Abstract
ATP-sensitive potassium (KATP) channels are required for maintenance of homeostasis during the metabolically demanding adaptive response to stress. However, in disease, the effect of cellular remodeling on KATP channel behavior and associated tolerance to metabolic insult is unknown. Here, transgenic expression of tumor necrosis factor α induced heart failure with typical cardiac structural and energetic alterations. In this paradigm of disease remodeling, KATP channels responded aberrantly to metabolic signals despite intact intrinsic channel properties, implicating defects proximal to the channel. Indeed, cardiomyocytes from failing hearts exhibited mitochondrial and creatine kinase deficits, and thus a reduced potential for metabolic signal generation and transmission. Consequently, KATP channels failed to properly translate cellular distress under metabolic challenge into a protective membrane response. Failing hearts were excessively vulnerable to metabolic insult, demonstrating cardiomyocyte calcium loading and myofibrillar contraction banding, with tolerance improved by KATP channel openers. Thus, disease-induced KATP channel metabolic dysregulation is a contributor to the pathobiology of heart failure, illustrating a mechanism for acquired channelopathy.
Keywords: ATP-sensitive potassium channel/energy metabolism/heart failure/potassium channel openers/TNFα
Introduction
Stress occurs as a threat to the physiological parameters necessary for survival, with the adaptive response incorporating alterations in bodily functions to sustain the intensified performance level necessary for confrontation or evasion. A critical component in maintaining homeostasis during this metabolically demanding adaptive reaction is the ATP-sensitive potassium (KATP) channel (Zingman et al., 2002a), a high-fidelity metabolic sensor that adjusts membrane potential-dependent cell functions to match metabolic state (Weiss and Lamp, 1987; Ashcroft, 1988; O’Rourke et al., 1994; Nichols et al., 1996). KATP channels are broadly represented in tissues that propagate the general adaptation reaction to stress, including nervous system (Amoroso et al., 1990; Miki et al., 2001; Yamada et al., 2001), vasculature (Yamada et al., 1997; Chutkow et al., 2002; Miki et al., 2002), heart (Inagaki et al., 1996), skeletal muscle (Vivaudou et al., 1991; Allard and Lazdunski, 1992) and pancreatic β-cells (Ashcroft et al., 1984; Inagaki et al., 1995; Koster et al., 2000; Aguilar-Bryan et al., 2001). While tissue-specific functions of KATP channels arise through distinctive properties of subunit isoforms, the channel role as membrane metabolic mediator is ubiquitous (Seino and Miki, 2003; Zingman et al., 2003).
In the heart, where KATP channels were originally discovered (Noma, 1983), the multimeric channel complex is assembled by physical association of the pore-forming Kir6.2 and regulatory SUR2A subunits (Inagaki et al., 1996; Lorenz and Terzic, 1999). Metabolic sensing occurs through modulation of the ATP sensitivity of Kir6.2 (Tucker et al., 1997) by the SUR2A subunit that harbors an intrinsic ATPase activity such that stabilization of SUR2A in a post-hydrolytic state favors K+ efflux through Kir6.2, leading to membrane hyperpolarization (Bienengraeber et al., 2000; Zingman et al., 2001, 2002b; Matsuo et al., 2002; Matsushita et al., 2002). Integration of nucleotide-dependent KATP channel gating with cellular metabolism occurs through interaction with membrane and cytosolic modulators (Shyng and Nichols, 1998; Beguin et al., 1999; Lin et al., 2000; Carrasco et al., 2001; Abraham et al., 2002; Crawford et al., 2002a). In particular, creatine kinase-catalyzed phosphotransfer bridges diffusional barriers between mitochondrial ATP production and cellular ATP-sensitive processes, securing over 90% of energetic distribution in the heart and supporting myocardial tolerance to metabolic insult (Wallimann et al., 1992; Dzeja et al., 1999; Saupe et al., 2000; Pucar et al., 2001; Crozatier et al., 2002). Indeed, the intimate relationship between the ATPase activity of SUR2A and creatine kinase phosphotransfer is fundamental to cardiac KATP channel translation of metabolic fluctuations into homeostatic changes in membrane excitability (Dzeja and Terzic, 1998; Bienengraeber et al., 2000; Zingman et al., 2001; Abraham et al., 2002; Crawford et al., 2002b).
The role of KATP channels as mediators of the response to stress is underscored in mice with genetic deletion of Kir6.2 that underperform on exercise stress test, a natural trigger of the general adaptation syndrome (Zingman et al., 2002a, 2003) The contribution of cardiac KATP channels is demonstrated by failure of Kir6.2 knockout hearts to shorten action potentials or to sustain augmented contractile performance, suffering Ca2+ overload, injury and increased susceptibility to fatal arrhythmia under adrenergic stress (Zingman et al., 2002a), with reduced tolerance to ischemic challenge (Suzuki et al., 2002). Furthermore, congenital KATP channel mutations lead to abnormal metabolic behavior, as has been shown in pancreatic β-cells (Thomas et al., 1995; Nichols et al., 1996; Aguilar-Bryan et al., 2001), and in heart, while no spontaneous mutations have been reported, engineered KATP channel mutants with abnormal sensitivity to ATP (Rajashree et al., 2002) or altered metabolic signaling to KATP channels by genetic deletion of creatine kinase (Abraham et al., 2002) respond aberrantly to myocardial metabolic inhibition. Yet, while the KATP channel metabolic integration and associated homeostatic role are increasingly elucidated, it is unestablished whether disease states due to an altered cellular environment will modify KATP channel behavior and the ability of diseased organisms to adapt to stress.
Cellular remodeling with consequent dysfunction of cellular processes is a fundamental feature of disease conditions. A paradigm of this principle is cardiomyocyte modification in heart failure. Structural and energetic remodeling of cardiomyocytes in failing hearts occurs in response to the altered mechanical, neurohumoral and/or inflammatory environment, and eventually becomes maladaptive, precipitating electrical and mechanical dysfunction (Towbin and Bowles, 2002). In this regard, remodeling in heart failure manifests with deficits in creatine kinase phosphotransfer (Liao et al., 1996; Nascimben et al., 1996; Tian et al., 1996; Neubauer et al., 1997; DeSousa et al., 1999; Dzeja et al., 2000; Ye et al., 2001), but it is unknown whether KATP channel gating and associated myocyte tolerance to stress is affected. This is of particular significance as cardiomyocytes in heart failure confront the metabolic insults of hypoxia, ischemia and adrenergic toxicity (Braunwald and Bristow, 2000; Bradham et al., 2002; Towbin and Bowles, 2002), such that altered stress tolerance would impact disease progression.
Therefore, to assess KATP channel behavior in a disease state, a model of heart failure, induced by the cytokine tumor necrosis factor-α (TNFα), was utilized. In normal heart, TNFα is not expressed; however, in heart failure, circulating TNFα levels correlate with disease severity and mortality (Levine et al., 1990; Torre-Amione et al., 1996; Deswal et al., 2001). Cardiac TNFα expression is triggered by biomechanical stress, and through cross-linkage of cardiomyocyte membrane receptors activates multiple kinases and transcriptional regulators that modulate growth and differentiation (McTiernan and Feldman, 2000; Mann, 2003). The role of this cytokine in promotion of cardiac remodeling is further evidenced by induction of dilated cardiomyopathy in response to constitutive cardiac TNFα overexpression (Bryant et al., 1998; Kadokami et al., 2000; Sivasubramanian et al., 2001). Here, we find in heart failure, induced by transgenic expression of TNFα, that cardiomyocyte remodeling did not affect intrinsic KATP channel properties, but hindered its metabolic regulation through alteration of signal communication. As a result KATP channels did not recognize cellular stress, resulting in failure of their homeostatic function. While the underlying signaling defects in heart failure created vulnerability to stress, tolerance was improved by direct pharmacological targeting of KATP channel proteins.
