Abstract
Deionized water was spiked with various concentrations of endotoxin and exposed to UV irradiation from medium-pressure UV lamps to assess endotoxin inactivation. It was found that endotoxin inactivation was proportional to the UV dose under the conditions examined. The inactivation rate was determined to be ∼0.55 endotoxin unit/ml per mJ/cm2 of irradiation delivered.
It has long been known that UV irradiation is an efficient technology for bacterial inactivation, but there had been concerns about its efficacy for protozoan cyst and oocyst inactivation (from the point of view of treating drinking water). Recent research has allayed these concerns by demonstrating, for example, that medium-pressure UV dosages as low as 3 mJ/cm2 in deionized water can provide 3.0-log-unit (99.9%) inactivation of Cryptosporidium parvum oocysts (9). Because Cryptosporidium oocysts are difficult to inactivate with chemical disinfectants, some drinking water treatment facilities are adding UV irradiation to augment existing disinfection and physical removal processes.
For disinfection of drinking water, a minimum UV dose (radiant exposure) of 40 mJ/cm2 has been established in at least two European jurisdictions (2, 11). In North America, regulations are being prepared. Guidelines ranging from 50 to 100 mJ/cm2 for reclaimed drinking water have been suggested (16).
The objectives of the research described herein were to assess the susceptibility of endotoxin to UV irradiation from medium-pressure UV lamps and to determine whether dosages being recommended for drinking water treatment will result in substantial inactivation of endotoxin.
Endotoxins are a component of the lipopolysaccharide complexes which make up part of the outer layer of the cell walls of most gram-negative bacteria (5, 18) and some cyanobacteria (8, 20). Lipopolysaccharide complexes are macromolecules composed of three main regions: lipid A, core polysaccharide, and O antigens (4). The lipid A component is critical for all biological responses to endotoxin (6, 15, 18). While ingestion is perhaps the most obvious route of exposure when considering the effect of endotoxins in water on humans, it has not been conclusively demonstrated that this route poses a health risk. Inhalation of moisture-saturated air in showers, swimming pools, hot tubs, saunas, etc. (1) and exposure to endotoxin in drinking water used to prepare or dilute solutions for intravenous injection (21) or dialysis (12) may be more important.
Symptoms of endotoxin exposure in humans are general and include fever, diarrhea, vomiting (5), hypotension, shock, intravascular coagulation, and death (4). The latter symptoms are exhibited only at elevated concentrations.
To date, outbreaks of endotoxin-related illness associated with drinking water have been documented infrequently (1, 12, 14; A. Muittari, R. Rylander, and M. Salkinoja-Salonen, Letter, Lancet ii:89, 1980). This may be due to the facts that many outbreaks of fever-related illness in water are never identified by routine medical and bacteriological analyses and that endotoxin-related fever symptoms are typically short-lived. Hindman et al. (12) documented endotoxin exposure in dialysis patients resulting in mild to moderate fever in 49 patients. One patient died of irreversible shock and cardiac arrest. Endotoxin was found in the tap water that was used to prepare the dialysate water. In 1996, two separate incidents in Brazil accounted for the deaths of 35 newborns and 33 infants (21). In both incidents, the cause of death was attributed to endotoxin-contaminated distilled water used to dilute intravenous medications. Unopened vials containing the distilled water were contaminated, indicating that endotoxins may have passed through the distillation process or that the vials may have been contaminated after distillation and before the vials were sealed. Endotoxins are heat resistant and are unaffected by distillation and autoclaving. Muittari et al. (letter) observed that fever was induced in subjects who had inhaled a calculated dosage of 10 to 30 ng of endotoxin/kg of body weight (a 50% retention of endotoxin was assumed when calculating this dosage). There is limited data for endotoxin concentrations in untreated water, but typically it appears to range from 1 to 400 endotoxin units (EU)/ml (1, 10, 13, 17, 19), although concentrations in excess of 38,000 EU/ml have been reported in freshwater cyanobacterial blooms (19).
Absorbance.
All absorbance values were determined with a Hewlett-Packard 8453 UV-Visible spectrophotometer (Mississauga, Ontario, Canada) using a 1-cm-long quartz cell.
Endotoxin.
