Google Scholar]
- Blair S.S. and Ralston,A. (1997) Smoothened-mediated Hedgehog signalling is required for the maintenance of the anterior–posterior lineage restriction in the developing wing of Drosophila. Development, 124, 4053–4063. [DOI] [PubMed] [Google Scholar]
- Broccoli V., Boncinelli,E. and Wurst,W. (1999) The caudal limit of Otx2 expression positions the isthmic organizer. Nature, 401, 164–168. [DOI] [PubMed] [Google Scholar]
- Ciruna B. and Rossant,J. (2001) FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell, 1, 37–49. [DOI] [PubMed] [Google Scholar]
- Crossley P.H. and Martin,G.R. (1995) The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development, 121, 439–451. [DOI] [PubMed] [Google Scholar]
- Crossley P.H., Martinez,S. and Martin,G.R. (1996) Midbrain development induced by FGF8 in the chick embryo. Nature, 380, 66–68. [DOI] [PubMed] [Google Scholar]
- Danielian P.S., Muccino,D., Rowitch,D.H., Michael,S.K. and McMahon,A.P. (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol., 8, 1323–1326. [DOI] [PubMed] [Google Scholar]
- Davis C.A. and Joyner,A.L. (1988) Expression patterns of the homeobox-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev., 2, 1736–1744. [DOI] [PubMed] [Google Scholar]
- Deng C.X., Wynshaw-Boris,A., Shen,M.M., Daugherty,C., Ornitz,D.M. and Leder,P. (1994) Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev., 8, 3045–3057. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Stewart,R.M. and Harland,R.M. (1990) Region-specific neural induction of an engrailed protein by anterior notochord in Xenopus. Science, 250, 800–802. [DOI] [PubMed] [Google Scholar]
- Henrique D., Adam,J., Myat,A., Chitnis,A., Lewis,J. and Ish-Horowicz,D. (1995) Expression of a Delta homologue in prospective neurons in the chick. Nature, 375, 787–790. [DOI] [PubMed] [Google Scholar]
- Irving C. and Mason,I. (2000) Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development, 127, 177–186. [DOI] [PubMed] [Google Scholar]
- Jungbluth S., Larsen,C., Wizenmann,A. and Lumsden,A. (2001) Cell mixing between the embryonic midbrain and hindbrain. Curr. Biol., 11, 204–207. [DOI] [PubMed] [Google Scholar]
- Kimmel R.A., Turnbull D.H., Blanquet V., Wurst W., Loomis C.A. and Joyner A.L. (2000) Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev., 14, 1377–1389. [PMC free article] [PubMed] [Google Scholar]
- Kitajima K., Koshimizu,U. and Nakamura,T. (1999) Expression of a novel type of classic cadherin, PB-cadherin in developing brain and limb buds. Dev. Dyn., 215, 206–214. [DOI] [PubMed] [Google Scholar]
- Lallemand Y., Luria,V., Haffner-Krausz,R. and Lonai,P. (1998) Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res., 7, 105–112. [DOI] [PubMed] [Google Scholar]
- Lee S.M., Danielian,P.S., Fritzsch,B. and McMahon,A.P. (1997) Evidence that FGF8 signalling from the midbrain–hindbrain junction regulates growth and polarity in the developing midbrain. Development, 124, 959–969. [DOI] [PubMed] [Google Scholar]
- Lilien J., Balsamo,J., Arregui,C. and Xu,G. (2002) Turn-off, drop-out: functional state switching of cadherins. Dev. Dyn., 224, 18–29. [DOI] [PubMed] [Google Scholar]
- Liu A. and Joyner,A.L. (2001) Early anterior/posterior patterning of the midbrain and cerebellum. Annu. Rev. Neurosci., 24, 869–896. [DOI] [PubMed] [Google Scholar]
- Lobe C.G., Koop,K.E., Kreppner,W., Lomeli,H., Gertsenstein,M. and Nagy,A. (1999) Z/AP, a double reporter for cre-mediated recombination. Dev. Biol., 208, 281–292. [DOI] [PubMed] [Google Scholar]
- Martinez S., Wassef,M. and Alvarado-Mallart,R.M. (1991) Induction of a mesencephalic phenotype in the 2-day-old chick prosencephalon is preceded by the early expression of the homeobox gene en. Neuron, 6, 971–981. [DOI] [PubMed] [Google Scholar]
- Martinez S., Marin,F., Nieto,M.A. and Puelles,L. (1995) Induction of ectopic engrailed expression and fate change in avian rhombomeres: intersegmental boundaries as barriers. Mech. Dev., 51, 289–303. [DOI] [PubMed] [Google Scholar]