Results
Transgenic cardiac TNFα expression recapitulates heart failure
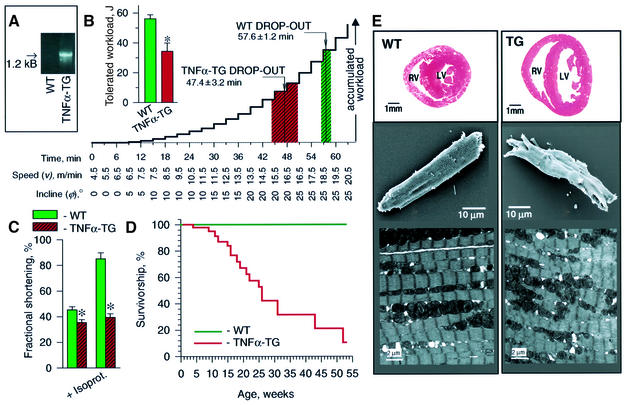
Mice engineered to overexpress TNFα (TNFα-TG) in the heart (Figure 1A; Sivasubramanian et al., 2001) were unable to match the physical exertion of wild-type (WT) littermates (Figure 1B). By 8–12 weeks of age, on treadmill, TNFα-TG dropped out earlier and at lower workloads than simultaneously exercised WT (n = 6 each, p < 0.05; Figure 1B). Tolerated workload was 34.3 ± 5.5 and 56.1 ± 2.7 J in TNFα-TG (n = 6) and WT (n = 6), respectively (p < 0.05, Figure 1B, inset). On echocardiography, left ventricular fractional shortening was significantly less in TNFα-TG (35.5 ± 2.1%, n = 3) than in WT (45.3 ± 2.4%, n = 4; p < 0.05; Figure 1C), despite similar heart rates (470 ± 16 b.p.m., n = 3 and 456 ± 37 b.p.m., n = 4, respectively). Under β-adrenergic challenge with isoproterenol, TNFα-TG (n = 3) failed to augment left ventricular fractional shortening (39 ± 3%) compared with the response in WT (85 ± 8%, n = 4; p < 0.05; Figure 1C), although increases in heart rate were similar (520 ± 29 and 518 ± 20 b.p.m., respectively). While there was no spontaneous death in WT mice observed for up to 53 weeks, TNFα-TG median survival was 26 weeks (Figure 1D). Remodeling of TNFα-TG myocardium resulted in dilated cardiomyopathy with chamber dilatation and left ventricular wall thinning (Figure 1E, top), cardiomyocyte ultrastructural deformation and irregular surface morphology (Figure 1E, middle), and myofibrillar disorganization (Figure 1E, bottom). Thus, exercise intolerance, ventricular dysfunction, compromised survival, with chamber and cardiomyocyte remodeling, key features of heart failure, were recapitulated in the transgenic TNFα heart failure model.
Fig. 1. Heart failure in TNFα transgenic mice. (A) The 1.2 kB band TNFα transgene in tail-cut PCR of transgenic (TNFα-TG) but not WT mice. (B) Exercise intolerance of TNFα-TG, compared with WT, with lower tolerated workload (inset) and earlier treadmill drop-out (p < 0.05). (C) Left ventricular fractional shortening, by echocardiography, was significantly less in TNFα-TG than WT (p < 0.05). In mice challenged with isoproterenol (0.5 µg i.p.), augmentation of fractional shortening was greater in WT compared with TNFα-TG (p < 0.05). (D) Mortality was greater in TNFα-TG (initial n = 135, 85% censored by 53 weeks) compared with WT (initial n = 175, 99% censored by 53 weeks) mice (p < 0.05). (E) Remodeling in 8-week-old TNFα-TG (TG) mice. Top: chamber dilation and reduced wall thickness at the base of TG versus WT hearts. Middle: distortion of architecture in TG versus rod-shaped WT ventricular cardiomyocytes on scanning microscopy. Bottom: myofibrillar disorganization in TG versus WT ventricular tissue by transmission electron microscopy.
KATP channels retain intrinsic gating properties but receive defective metabolic signaling in failing hearts
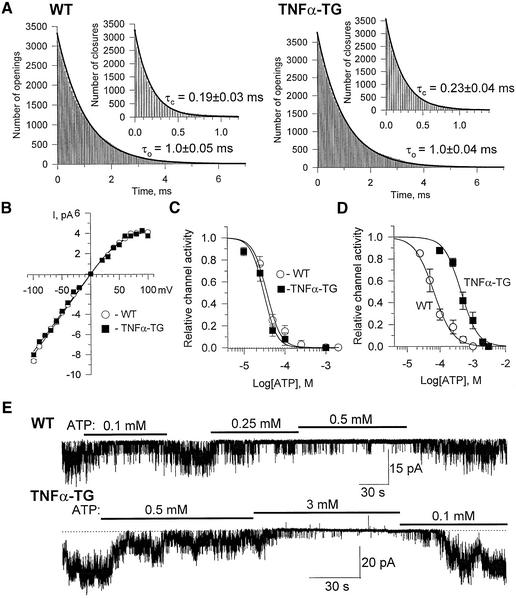
To distinguish intrinsic channel properties from the regulatory contribution of the cellular milieu, KATP channel behavior was compared in excised membrane patches versus the open cell-attached configuration that allows retention of cellular infrastructure, including organelles and metabolic enzymes, while permitting exchange of ions and metabolic ligands. In membrane patches excised from the cell environment, the kinetic behavior within a burst of KATP channel opening (Figure 2A), the channel current–voltage relationship (Figure 2B) and the concentration dependence to ATP (IC50 = 31 ± 3 versus 37 ± 3 µM; Figure 2C) were virtually identical for TNFα-TG (n = 4) and WT (n = 3). While cytosolic ATP levels were preserved in the TNFα-TG compared with the WT (5.4 ± 0.2 versus 5.8 ± 0.3 mM; n = 4 each), and no spontaneous channel activity was observed in unpermeabilized TNFα-TG or WT cardiomyocytes, in open cell-attached patches the concentration dependence of KATP channel activity to ATP was significantly altered in TNFα-TG compared with WT (Figure 2D and E). Applied ATP (0.1–0.5 mM) effectively blocked channel activity in WT, but not in TNFα-TG (Figure 2E), and consequently the concentration–response curves defining the ATP sensitivity of KATP channels were significantly different between WT (n = 3) and TNFα-TG (n = 3), with an IC50 of 66 ± 5 and 443 ± 30 µM, respectively (p < 0.05; Figure 2D). Thus, although the biophysical properties of KATP channels were intact and excised channels properly measured ATP, within the cellular milieu of the failing cardiomyocyte recognition by the channel of this major metabolic ligand was altered.
Fig. 2. Intact KATP channels in TNFα-TG cardiomyocytes receive altered ATP signal. (A) Intraburst KATP channel kinetics were indistinguishable in excised WT and TNFα-TG patches, with characteristic open and closed times (τo and τc), derived from the best-fit of corresponding distributions, not significantly different (n = 3). (B) KATP channel current–voltage relationships (n = 4) with identical channel conductance and rectification in WT and TNFα-TG patches. (C) KATP channel activity in excised membrane patches calculated relative to activity in the absence of ATP, and fitted by the Hill equation 1/[1 = x/IC50]k (solid curves), where x is ATP concentration, h the Hill coefficient and IC50 the half-maximal inhibition concentration. (D and E) KATP channels, in open cell-attached mode, show altered effect of ATP in TNFα-TG compared with WT.
