Abstract
Cryptosporidium parvum represents a challenge to the water industry and a threat to public health. In this study, we developed a cell culture-quantitative PCR assay to evaluate the inactivation of C. parvum with disinfectants. The assay was validated by using a range of disinfectants in common use in the water industry, including low-pressure UV light (LP-UV), ozone, mixed oxidants (MIOX), and chlorine. The assay was demonstrated to be reliable and sensitive, with a lower detection limit of a single infectious oocyst. Effective oocyst inactivation was achieved (>2 log10 units) with LP-UV (20 mJ/cm2) or 2 mg of ozone/liter (for 10 min). MIOX and chlorine treatments of oocysts resulted in minimal effective disinfection, with <0.1 log10 unit being inactivated. These results demonstrate the inability of MIOX to inactivate Cryptosporidium. The assay is a valuable tool for the evaluation of disinfection systems for drinking water and recycled water.
Cryptosporidium is recognized as a frequent cause of waterborne disease in humans (3). The disease has been documented worldwide, with speculation that many undiagnosed cases of gastroenteritis may have been caused by this parasite (21, 38). Two means of transmission have been shown to be contaminated drinking water in distribution systems (25, 28) and swimming pools (17). Cryptosporidium oocysts are resistant to standard chlorine-based disinfection procedures that are a key barrier in the transmission of other waterborne pathogens in potable water. Although some water treatment processes remove oocysts, any oocysts that break through represent a potential threat to human health due to their relative chlorine resistance. Various methods of disinfection have been investigated by a number of research groups; these methods include UV light (4, 10, 11, 13, 14, 23, 35), ozone (5, 18, 22, 29), chlorine dioxide (8, 12, 22, 30, 34), mixed oxidants (MIOX) (6, 40), and chlorine (7, 22, 17). Many disinfection studies have used animal infectivity or surrogate in vitro assays to determine the viability of oocysts after disinfection. Animal bioassays are considered the “gold standard” for assessing Cryptosporidium oocyst infectivity, and the neonatal mouse model has been used extensively in the assessment of oocyst disinfection. However, this model has limited applications for the assessment of waterborne oocysts because type 1 (human) Cryptosporidium parvum cannot infect mice. The human genotype can be cultured in gnotobiotic pigs (41), and this model has been used to assess drug efficacy (37).
Significant developments in determining oocyst infectivity have included cell culture (CC) assays for type 1 oocysts (20) and for type 2 oocysts (14, 15, 20, 31, 36, 39). These methods have used culturing of oocysts in HCT-8 (human ileocecal adenocarcinoma) cells. Evaluation of these methods has shown the CC assay with the HCT-8 cell line to be equivalent to the gold standard neonatal mouse infectivity assay (32, 35). Shim et al. (35) demonstrated that CC assays provide a level of sensitivity similar to that of mouse bioassays when low-pressure UV light (LP-UV) is used for the disinfection of C. parvum. Rochelle et al. (32) compared a range of cell lines with the CD-1 mouse bioassay for determining dose response and 50% infective dose. The correlation between infectivity in CD-1 mice and three CC models demonstrated that HCT-8 cells had infectivity measurements matching those in CD-1 mice for untreated oocysts (r = 0.85, n = 25) and for oocysts exposed to ozone and UV light (r = 0.85, n = 25). These results demonstrated that in vitro cell culturing was equivalent to the gold standard mouse infectivity assay and should be considered a practical alternative for assessing oocyst infectivity and inactivation (32). The use of a continuous cell line removes the issues related to animal ethics.