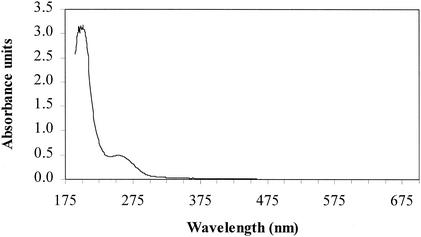
Endotoxin concentrations were determined by the QCL-1000 chromogenic Limulus amebocyte lysate tube method (BioWhittaker, Inc., Walkersville, Md.). Endotoxin from Escherichia coli strain O55:B5 was used for spiking experiments (1 ng = 8 EU for the lot used [8L2670]), and endotoxin from E. coli strain O111:B4 was used for triplicate calibration curves (both obtained from BioWhittaker, Inc.). UV absorbance of endotoxin from E. coli strain O55:B5 (500 μg/ml of deionized water) was measured and is shown in Fig. 1. It absorbs strongly between 190 and 205 nm. Absorbance then decreases between 205 and 240 nm, and a second, smaller absorbance peak is observed at 255 nm. Make-up water absorbance was less than 0.0002 absorbance unit at all wavelengths.
FIG. 1.
E. coli strain O55:B5-derived endotoxin absorbance between wavelengths of 190 and 700 nm.
Glassware preparation.
All glassware was rendered pyrogen free by heating at 350 to 400°C for at least 30 min. Some products, such as micropipetter tips and water, were purchased pyrogen free.
UV irradiation.
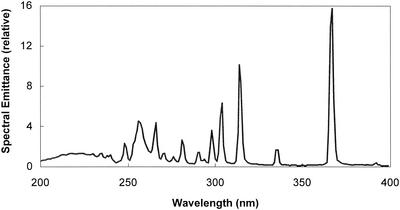
Experiments involving UV irradiation were performed with a collimated beam apparatus (Calgon Carbon Corporation, Pittsburgh, Pa.). A Rayox 1-kW medium-pressure mercury lamp emitting UV light over a broad range of wavelengths from approximately 185 to 1,400 nm was housed above a 93-cm-long polyvinyl chloride collimating tube. The relative spectral emittance of a medium-pressure UV lamp in the wavelength range from 200 to 400 nm is shown in Fig. 2. The UV fluence (dose) was determined by the method of J. R. Bolton (ultraviolet applications handbook, Bolton Photosciences Inc., Ayr, Ontario, Canada), Bolton and Linden (3), and Bukhari et al. (7) and calculated by using software produced by Bolton Photosciences Inc. The UV fluence is expressed as the product of UV intensity, expressed in milliwatts per square centimeter, and the exposure time of the fluid to be treated, expressed in seconds. The units of UV fluence expressed herein are millijoules per square centimeter, which is equivalent to milliwatt seconds per square centimeter. To convert from millijoules per square centimeter to joules per square meter, multiply the millijoule per square centimeter value by 10. All dosages expressed here are measured dosages (maximum unweighted fluence), determined by calculation after measuring the irradiance (fluence rate) at 254 nm with a radiometer (model IL-1700 research radiometer with SED240 narrow-band germicidal probe; International Light, Inc., Newburyport, Mass.). To determine the average irradiance in the test solution, the irradiance at the center of the sample surface was corrected for (i) variation in the irradiance across the petri dish, (ii) UV absorption within the liquid (water factor, measured in the 200- to 300-nm wavelength range), (iii) reflection of UV at the liquid surface (reflection factor constant of 0.975), and (iv) variation in the sensitivity of the detector (sensor factor [provided by manufacturer] of 1.206). During periods of UV irradiation, all samples were continuously stirred in a 50-mm-diameter petri dish for predetermined exposure times. The sample volume was 5 ml, resulting in a depth of 0.28 cm. The lid of the petri dish was removed during irradiation.
FIG. 2.
Relative spectral emittance of medium-pressure UV lamp in the wavelength range from 200 to 400 nm. (Adapted from reference 2a with the permission of the publisher.)
Irradiation conditions.
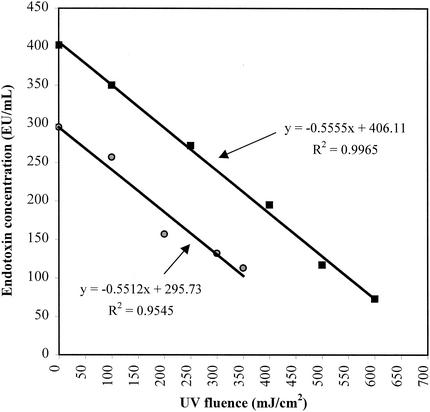
Endotoxin was added to Milli-Q Plus (Millipore, Bedford, Mass.) deionized water to achieve target concentrations of 300 and 400 EU/ml for two separate experiments. The pH of the deionized water was 5.9, and the experiments were conducted at ambient temperature (22 to 24°C). Deionized water was used to avoid potential matrix problems, limit irradiance of OH radical precursors, and allow for comparison in the future of endotoxins from various strains of bacteria and cyanobacteria. All 5-ml aliquots of water to be irradiated were removed from a single flask containing a known amount of endotoxin immediately prior to irradiation of the first sample. Included in these aliquots was a nonirradiated sample that was placed in a petri dish at the same time. All dishes were kept covered in the dark, except while being irradiated (including the control). The nonirradiated samples (the values shown at 0 mJ/cm2 in Fig. 3) are water samples that were placed in the petri dishes at the start of the experiments. All samples, including the nonirradiated control, were in the petri dishes for exactly the same amount of time. If there were losses to the plastic, they should have been uniform in all samples. The calculated inactivation rates should therefore be real and unaffected or equally affected by adsorption (i.e., slope should not change).