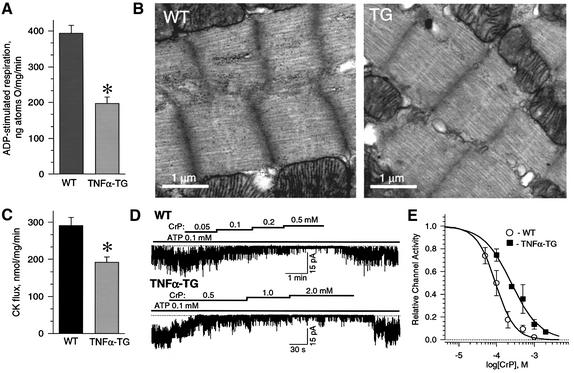
Energetic signaling to KATP channels principally depends on generation of ATP by oxidative phosphorylation in concert with high-energy phosphoryl transfer through the creatine kinase system (Sasaki et al., 2001; Abraham et al., 2002; Crawford et al., 2002b). Here, mitochondria from TNFα-TG hearts had depressed ADP-stimulated respiration, a measure of oxidative phosphorylation potential (Ozcan et al., 2002), with 197 ± 18 ng atoms O/mg/min (n = 7) utilized versus 394 ± 22 ng atoms O/mg/min (n = 7) in WT (p < 0.05; Figure 3A). Glycogen, a substrate reservoir for oxidative phosphorylation (Goodwin et al., 1996), was abundant in electron micrographs of WT hearts (68 ± 18 per µm2, n = 3) forming profuse electron-dense particles (Figure 3B, left). However, glycogen granules were less common in TNFα-TG (12 ± 9 per µm2, n = 3) myocardium (p < 0.05; Figure 3B, right). Moreover, creatine kinase flux in TNFα-TG (n = 3) was significantly lower than in WT (n = 4) hearts (192 ± 14 versus 291 ± 22 nmol/mg/min, p < 0.05; Figure 3C), with the responsiveness of KATP channels to creatine kinase signaling, induced in the open cell-attached mode by application of its substrate creatine phosphate, blunted in TNFα-TG cardiomyocytes (Figure 3D). Indeed, the channel IC50 to creatine phosphate was 250 ± 18 µM (n = 3) in TNFα-TG versus 94 ± 5 µM (n = 3) in the WT (p < 0.05; Figure 3E). Thus, cardiomyocytes from failing myocardium displayed a deficit in the potential for metabolic signal production and transmission responsible for optimal KATP channel regulation.
Fig. 3. Depressed bioenergetic components create conditions impeding signaling to KATP channels in TNFα-TG hearts. (A) Mitochondrial ADP-stimulated respiration is significantly depressed in isolated mitochondria from TNFα-TG compared with WT hearts. (B) While in WT glycogen granules are abundant in electron micrography, in TNFα-TG they are sparse. (C) Creatine kinase (CK) flux, by 18O-assisted NMR spectroscopy, was significantly reduced in TNFα-TG compared with WT hearts. (D) In the open cell-attached mode, the creatine phosphate (CrP)/creatine kinase system effectively regulated KATP channel activity in the WT (upper), but not TNFα-TG (lower). (E) Concentration-response of CrP-stimulated KATP channel inhibition in open cell-attached patches. Data fitted by the Hill equation (solid curves) show a significant increase in the IC50 for CrP-induced channel inhibition in TNFα-TG versus WT. The asterisk in (A) and (C) indicates p < 0.05.
Dysregulated KATP channels unable to adjust membrane excitability under stress in cardiomyocytes of failing hearts
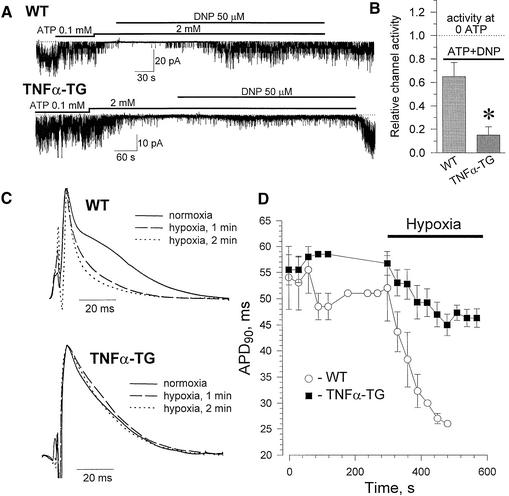
The deficit in the potential for energetic communication in the remodeled TNFα-TG cardiomyocyte could indicate impaired integration of KATP channels with the cellular environment. This would hamper the delivery of metabolic stress-induced signals. Yet, proper KATP channel response is required for cellular homeostasis under stress, with channel opening responsible for protective shortening of the cardiac action potential (Nichols and Lederer, 1991; Rajashree et al., 2002; Suzuki et al., 2002; Zingman et al., 2002a). Here, hypoxic stress, simulated by the mitochondrial uncoupler dinitrophenol (DNP), induced vigorous KATP channel opening in cardiomyocytes from WT, but not TNFα-TG hearts (Figure 4A). On average, KATP channel activity was 4-fold higher in WT (n = 3) than TNFα-TG (n = 3; Figure 4B), with DNP-induced channel opening abolished by the mitochondrial F0F1-ATPase inhibitor oligomycin (1 µg/ml). Thus, KATP channels in the failing cardiomyocyte did not appropriately recognize metabolic stress. As a consequence, monophasic action potential shortening in hypoxia was blunted in TNFα-TG compared with WT hearts (Figure 4C). By 3 min into hypoxia, monophasic action potential duration at 90% repolarization (APD90) was significantly shorter (p < 0.05) in WT (27 ± 1 ms, n = 3) than in TNFα-TG hearts (47 ± 2 ms; n = 4), despite similar pre-hypoxic APD90 (52 ± 6 versus 57 ± 2 ms, p > 0.05; Figure 4D). Therefore, in the failing heart, KATP channels behaved as if uncoupled from cellular metabolic signals, compromising protective KATP channel-dependent regulation of membrane excitability under stress.
Fig. 4. KATP channel-dependent membrane control under stress defective in TNFα-TG hearts. (A) In the open cell-attached mode, with spontaneous channel opening suppressed by ATP, dinitrophenol (DNP) induced a vigorous KATP channel response in WT, but not TNFα-TG. (B) DNP-induced KATP channel activity in WT versus TNFα-TG (p < 0.05). (C) Monophasic action potentials under normoxia (O2 content = 32 mg/l) and hypoxia (O2 content = 3.1 mg/l). (D) Under hypoxia, APD90 markedly shortened in WT, but not TNFα-TG hearts.