- Martinez S., Crossley,P.H., Cobos,I., Rubenstein,J.L. and Martin,G.R. (1999) FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development, 126, 1189–1200. [DOI] [PubMed] [Google Scholar]
- Maruoka Y., Ohbayashi,N., Hoshikawa,M., Itoh,N., Hogan,B.M. and Furuta,Y. (1998) Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech. Dev., 74, 175–177. [DOI] [PubMed] [Google Scholar]
- McMahon A.P., Joyner,A.L., Bradley,A. and McMahon,J.A. (1992) The midbrain–hindbrain phenotype of Wnt-1–/Wnt-1– mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell, 69, 581–595. [DOI] [PubMed] [Google Scholar]
- Meyers E.N., Lewandoski,M. and Martin,G.R. (1998) An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet., 18, 136–141. [DOI] [PubMed] [Google Scholar]
- Millet S., Bloch-Gallego,E., Simeone,A. and Alvarado-Mallart,R.M. (1996) The caudal limit of Otx2 gene expression as a marker of the midbrain/hindbrain boundary: a study using in situ hybridisation and chick/quail homotopic grafts. Development, 122, 3785–3797. [DOI] [PubMed] [Google Scholar]
- Millet S., Campbell,K., Epstein,D.J., Losos,K., Harris,E. and Joyner,A.L. (1999) A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature, 401, 161–164. [DOI] [PubMed] [Google Scholar]
- Minowada G., Jarvis,L.A., Chi,C.L., Neubuser,A., Sun,X., Hacohen,N., Krasnow,M.A. and Martin,G.R. (1999) Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development, 126, 4465–4475. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Takagi,S., Tsuji,T., Matsui,K.A. and Fujisawa,H. (1988) The prosencephalon has the capacity to differentiate into optic tectum: analysis in quail-chick-chimeric brains. Dev. Growth Differ., 30, 717–725. [DOI] [PubMed] [Google Scholar]
- Partanen J., Schwartz,L. and Rossant,J. (1998) Opposite phenotypes of hypomorphic and Y766 phosphorylation site mutations reveal a function for Fgfr1 in anteroposterior patterning of mouse embryos. Genes Dev., 12, 2332–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifers F., Bohli,H., Walsh,E.C., Crossley,P.H., Stainier,D.Y. and Brand,M. (1998) Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain–hindbrain boundary development and somitogenesis. Development, 125, 2381–2395. [DOI] [PubMed] [Google Scholar]
- Rhinn M., Dierich,A., LeMeur,M. and Ang,S.-L. (1999) Cell autonomous and non-cell autonomous functions of Otx2 in patterning the rostral brain. Development, 126, 4295–4304. [DOI] [PubMed] [Google Scholar]
- Rodriguez I. and Basler,K. (1997) Control of compartmental affinity boundaries by hedgehog. Nature, 389, 614–618. [DOI] [PubMed] [Google Scholar]
- Shamim H., Mahmood,R., Logan,C., Doherty,P., Lumsden,A. and Mason,I. (1999) Sequential roles for Fgf4, En1 and Fgf8 in specification and regionalisation of the midbrain. Development, 126, 945–959. [DOI] [PubMed] [Google Scholar]
- Trokovic N., Trokovic,R., Mai,P. and Partanen,J. (2003) Fgfr1 regulates patterning of the pharyngeal region. Genes Dev., 17, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshe J. and Mason,I. (2000) Expression of FGFR1, FGFR2 and FGFR3 during early neural development in the chick embryo. Mech. Dev., 90, 103–110. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.G. and Green,J. (1990) In situ hybridization and the three-dimensional construction of serial sections. In A.J. Copp and D.L. Cockroft (eds), Postimplantation Mammalian Embryos. Oxford University Press, Oxford, UK, p. 155–171.
- Wurst W. and Bally-Cuif,L. (2001) Neural plate patterning: upstream and downstream of the isthmic organizer. Nat. Rev. Neurosci., 2, 99–108. [DOI] [PubMed] [Google Scholar]
- Xu J., Liu,Z. and Ornitz,D.M. (2000) Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development, 127, 1833–1843. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.P., Conlon,R.A. and Rossant,J. (1992) Expression of the fibroblast growth factor receptor FGFR-1/flg during gastrulation and segmentation in the mouse embryo. Dev. Biol., 152, 75–88. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.P., Harpal,K., Henkemeyer,M. and Rossant,J. (1994) fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev., 8, 3032–3044. [DOI] [PubMed] [Google Scholar]