A range of methods, including reverse transcriptase PCR (33), immunofluorescence microscopy (36), and colorimetric in situ hybridization (32), have been applied for the analysis of CC infection. These methods are often time-consuming, involving extraction of mRNA or considerable amounts of scanning on a microscope. The advent of “real-time” quantitative PCR (Q-PCR) offers the prospect of a faster analytical procedure for the detection and quantification of Cryptosporidium CC infectivity. Q-PCR allows real-time quantitation of PCR amplicons without the need for electrophoresis and densitometry (19). A Q-PCR assay was recently developed for the assessment of drug efficacy against C. parvum (24) and demonstrated reproducibility and a high level of sensitivity. In this study, we present a rapid method that allows quantitation of the level of infection within a CC. This goal was achieved by combining standard CC techniques with a Taqman PCR that allows real-time evaluation of oocyst infectivity. The assay was tested against a range of disinfection methods, including UV light, ozone, MIOX, and sodium hypochlorite.
MATERIALS AND METHODS
C. parvum oocysts.
C. parvum cattle isolate (Swiss cattle C26) oocysts were purchased from U. Ryan, Department of Veterinary and Biomedical Sciences, Murdoch University, Perth, Australia. Oocysts were passaged through mice as described by Meloni and Thompson (26) and Hijjawi et al. (20). Oocysts were stored in sterile phosphate-buffered saline (PBS) supplemented with antibiotic solution (15 μl/ml) containing ampicillin (10 mg/ml) and lincomycin (4 mg/ml). All experiments were carried out within 6 weeks after purification of oocysts with greater than 80% viability, as determined by in vitro excystation (22).
Enumeration of oocysts.
Standard counts were determined for all oocyst stocks. Counts were determined by serial dilution in sterile double-distilled water (SDDW) and placement of replicate aliquots on polycarbonate black 0.8-μm-pore-size membrane filters (Osmonics Inc.) by using a Qiavac manifold (Qiagen, Clifton Hill, Victoria, Australia) fitted with Swinnex filter holders (Millipore) at a vacuum pressure of 200 mbar (1 bar = 105 Pa). Monoclonal antibody AusFlowCry104 (Macquarie University, Sydney, New South Wales, Australia) was diluted to a concentration of 6.6 μg/ml in antibody buffer (5% [wt/vol] bovine serum albumin in Isoton II [Beckman Coulter, Glodesville, New South Wales, Australia]). An aliquot (100 μl) was applied to oocysts on the membranes and incubated at room temperature for 15 min. The antibody solution was drawn through the membranes via a vacuum, and the membranes were washed with Isoton II solution (250 μl). The membranes were mounted on glass microscope slides with 4 μl of mounting medium (glycerol [nonphotoreactive], 2 ml; 100 mg of DABCO {1,4-diazabicyclo[2,2,2]octane} per ml of SDDW, 2.4 ml; 0.1 M Tris buffer, 4.8 ml; formalin, 0.5 ml; and 5 M NaCl, 0.5 ml) and sealed with coverslips and clear nail varnish. Entire membranes were scanned and all oocysts were counted by fluorescence microscopy (Olympus Vanox BX50). All counts were determined in triplicate.
Disinfection experiments. (i) UV light disinfection experiments.
UV light was applied to aliquots of 10,000 oocysts by using a Starkey UV sterilization cabinet consisting of two 15-W low-pressure mercury vapor germicidal lamps emitting nearly monochromatic UV irradiation at 253.7 nm. UV irradiance was measured with an IL-400A photometer (International Light, Newburyport, Mass.) at the point of irradiation. Oocysts were diluted in 0.01 M potassium phosphate buffer (phosphate buffer) from stock buffer (0.1 M; 39 ml of 0.2 M KH2PO4 and 61 ml of 0.2 M K2HPO4 [pH 7.0], diluted in MQ water to 0.01 M) to a final pH of 7.0. Aliquots (100 μl) were placed in 25-mm disposable petri dishes (lids off) at 22°C in a static system, and UV light was applied at various doses from 3 to 1,000 mJ/cm2 (see Table 2).
TABLE 2.