KATP channel openers restore tolerance to stress
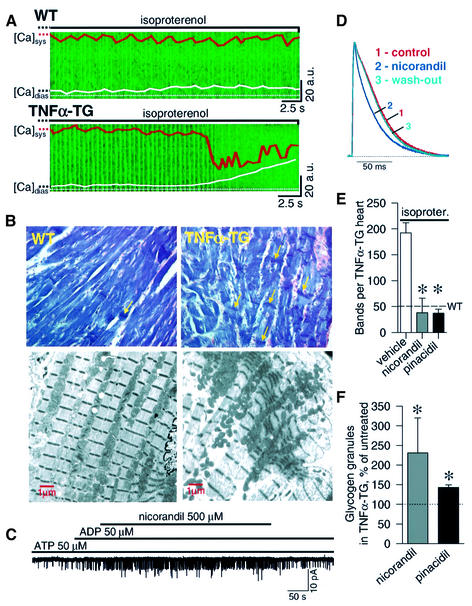
Hearts lacking KATP channels are susceptible to adrenergic stress-induced calcium overload and associated myocyte injury (Zingman et al., 2002a). Here, WT cardiomyocytes tolerated sympathetic challenge (n = 6), while all TNFα-TG cardiac cells (n = 6) developed intracellular calcium overload, precipitating contracture and ultimately cell death (Figure 5A). Morever, in animals stressed with isoproterenol (0.8–2.0 mg i.p.), contraction bands, a result of calcium overload (Karch and Billingham, 1986), were rare in WT (50 ± 11/heart), but were four times more frequent in the TNFα-TG (192 ± 20/heart) myocardium (n = 5, p < 0.05; Figure 5B, upper), seen on electron microscopy as sarcomere shortening (Figure 5B, lower). Potassium channel-opening drugs increase KATP channel activity through binding to channel proteins (Schwanstecher et al., 1998; Hambrock et al., 1999; Ashcroft and Gribble, 2000; Moreau et al., 2000). The clinically available potassium channel opener nicorandil activated KATP channels in membrane patches from failing hearts (n = 3; Figure 5C), and reversibly shortened action potential duration in isolated TNFα-TG heart (Figure 5D). Treatment of TNFα-TG animals with nicorandil (n = 4), as well as the structurally distinct KATP channel opener pinacidil (n = 3), reduced isoproterenol-induced contraction bands to a level comparable to the WT (Figure 5E), and improved energetic reserve assessed by storage of glycogen (Figure 5F). Thus, in failing hearts, improved tolerance to stress afforded by potassium channel openers underscores the contribution of KATP channel dysregulation to increased myocyte vulnerability in a disease environment.
Fig. 5. Vulnerability to stress in TNFα-TG hearts attenuated by potassium channel openers. (A) Fluo-3-loaded and paced WT cardiomyocytes (upper) tolerated isoproterenol (1 µM) challenge without significant change in maximal systolic and diastolic Ca2+ levels. TNFα-TG cardiomyocytes (lower), under isoproterenol stress, developed diastolic Ca2+ overload with cell contracture. Ca2+-induced fluorescence in a transverse cellular plane versus time is shown in green. Orange and white traces are deconvoluted fluorescent frames, and represent average Ca2+ maxima (systole, sys) and minima (diastole, dias). (B) Upper: photomicrographs (40×) of phosphotungstic acid hematoxylin-stained left ventricle 45 min after isoproterenol (2 mg i.p.) with contraction bands (arrows) in TNFα-TG, but not WT. Lower: electron microscopy of a contraction band in TNFα-TG (right) compared with normal sarcomeric pattern in WT (left). (C and D) In TNFα-TG, nicorandil (500 µM) activated KATP channels in excised patches (C), and shortened action potential duration (D). (E) Nicorandil (2 mg i.p; n = 4) or pinacidil (0.1 mg i.p; n = 3) versus vehicle (n = 5), 30 s prior to isoproterenol (isoproter.) challenge (0.8–2 mg i.p.), significantly reduced contraction bands in TNFα-TG mice (p < 0.05). Dotted line: average bands in vehicle-treated, isoproterenol-stressed, WT (n = 3). (F) Treatment of TNFα-TG mice twice daily for 1 week with nicorandil (0.5 mg/kg, s.c.; n = 3) or pinacidil (1 mg/kg, s.c.; n = 3) improved glycogen storage (p < 0.05) expressed relative to vehicle-treated TNFα-TG mice (n = 3; dotted line).
Discussion
The ability of organisms to effectively respond to stress is crucial for health and survival, with an emerging recognition of the role of KATP channels in execution of this process (Zingman et al., 2003). While altered ATP responsiveness of KATP channels in hypertophied cardiomyocytes has been reported (Cameron et al., 1988; Yuan et al., 1997), it has remained unclear how disease states with consequent cellular remodeling affect the homeostatic function of KATP channels. Here, in a transgenic heart failure model, dysregulation of the KATP channel disrupted stress tolerance. This dysregulation was not due to alterations in the intrinsic biophysical properties of the channel, but rather to aberrant metabolic signaling to the channel preventing translation of distress under metabolic challenge into a protective membrane response. Disruption of this critical homeostatic mechanism exposes cells to injury in the disease environment of continuous stress confrontation, expanding the risk of disease progression.
The mitigated KATP channel response to cellular metabolic insult, despite intact basic properties of the channel, implicates alterations proximal to the channel, i.e. in ATP production and/or in transmission of energetic signals to the channel site, as the source of channel dysregulation. Indeed, the reduced potential for ATP production observed here is consistent with similar findings in human or experimental heart failure (Sharov et al., 2000; Liu et al., 2001), and TNFα-induced damage to mitochondria through impaired mitochondrial DNA repair (Li et al., 2001). Normally, the majority of ATP is conveyed by creatine kinase to KATP channels, thereby overcoming time delays, concentration gradients and filtering effects of passive diffusion (Abraham et al., 2002). Modifications of this norm through remodeling in heart failure can occur by several mechanisms. As is shown here, and as has been established in general, flux or activity of creatine kinase is significantly diminished in failing hearts (Nascimben et al., 1996; Dzeja et al., 1999a). In addition, architectural alterations in the myocyte (this study; see also Chien, 1999; Hein et al., 2000; Bradham et al., 2002) could contribute to physical disruption of the phosphotransfer network and alter creatine kinase-dependent gating of KATP channels. These structural changes in failing myocytes would also heighten barriers to nucleotide diffusion and influence direct energetic crosstalk between cellular compartments (Kaasik et al., 2001; Sasaki et al., 2001), impeding KATP channel responsiveness to ATP. In fact, previous works demonstrate the dependence of proper KATP channel gating on the structural integrity of the myocyte (Brady et al., 1996; Furukawa et al., 1996; Terzic and Kurachi, 1996). Whether single or multiple alterations underlie defective signaling in heart failure, the inability of the creatine kinase system to properly regulate channel function relinquishes channel control to less efficient energy transfer, compromising the fidelity of the KATP channel response to cellular metabolic fluctuations under stress.
In heart failure, myocytes confront the metabolic insults of hypoxia, ischemia and adrenergic toxicity (Chien, 1999; Braunwald and Bristow, 2000; Bradham et al., 2002; Towbin and Bowles, 2002). Normal myocytes respond to such stressors by early activation of KATP channel-mediated K+ conductance, resulting in action potential duration shortening, limitation of Ca2+ entry and myocardial protection (Nichols and Lederer, 1991). However, strikingly similar to the case of genetic deletion of KATP channels (Suzuki et al., 2002; Zingman et al., 2002a; Seino and Miki, 2003), the membrane response in TNFα-TG hearts to metabolic stress was characterized by a deficit in KATP channel opening and resultant action potential shortening. Accordingly, specific KATP channel blockers are unable to alter membrane repolarization in stressed failing hearts (Saavedra et al., 2002). Thus, dramatically blunted and/or delayed membrane response to metabolic challenge provides a mechanistic basis for vulnerability to stress of cardiomyocytes in failing heart due to KATP channel dysregulation.