FIG. 3.
UV fluence versus endotoxin remaining in spiked deionized water samples. Results from two separate experiments with differing initial endotoxin concentrations (multiply the millijoule per square centimeter value by 10 to convert to joules per square meter).
Endotoxin inactivation.
Endotoxin inactivation was found to be proportional to UV fluence between 100 and 600 mJ/cm2. Figure 3 shows the results of two experiments with a regression line and equation for each experiment. The linear regression equations show that endotoxin from E. coli strain O55:B5 is removed at a rate of approximately 0.55 (EU/ml)/(mJ/cm2) of medium-pressure UV fluence. UV disinfection treatment of drinking water will likely vary from 40 to 100 mJ/cm2. Therefore, in practical terms, an inactivation rate of 0.55 (EU/ml)/(mJ/cm2) has the potential to completely remove or substantially reduce endotoxin levels (Table 1) when the initial concentration is in the lower end of the range (1 to 50 EU/ml) typically found in untreated water. Removal ranging from 11 to 55% can be expected in the range from 50 to 200 EU/ml. An OH− scavenger (2-methylpropan-2-ol) was added in one series of tests to confirm that hydrogen peroxide-mediated reactions were not affecting the inactivation rate, and it was found that they were not (data not shown). This research suggests that typical endotoxin concentrations in drinking water could be effectively completely inactivated by applying UV fluences of up to 500 mJ/cm2 (assuming that all endotoxins are inactivated equally). This previously unreported finding is significant because of the rapidly increasing use of UV for drinking water disinfection, although the economic feasibility of such high doses for a given plant would need to be considered. To provide a greater quantitative database, this research should be repeated for endotoxin isolated from other strains of bacteria and/or cyanobacteria and for different water types (with different transmittance and/or turbidity values). Additional confirmation could be obtained by evaluating some full-scale drinking water treatment plant units when they become accessible for testing. The present work may also have implications with regard to dialysis issues (e.g., point-of-use applications) and the potential for advances in the treatment of liquids and equipment for routine medical applications involving both healthy and immunosuppressed patients.
TABLE 1.
Theoretical endotoxin concentration after UV exposure
| UV fluence (mJ/cm2) | Theoretical endotoxin concn (EU/ml) after UV irradiation of samples with an initial endotoxin concn (EU/ml) of:
|
|||||
|---|---|---|---|---|---|---|
| 10 | 25 | 50 | 100 | 150 | 200 | |
| 40 | 0 | 3 | 28 | 78 | 128 | 178 |
| 60 | 0 | 0 | 17 | 67 | 117 | 167 |
| 80 | 0 | 0 | 6 | 56 | 106 | 156 |
| 100 | 0 | 0 | 0 | 45 | 95 | 145 |
Acknowledgments
Funding for this project was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC).
We thank Janis L. Zimmer and James R. Bolton for assistance with the collimated beam apparatus operation and associated dose calculations. We thank Calgon Carbon Corporation (Pittsburgh, Pa.) for access to the collimated beam apparatus.
REFERENCES
- 1.Anderson, W. B., R. M. Slawson, and C. I. Mayfield. 2002. A review of drinking water-associated endotoxin including potential routes of human exposure. Can. J. Microbiol. 48:567-587. [DOI] [PubMed] [Google Scholar]
- 2.Austrian Standards Institute. 2001. Plants for the disinfection of water using ultraviolet radiation, requirements and testing: low pressure mercury lamp plants. Technical Committee 140, Water Quality and Water Treatment. ÖNORM M5873-1. Österreichisches Normungsinstitut, Vienna, Austria.
- 2a.Bolton, J. R. 2001. Ultraviolet applications handbook, 2nd ed., p. 18. Bolton Photosciences Inc., Ayr, Ontario, Canada.
- 3.Bolton, J. R., and K. G. Linden. 2001. Determination of fluence (UV dose) in a bench-scale collimated beam apparatus for monochromatic and broadband UV lamps. Proceedings of the American Waterworks Association Water Quality Technology Conference. American Water Works Association, Denver, Colo.