In contrast to KATP channel knockout hearts, failing TNFα-TG hearts retain intact intrinsic KATP channel properties, and thereby the potential for channel manipulation. The clinically used potassium channel opener nicorandil, in combination with mitochondrial preservation and nitrate-like effects, significantly activates sarcolemmal KATP channels in metabolically challenged cardiomyocytes (Shen et al., 1991; Jahangir et al., 1994), resulting in improved tolerance to insult (Tsuchida et al., 2002). Here, in failing TNFα-TG hearts, nicorandil shortened action potential duration, attenuated calcium overload-associated contraction banding under stress, and improved glycogen stores. This is in line with recent clinical studies in which cardioprotection conferred by nicorandil therapy was demonstrated in patients with ischemic heart disease (Patel et al., 1999; The IONA Study Group, 2002). The beneficial action of potassium channel openers in TNFα-TG failing hearts was further verified by pinacidil, a structurally distinct class member. Thus, potassium channel openers, in addition to their protective effect in cardiac ischemia, may have a role in attenuating myocardial injury in the setting of heart failure.
In summary, since their discovery two decades ago, KATP channels have been recognized as metabolic mediators with recent emphasis on their role in the adaptive response to stress. However, in disease states in which cells are remodeled under altered environmental demands, understanding of KATP channel behavior has thus far been limited. Here, in transgenic cytokine-induced heart failure, key features of human disease were recapitulated, including typical structural and energetic pathology. This remodeling, without influencing intrinsic KATP channel properties, translated into deficits in the potential for production and transmission of metabolic signals, thus compromising recognition of stress and adequate membrane homeostatic response. As continual cellular confrontation of stressors is characteristic of heart failure, loss of protective mechanisms expands the risk of disease progression. Thus, KATP channel metabolic dysregulation created by the disease state is a contributor to dysfunction and vulnerability in heart failure, illustrating a mechanism for acquired channelopathy in the absence of channel mutations. As deficits are proximal to channel proteins, the retained intrinsic KATP channel properties offer a therapeutic target for improved cell tolerance in disease.
Materials and methods
Transgenic mice
Heart failure was induced by cardiac-restricted overexpression of the cytokine TNFα using the α-myosin heavy chain promoter linked to the TNFα transgene (Sivasubramanian et al., 2001). Wild-type females were bred with transgenic males, and resultant heterozygous transgenic offspring (TNFα-TG), identified by tail-cut PCR, compared with WT littermates. Protocols were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic.
Treadmill
TNFα-TG and WT mice were simultaneously exercised with increases in incline or velocity at 3 min intervals, on a two-track treadmill with a rear shock grid to enforce running (Columbus Instruments, Columbus, OH). Failure to exercise despite five shocks in 1 min defined time of drop-out. Tolerated workload (J) is the sum of kinetic (Ek = m·v2/2) and potential (Ep = m·g·v·t·sinφ) energy, where m is animal mass, v is running velocity, g is acceleration due to gravity, t is elapsed time at a protocol level and φ is angle of incline.
Echocardiography
Two-dimensional M-mode echocardiographic images (Vingmed System FiVe; GE Medical Systems, Milwaukee, WI) in isoflurane-anesthetized mice were obtained at the mid-left ventricle from the parasternal long-axis view using a 10 MHz probe and a 2 cm gel stand-off (Parker Laboratories, Inc., Fairfield, NJ). Fractional shortening (%FS) was calculated as %FS = [(S – D)/D × 100], where S is the end-systolic and D is the end-diastolic left ventricular chamber dimension (in cm) calculated using the leading-edge convention of the American Society of Echocardiography.
Isolated cardiomyocytes
The aorta was cannulated in situ, heart rapidly excised and retrogradely perfused at 90 mmHg for 5 min with HEPES buffer (Medium 199; Sigma), 1 min with a ‘low-calcium’ medium (100 mM NaCl, 10 mM KCl, 1.2 mM KH2PO4, 5 mM MgSO4, 20 mM glucose, 50 mM taurine, 10 mM HEPES) supplemented with 0.13 mM CaCl2, 2.1 mM EGTA, then 13 min with ‘low-calcium’ medium supplemented with 1% BSA, 0.2 mM CaCl2, collagenase (type IV, 22 U/ml; Worthington) and pronase (100 µg/ml; Serva). Perfusion solutions were bubbled with 100% O2. Ventricles were removed, cut into pieces (∼3 × 3 mm) and incubated at 37°C for 15 min in the enzyme solution with gentle stirring. To harvest dissociated cardiomyocytes, supernatant was centrifuged at 500 r.p.m. for 1 min. The pellet was washed in ‘low-calcium’ medium supplemented with 0.2 mM CaCl2 (‘wash’), and again centrifuged. Finally, the pellet was suspended in ‘wash’ and kept at room temperature for 0–2 h until use. The harvest procedure was repeated three to five times on the incubated ventricle pieces to maximize yield. All solutions were at pH 7.25.
Microscopy
Paraffin sections of myocardium, stained with hematoxylin/eosin or Mallory’s phosphotungstic acid hematoxylin, were examined by light microscopy. For field-emission scanning electron microscopy, isolated cardiomyocytes were fixed in PBS containing 1% glutaraldehyde and 4% formaldehyde (pH 7.2). Cells were dehydrated with ethanol and dried in a critical point dryer, coated with platinum using an Ion Tech indirect argon ion-beam sputtering system (VCR Group, San Francisco, CA), operating at accelerating voltages of 9.5 kV and 4.2 mA, and examined on a Hitachi 4700 field-emission scanning microscope (Perez-Terzic et al., 2001). For transmitted scanning electron microscopy, cells were post-fixed in phosphate-buffered 1% OsO4, stained en bloc with 2% uranyl acetate, dehydrated in ethanol and propylene oxide, and embedded in low-viscosity epoxy resin. Thin (90 nm) sections were cut on an ultramicrotome (Reichert Ultracut E), placed on 200 µm mesh copper grids and stained with lead citrate. For glycogen analysis, tissue sections were processed with 2% uranyl acetate simultaneous with lead citrate. Micrographs were taken on a JEOL 1200 EXII electron microscope operating at 60 kV.
Patch–clamp electrophysiology
Isolated ventricular cadiomyocytes (∼70 µm long) were bathed at 30 ± 1°C in 140 mM KCl, 10 mM HEPES, 5 mM glucose, 1 mM malate, 5 mM pyruvate, 5 mM EGTA and 1 mM MgCl2 pH 7.4, and patched in the ‘cell-attached’ followed by the ‘open cell-attached’ or excised ‘inside-out’ mode using 5–10 MΩ pipettes filled with 140 mM KCl, 5 mM HEPES, 1 mM MgCl2, 1 mM CaCl2 and 5 mM glucose, at –60 mV. Following seal formation with the patch pipette in the cell-attached mode, the inside-out mode was obtained by patch excision whereas the open cell-attached mode was created by remote cell membrane permeabilization using digitonin (5–8 µg/ml) applied to the cell surface by a superfusion pipette (filled with 5 µg/ml propidium iodide and 0.5 µg/ml rhodamine). Formation of the open cell-attached patch configuration was indicated, under ultraviolet light, by propidium iodide staining of the cell nucleus, while rhodamine was used to indicate superfusion flow (Abraham et al. 2002). Single channel kinetics were analyzed within bursts of channel opening (Alekseev et al., 1998).
Mitochondria
Ventricles rapidly excised from anesthetized mice were removed into an ice-cold buffer composed of 50 mM sucrose, 200 mM mannitol, 5 mM KH2PO4, 1 mM EGTA, 5 mM 3-(N-morpholino) propanesulfonic acid (MOPS) pH 7.3, with 0.2% BSA. Tissue was homogenized (PT 10/35 Polytron; Brinkman Instruments, Westbury, NY), and the mitochondrial fraction obtained by differential centrifugation (Sorvall RCSC; Kendro Laboratory Products, Newtown, CT). Mitochondria were washed, suspended in isolation buffer (without EGTA and BSA) and kept on ice. Mitochondrial protein concentration was determined with a DC protein kit (Bio-Rad, Hercules, CA). Mitochondrial oxygen consumption was monitored with an oxygen-sensitive electrode, and data processed with the use of Bioquest software. State 3 (V3) respiration was determined in the presence of 500 µM ADP (Ozcan et al., 2002).
Nuclear magnetic resonance
The aorta was cannulated in situ, heart excised and labeled for 30 s by perfusion with Krebs–Henseleit buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 0.5 mM Na-EDTA, 25 mM NaHCO3, 2.5 mM CaCl2, 11 mM glucose, 1 mM malate and 5 mM pyruvate), containing 30% [18O]water (Isotec), freeze-clamped, pulverized under liquid N2 and then extracted in a solution containing 0.6 mM HClO4 and 1 mM EDTA (Pucar et al., 2001). Protein content was determined with a DC Protein Assay kit (Bio-Rad). High-resolution 31P NMR spectra of tissue extracts were recorded on an Bruker 11 T spectrometer (Avance) at 202.5 MHz. Peak integrals were determined with a built-in integration routine (Xwinnmr 2.5 software; Bruker). Creatine kinase phosphotransfer rate was determined from the appearance rate of 18O-labeled creatine phosphate species using pseudo-linear approximation, and ATP levels determined using methylene diphosphonic acid as standard (Pucar et al., 2001).
Monophasic action potentials
The aorta was cannulated in situ, heart excised and retrogradely perfused at 90 mmHg with Krebs–Henseleit buffer filtered at 0.22 µm and bubbled with 95% O2/5% CO2 at 37°C and pH 7.4. Hearts were paced (A310 Accupulser; World Precision Instruments, Sarasota, FL) by a catheter positioned in the right ventricular apex (NuMed, Hopkinton, NY). A monophasic action potential probe (EP Technologies, San Jose, CA) was maintained in a single position on the left ventricular epicardium, and the amplified signals (IsoDam; World Precision Instruments) acquired at 11.8 kHz. For hypoxia, the perfusate was bubbled with 90% N2/5% O2/5% CO2.
Calcium imaging
Scanning confocal images (256 × 256 pixels) of isolated cardiomyocytes, loaded with Fluo3-AM (3 µM; Molecular Probes) and paced at 2 Hz, were acquired every 328 ms using the 488 nm line of an argon/krypton laser. Images were analyzed by MetaMorph software (Visitron Universal Imaging, Downingtown, PA).
Statistics
Data are expressed as mean ± SE. Comparisons are by paired Student’s t-test or analysis of variance. Survival is presented as the Kaplan–Meier product-limit estimate from which killed mice were censored, and differences determined by log-rank test. A p value <0.05 is considered significant.
Acknowledgments
Acknowledgements
Supported by the NIH (HL64822, HL-07111, GM-08685), American Heart Association, Marriott Foundation, Miami Heart Research Institute, Bruce and Ruth Rappaport Program, American Physicians Fellowship for Medicine in Israel, and Mayo Foundation Clinician-Investigator Program. A.T. is an Established Investigator of the American Heart Association.
References
- Abraham M.R., Selivanov,V.A., Hodgson,D.M., Pucar,D., Zingman,L.V., Wieringa,B., Dzeja,P.P., Alekseev,A.E. and Terzic,A. (2002) Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J. Biol. Chem., 277, 24427–24434. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L., Bryan,J. and Nakazaki,M. (2001) Of mice and men: KATP channels and insulin secretion. Recent Prog. Horm. Res., 56, 47–68. [DOI] [PubMed] [Google Scholar]
- Alekseev A.E., Brady,P.A. and Terzic,A. (1998) Ligand-insensitive state of cardiac ATP-sesitive K+ channels. Basis for channel opening. J. Gen. Physiol., 111, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B. and Lazdunski,M. (1992) Nucleotide diphosphates activate the ATP-sensitive potassium channel in mouse skeletal muscle. Pflugers Arch., 422, 185–192. [DOI] [PubMed] [Google Scholar]
- Amoroso S., Schmid-Antomarchi,H., Fosset,M. and Lazdunski,M. (1990) Glucose, sulfonylureas, and neurotransmitter release: Role of ATP-sensitive K+ channels. Science, 247, 852–854. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. (1988) Adenosine 5′-triphosphate-sensitive potassium channels. Ann. Rev. Neurosci., 11, 97–118. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. and Gribble,F.M. (2000) New windows on the mechanism of action of KATP channel openers. Trends Pharmacol. Sci., 21, 439–445. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M., Harrison,D.E. and Ashcroft,S.J. (1984) Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature, 312, 446–448. [DOI] [PubMed] [Google Scholar]
- Beguin P., Nagashima,K., Nishimura,M., Gonoi,T. and Seino,S. (1999) PKA-mediated phosphorylation of the human KATP channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J., 18, 4722–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienengraeber M., Alekseev,A.E, Abraham,M.R., Carrasco,A., Moreau,C., Vivaudou,M., Dzeja,P.P and Terzic,A. (2000) ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J., 14, 1943–1952. [DOI] [PubMed] [Google Scholar]
- Bradham W.S., Bozkurt,B., Gunasinghe,H., Mann,D.L. and Spinale,F.G. (2002) Tumor necrosis factor-α and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc. Res., 53, 822–830. [DOI] [PubMed] [Google Scholar]
- Brady P.A., Alekseev,A.E., Aleksandrova,L.A., Gomez,L.A. and Terzic,A. (1996) A disrupter of actin microfilaments impairs sulfonylurea-inhibitory gating of cardiac KATP channels. Am. J. Physiol., 271, H2710–H2716. [DOI] [PubMed] [Google Scholar]
- Braunwald E. and Bristow,M.R. (2000) Congestive heart failure: fifty years of progress. Circulation, 102, IV14–IV23. [DOI] [PubMed] [Google Scholar]
- Bryant D., Becker,L., Richardson,J., Shelton,J., Franco,F., Peshock,R., Thompson,M. and Giroir,B. (1998) Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-α. Circulation, 97, 1375–1381. [DOI] [PubMed] [Google Scholar]
- Cameron J.S., Kimura,S., Jackson-Burns,D.A., Smith,D.B. and Bassett,A.L. (1988) ATP-sensitive K+ channels are altered in hypertrophied ventricular myocytes. Am. J. Physiol., 255, H1254–H1258. [DOI] [PubMed] [Google Scholar]
- Carrasco A.J. et al. (2001) Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc. Natl Acad. Sci. USA, 98, 7623–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K.R. (1999) Stress pathways and heart failure. Cell, 98, 555–558. [DOI] [PubMed] [Google Scholar]
- Chutkow W.A., Pu,J., Wheeler,M.T., Wada,T., Makielski,J.C., Burant,C.F. and McNally,E.M. (2002) Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J. Clin. Invest., 110, 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R.M., Budas,G.R., Jovanovic,S., Ranki,H.J., Wilson,T.J., Davies,A.M. and Jovanovic,A. (2002a) M-LDH serves as a sarcolemmal KATP channel subunit essential for cell protection against ischemia. EMBO J., 21, 3936–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R.M., Ranki,H.J., Botting,C.H., Budas,G.R. and Jovanovic,A. (2002b) Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J., 16, 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier B., Badoual,T., Boehm,E., Ennezat,P.V., Guenoun,T., Su,J., Veksler,V., Hittinger,L. and Ventura-Clapier,R. (2002) Role of creatine kinase in cardiac excitation–contraction coupling: studies in creatine kinase-deficient mice. FASEB J., 16, 653–660. [DOI] [PubMed] [Google Scholar]