- 4.Braude, A. I. 1982. Bacterial endotoxins, p. 63-74. In C. E. Davis and J. Fierer (ed.), Microbiology. W. B. Saunders Company, Toronto, Ontario, Canada.
- 5.Brock, T. D., and M. T. Madigan. 1991. Biology of microorganisms, 6th ed. Prentice Hall, Englewood Cliffs, N.J.
- 6.Brooks, G. F., J. S. Butel, L. N. Ornston, E. Jawetz, J. L. Melnick, and E. A. Adelberg. 1995. Jawetz, Melnick & Adelberg's medical microbiology, 20th ed. Appleton & Lange, Stamford, Conn.
- 7.Bukhari, Z., T. M. Hargy, J. R. Bolton, B. W. Dussert, and J. L. Clancy. 1999. Medium-pressure UV for oocyst inactivation. J. Am. Water Works Assoc. 93:86-94. [Google Scholar]
- 8.Buttke, T. M., and L. O. Ingram. 1975. Comparison of lipopolysaccharides from Agmenellum quadruplicatum to Escherichia coli and Salmonella typhimurium by using thin layer chromatography. J. Bacteriol. 124:1566-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy, J. L., Z. Bukhari, T. M. Hargy, J. R. Bolton, B. W. Dussert, and M. M. Marshall. 2000. Using UV to inactivate Cryptosporidium. J. Am. Water Works Assoc. 92:97-104. [Google Scholar]
- 10.Evans, T. M., J. E. Schillinger, and D. G. Stuart. 1978. Rapid determination of bacteriological water quality by using Limulus lysate. Appl. Environ. Microbiol. 35:376-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.German Association on Gas and Water (Deutscher Verein des Gas- und Wasserfaches e.V.). 1997. UV systems for disinfection in drinking water supplies—requirements and testing. Technical standard W 294. [Online.] Deutscher Verein des Gas- und Wasserfaches e.V., Bonn, Germany. http://www.dvgw.de.
- 12.Hindman, S. H., M. S. Favero, L. A. Carson, N. J. Peterson, L. B. Schonberger, and J. T. Solano. 1975. Pyrogenic reactions during haemodialysis caused by extramural endotoxins. Lancet ii:732-734. [DOI] [PubMed]
- 13.Korsholm, E., and H. Søgaard. 1988. An evaluation of direct microscopical counts and endotoxin measurements as alternatives for total plate counts. Water Res. 22:783-788. [Google Scholar]
- 14.McGregor, F. R., W. D. Walenczak, R. Rogers, and L. Magnetti. 1993. Case study: ozone-based water treatment for high quality air and water in a municipal swimming center, p. S-12A-1-S-12A-8. In Ozone in water and wastewater treatment. Proceedings of the International Ozone Association's 11th Ozone World Congress. International Ozone Association, Lille, France.
- 15.Morrison, D. C., R. L. Danner, C. A. Dinarello, R. S. Munford, C. Natanson, M. Pollack, J. J. Spitzer, R. J. Ulevitch, S. N. Vogel, and E. McSweegan. 1994. Bacterial endotoxins and pathogenesis of Gram-negative infections: current status and future direction. J. Endotoxin Res. 1:71-83.
- 16.National Water Research Institute/American Water Works Association Research Foundation. 2000. Ultraviolet disinfection guidelines for drinking water and water reuse. National Water Research Institute, Fountain Valley, Calif.
- 17.Peppler, M. S., P. M. Huck, G. Flowerdew, and W. B. Anderson. 1994. Production of endotoxin in a biological drinking water treatment process, p. 1365-1396. Proceedings of the American Waterworks Association Water Quality Technology Conference. American Water Works Association, Denver, Colo.
- 18.Prescott, L. M., J. P. Harley, and D. A. Klein. 1993. Microbiology, 2nd ed. William C. Brown Publishers, Dubuque, Iowa.
- 19.Rapala, J., K. Lahti, L. A. Räsänen, A.-L. Esala, S. I. Niemelä, and K. Sivonen. 2002. Endotoxins associated with cyanobacteria and their removal during drinking water treatment. Water Res. 36:2627-2635. [DOI] [PubMed]
- 20.Sykora, J. L., and G. Keleti. 1981. Cyanobacteria and endotoxins in drinking water supplies, p. 285-301. In W. W. Carmichael (ed.), The water environment, algal toxins and health. Plenum Press, New York, N.Y.
- 21.Williams, K. L. 2001. Endotoxins, pyrogens, LAL testing, and depyrogenation, 2nd ed., p. 120. Marcel Dekker, Inc., New York, N.Y.