- DeSousa E. et al. (1999) Subcellular creatine kinase alterations—implications in heart failure. Circ. Res., 85, 68–76. [DOI] [PubMed] [Google Scholar]
- Deswal A., Petersen,N.J., Feldman,A.M., Young,J.B., White,B.G. and Mann,D.L. (2001) Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation, 103, 2055–2059. [DOI] [PubMed] [Google Scholar]
- Dzeja P.P. and Terzic,A. (1998) Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J., 12, 523–529. [DOI] [PubMed] [Google Scholar]
- Dzeja P.P., Vitkevicius,K.T., Redfield,M.M., Burnett,J.C. and Terzic,A. (1999) Adenylate kinase-catalyzed phosphotransfer in the myocardium: increased contribution in heart failure. Circ. Res., 84, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Dzeja P.P., Redfield,M.M., Burnett,J.C. and Terzic,A. (2000) Failing energetics in failing hearts. Curr. Cardiol. Rep., 2, 212–217. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Yamane,T., Terai,T., Katayama,Y. and Hiraoka,M. (1996) Functional linkage of the cardiac ATP-sensitive K+ channel to the actin cytoskeleton. Pflugers Arch., 431, 504–512. [DOI] [PubMed] [Google Scholar]
- Goodwin G.W., Ahmad,F. and Taegtmeyer,H. (1996) Preferential oxidation of glycogen in isolated working rat heart. J. Clin. Invest., 97, 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrock A., Loffler-Walz,C., Kloor,D., Delabar,U., Horio,Y., Kurachi,Y. and Quast,U. (1999) ATP-sensitive K+ channel modulator binding to sulfonylurea receptors SUR2A and SUR2B: opposite effects of MgADP. Mol. Pharmacol., 55, 832–840. [PubMed] [Google Scholar]
- Hein S., Kostin,S., Heling,A., Maeno,Y. and Schaper,J. (2000) The role of the cytoskeleton in heart failure. Cardiovasc. Res., 45, 273–278. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi,T., Clement,J.P., Namba,N., Inazawa,J., Gonzalez,G., Aguilar-Bryan,L., Seino,S., and Bryan,J. (1995) Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science, 270, 1166–1170. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi,T., Clement,J.P., Wang,C.Z., Aguilar-Bryan,L., Bryan,J. and Seino,S. (1996) A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron, 16, 1011–1017. [DOI] [PubMed] [Google Scholar]
- Jahangir A., Terzic,A. and Kurachi,Y. (1994) Intracellular acidification and ADP enhance nicorandil induction of ATP-sensitive potassium channel current in cardiomyocytes. Cardiovasc. Res., 28, 831–835. [DOI] [PubMed] [Google Scholar]
- Kaasik A., Veksler,V., Boehm,E., Novotova,M., Minajeva,A. and Ventura-Clapier,R. (2001) Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ. Res., 89, 153–159. [DOI] [PubMed] [Google Scholar]
- Kadokami T., McTiernan,C.F., Kubota,T., Frye,C.S. and Feldman,A.M. (2000) Sex-related survival differences in murine cardiomyopathy are associated with differences in TNF-receptor expression. J. Clin. Invest., 106, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch S.B. and Billingham,M.E. (1986) Myocardial contraction bands revisited. Hum. Pathol., 17, 9–13. [DOI] [PubMed] [Google Scholar]
- Koster J.C., Marshall,B.A., Ensor,N., Corbett,J.A. and Nichols,C.G. (2000) Targeted overactivity of β cell KATP channels induces profound neonatal diabetes. Cell, 100, 645–654. [DOI] [PubMed] [Google Scholar]
- Levine B., Kalman,J., Mayer,L., Fillit,H.M. and Packer,M. (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med., 323, 236–241. [DOI] [PubMed] [Google Scholar]
- Li Y.Y., Chen,D., Watkins,S.C. and Feldman,A.M. (2001) Mitochondrial abnormalities in tumor necrosis factor-α-induced heart failure are associated with impaired DNA repair activity. Circulation, 104, 2492–2497. [DOI] [PubMed] [Google Scholar]
- Liao R., Nascimben,L., Friedrich,J., Gwathmey,J.K. and Ingwall,J.S. (1996) Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ. Res., 78, 893–902. [DOI] [PubMed] [Google Scholar]
- Lin Y.F., Jan,Y.N. and Jan,L.Y. (2000) Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J., 19, 942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. et al. (2001) Mitochondrial ATPase and high-energy phosphates in failing hearts. Am. J. Physiol., 281, H1319–H1326. [DOI] [PubMed] [Google Scholar]
- Lorenz E. and Terzic,A. (1999) Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. J. Mol. Cell. Cardiol., 31, 425–434. [DOI] [PubMed] [Google Scholar]
- Mann D.L. (2003) Stress-activated cytokines and the heart: From adaptation to maladaptation. Annu. Rev. Physiol., 65, 81–101. [DOI] [PubMed] [Google Scholar]
- Matsuo M., Dabrowski,M., Ueda,K. and Ashcroft,F.M. (2002) Mutations in the linker domain of NBD2 of SUR inhibit transduction but not nucleotide binding. EMBO J., 21, 4250–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Kinoshita,K., Matsuoka,T., Fujita,A., Fujikado,T., Tano,Y., Nakamura,H. and Kurachi,Y. (2002) Intramolecular interaction of SUR2 subtypes for intracellular ADP-induced differential control of KATP channels. Circ. Res., 90, 554–561. [DOI] [PubMed] [Google Scholar]
- McTiernan C.F. and Feldman,A.M. (2000) The role of tumor necrosis factor α in the pathophysiology of congestive heart failure. Curr. Cardiol. Rep., 2, 189–197. [DOI] [PubMed] [Google Scholar]
- Miki T. et al. (2001) ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat. Neurosci., 4, 507–512. [DOI] [PubMed] [Google Scholar]
- Miki T. et al. (2002) Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat. Med., 8, 466–472. [DOI] [PubMed] [Google Scholar]
- Moreau C., Jacquet,H., Prost,A.L., D’hahan,N. and Vivaudou,M. (2000) The molecular basis of the specificity of action of KATP channel openers. EMBO J., 19, 6644–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimben L., Ingwall,J.S., Pauletto,P., Friedrich,J., Gwathmey,J.K., Saks,V., Pessina,A.C and Allen,P.D. (1996) Creatine kinase system in failing and nonfailing human myocardium. Circulation, 94, 1894–1901. [DOI] [PubMed] [Google Scholar]
- Neubauer S. et al. (1997) Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation, 96, 2190–2196. [DOI] [PubMed] [Google Scholar]
- Nichols C.G. and Lederer,W.J. (1991) Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am. J. Physiol., 261, H1675–H1686. [DOI] [PubMed] [Google Scholar]
- Nichols C.G., Shyng,S.L., Nestorowicz,A., Glaser,B., Clement,J.P., Gonzalez,G., Aguilar-Bryan,L., Permutt,M.A. and Bryan,J. (1996) Adenosine diphosphate as an intracellular regulator of insulin secretion. Science, 272, 1785–1787. [DOI] [PubMed] [Google Scholar]
- Noma A. (1983) ATP-regulated K+ channels in cardiac muscle. Nature, 305, 147–148. [DOI] [PubMed] [Google Scholar]
- O’Rourke B., Ramza,B.M. and Marban,E. (1994) Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science, 265, 962–966. [DOI] [PubMed] [Google Scholar]
- Ozcan C., Bienengraeber,M., Dzeja,P.P. and Terzic,A. (2002) Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am. J. Physiol., 282, H531–H539. [DOI] [PubMed] [Google Scholar]
- Patel D.J., Purcell,H.J. and Fox,K.M. (1999) Cardioprotection by opening of the KATP channel in unstable angina. Eur. Heart J., 20, 51–57. [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C., Gacy,A.M., Bortolon,R., Dzeja,P.P., Puceat,M., Jaconi,M., Prendergast,F.G. and Terzic,A. (2001) Direct inhibition of nuclear import in cellular hypertrophy. J. Biol. Chem., 276, 20566–20571. [DOI] [PubMed] [Google Scholar]
- Pucar D., Dzeja,P.P., Bast,P., Juranic,N., Macura,S. and Terzic,A. (2001) Cell energetics in the preconditioned state: protective role for phosphotransfer reactions captured by 18O-assisted 31P NMR. J. Biol. Chem., 276, 44812–44819. [DOI] [PubMed] [Google Scholar]
- Rajashree R., Koster,J.C., Markova,K.P., Nichols,C.G. and Hofmann,P.A. (2002) Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal KATP channels. Am. J. Physiol., 283, H584–H590. [DOI] [PubMed] [Google Scholar]
- Saavedra W.F., Paolocci,N. and Kass,D.A. (2002) Effects of cardioselective KATP channel antagonism on basal, stimulated, and ischaemic myocardial function in in vivo failing canine heart. Br. J. Pharmacol., 135, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N., Sato,T., Marban,E. and O’Rourke,B. (2001) ATP consumption by uncoupled mitochondria activates sarcolemmal KATP channels in cardiac myocytes. Am. J. Physiol., 280, H1882–H1888. [DOI] [PubMed] [Google Scholar]
- Saupe K.W., Spindler,M., Hopkins,J.C., Shen,W. and Ingwall,J.S. (2000) Kinetic, thermodynamic, and developmental consequences of deleting creatine kinase isoenzymes from the heart. J. Biol. Chem., 275, 19742–19746. [DOI] [PubMed] [Google Scholar]
- Schwanstecher M., Sieverding,C., Dorschner,H., Gross,I., Aguilar-Bryan,L., Schwanstecher,C. and Bryan,J. (1998) Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J., 17, 5529–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S. and Miki,T. (2003) Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol., 81, 133–176. [DOI] [PubMed] [Google Scholar]
- Sharov V.G., Todor,A.V., Silverman,N., Goldstein,S. and Sabbah,H.N. (2000) Abnormal mitochondrial respiration in failed human myocardium. J. Mol. Cell. Cardiol., 32, 2361–2367. [DOI] [PubMed] [Google Scholar]
- Shen W.K., Tung,R.T., Machulda,M.M. and Kurachi,Y. (1991) Essential role of nucleotide diphosphates in nicorandil-mediated activation of cardiac ATP-sensitive K+ channel. Circ. Res., 69, 1152–1158. [DOI] [PubMed] [Google Scholar]
- Shyng S.L. and Nichols,C.G. (1998) Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science, 282, 1138–1141. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian N., Coker,M.L., Kurrelmeyer,K.M., MacLellan,W.R., DeMayo,F.J., Spinale,F.G. and Mann,D.L. (2001) Left-ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation, 104, 826–831. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Sasaki,N., Miki,T., Sakamoto,N., Ohmoto-Sekine,Y., Tamagawa,M., Seino,S., Marban,E. and Nakaya,H. (2002) Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J. Clin. Invest., 109, 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic A. and Kurachi,Y. (1996) Actin microfilament disrupters enhance KATP channel opening in patches from guinea-pig cardiomyocytes. J. Physiol., 492, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The IONA Study Group (2002) Effect of nicorandil on coronary events in patients with stable angina: the Impact of Nicorandil in Angina (IONA) randomised trial. Lancet, 359, 1269–1275. [DOI] [PubMed] [Google Scholar]
- Thomas P.M., Cote,G.J., Wohllk,N., Haddad,B., Mathew,P.M., Rabl,W., Aguilar-Bryan,L., Gagel,R.F. and Bryan,J. (1995) Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science, 268, 426–429. [DOI] [PubMed] [Google Scholar]
- Tian R., Nascimben,L., Kaddurah-Daouk,R. and Ingwall,J.S. (1996) Depletion of energy reserve via the creatine kinase reaction during the evolution of heart failure in cardiomyopathic hamsters. J. Mol. Cell. Cardiol., 28, 755–765. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G., Kapadia,S., Lee,J., Durand,J.B., Bies,R.D., Young,J.B. and Mann,D.L. (1996) Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation, 93, 704–711. [DOI] [PubMed] [Google Scholar]
- Towbin J.A. and Bowles,N.E. (2002) The failing heart. Nature, 415, 227–233. [DOI] [PubMed] [Google Scholar]
- Tsuchida A. et al. (2002) Infarct size limitation by nicorandil: Roles of mitochondrial KATP channels, sarcolemmal KATP channels, and protein kinase C. J. Am. Coll. Cardiol., 40, 1523–1530. [DOI] [PubMed] [Google Scholar]
- Tucker S.J., Gribble,F.M., Zhao,C., Trapp,S. and Ashcroft,F.M. (1997) Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature, 387, 179–183. [DOI] [PubMed] [Google Scholar]
- Vivaudou M.B., Arnoult,C. and Villaz,M. (1991) Skeletal muscle ATP-sensitive K+ channels recorded from sarcolemmal blebs of split fibers: ATP inhibition is reduced by magnesium and ADP. J. Membr. Biol., 122, 165–175. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Wyss,M., Brdiczka,D., Nicolay,K. and Eppenberger,H.M. (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J., 281, 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J.N. and Lamp,S.T. (1987) Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science, 238, 67–69. [DOI] [PubMed] [Google Scholar]
- Yamada K., Ji,J.J., Yuan,H., Miki,T., Sato,S., Horimoto,N., Shimizu,T., Seino,S. and Inagaki,N. (2001) Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science, 292, 1543–1546. [DOI] [PubMed] [Google Scholar]
- Yamada M., Isomoto,S., Matsumoto,S., Kondo,C., Shindo,T., Horio,Y. and Kurachi,Y. (1997) Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J. Physiol., 499, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Gong,G., Ochiai,K., Liu,J. and Zhang,J. (2001) High-energy phosphate metabolism and creatine kinase in failing hearts—a new porcine model. Circulation, 103, 1570–1576. [DOI] [PubMed] [Google Scholar]
- Yuan F., Brandt,N.R., Pinto,J.M.B., Wasserlauf,B.J., Myerburg,R.J. and Bassett,A.L. (1997) Hypertrophy decreases cardiac KATP channel responsiveness to exogenous and locally generated (glycolytic) ATP. J. Mol. Cell. Cardiol., 29, 2837–2848. [DOI] [PubMed] [Google Scholar]
- Zingman L.V., Alekseev,A.E., Bienengraeber,M., Hodgson,D., Karger,A.B., Dzeja,P.P. and Terzic,A. (2001) Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron, 31, 233–245. [DOI] [PubMed] [Google Scholar]
- Zingman L.V. et al. (2002a) Kir6.2 is required for adaptation to stress. Proc. Natl Acad. Sci. USA, 99, 13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman L.V., Hodgson,D.M., Bienengraeber,M., Karger,A.B., Kathmann,E.C., Alekseev,A.E. and Terzic,A. (2002b) Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J. Biol. Chem., 277, 14206–14210. [DOI] [PubMed] [Google Scholar]
- Zingman L.V., Hodgson,D.M., Alekseev,A.E., and Terzic,A. (2003) Stress without distress: homeostatic role for KATP channels. Mol. Psych., 8, in press. [DOI] [PubMed] [Google Scholar]