Inactivation of C. parvum oocysts by LP-UV (Starkey cabinet), as determined by the CC-Taqman PCR
| UV dose (mJ/cm2) | Log10 reduction in C. parvum oocyst infectivity in expt:
|
Avg for all expts: | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 0 | 0 | 0 | 0 | 0 |
| 3 | 1.27 | 1.28 | 2.04 | 1.53 |
| 6 | 1.57 | 1.59 | 2.25 | 1.80 |
| 12 | 1.66 | 1.79 | 1.87 | 1.77 |
| 20 | 2.07 | 2.2 | 2.2 | 2.16 |
| 30 | 1.96 | 2.4 | 2.18 | |
| 500 | 3.70 | 3.85 | 3.69 | 3.75 |
| 1,000 | 4.0 | 4.0 | 4.0 | 4.0 |
(ii) Ozone disinfection experiments.
Ozonated water was generated on site by using an Ozat CFS apparatus (Ozonia, Ltd., Duebendorf, Switzerland). The ozone gas concentration was measured by the indigo trisulfonate method and on the assumption of a molar absorptivity of 23,150 M−1 cm−1 (9). The ozone concentration was determined per milliliter of ozonated water and allowed calculation of the volume required to add the appropriate dose to individual reaction vessels. The reactors were set up by using 250-ml Schott bottles and 0.01 M phosphate buffer (pH 7.0) (previously described). Experiments were started by the addition of a suspension of 10,000 oocysts to a reaction vessel. Ozone was applied at 1.0, 2.0, or 5.0 mg/liter, and the final reactor volume was 200 ml. The reactors were immediately mixed by inversion (five times) and incubated for 10 min at 22°C. All reactions (including the ozone-free control) were quenched by using sodium thiosulfate (2 ml of a 1.67% [wt/vol] solution). The suspension was decanted into a centrifuge tube, the reaction vessel was rinsed with 50 ml of 0.01% Tween 20, and oocysts were pelleted by centrifugation (1,800 × g for 10 min). The majority of the supernatant was discarded, the pellet was resuspended in the remaining supernatant, and the mixture was transferred to a 10-ml sterile tube. Samples were centrifuged (10 min at 1,800 × g), the majority of the supernatant was discarded, and the resuspended pellet was transferred to a 1.5-ml centrifuge tube. Samples were brought to 1 ml with sterile PBS, and an aliquot (100 μl) was set aside for staining with the antibody solution for enumeration by microscopy. The remainder was concentrated by centrifugation (1,800 × g for 10 min). The majority of the supernatant was removed, and samples were prepared for the CC-Taqman PCR assay by incubation in acidified water-trypsin (see description of in vitro culturing and infection of the cell line below).
(iii) MIOX disinfection experiments.
MIOX were generated by using a MIOX brine pump system (BPS) disinfection unit (MIOX Corp., Albuquerque, N.Mex.). Brine (NaCl, 11 g/liter) was prepared in a 1-liter Schott bottle wrapped in aluminum foil 1 h prior to the experiments. The brine solution was passed through the BPS at a rate of 19 liters/h, 12 V, and 6 A (a function of the brine concentration). The MIOX solution was collected in a sterile glass bottle from either the anode flow (MIOXa) or the combined anode and cathode flows (MIOXac) from the electrochemical cell and stored in the dark until used. Total oxidants were measured for MIOX as total chlorine by using diethyl-p-phenylenediamine as an indicator (1); the concentration was expressed in milligrams per liter. Disinfection experiments were carried out with 10-ml reaction vessels containing 5 ml of oocyst suspension (10,000 oocysts/ml in 0.01 M phosphate buffer [pH 7.0]). MIOX were applied at a concentration of 2, 5, or 10 mg/liter, and samples were mixed by brief vortexing and incubated at 22°C for between 1 and 24 h. Aliquots (1 ml) were taken after brief vortexing to resuspend oocysts and were neutralized with an equal volume of 1% (wt/vol) sodium thiosulfate. Oocysts were recovered by centrifugation (1,800 × g for 10 min), and the majority of the supernatant was discarded. The entire sample was prepared for the CC-PCR assay.
(iv) Sodium hypochlorite.
Standard pool chlorine (125 g of total chlorine/liter) was used as a control sodium hypochlorite mixture. Chlorine was freshly diluted in sterile MQ water, and total chlorine was determined by using diethyl-p-phenylenediamine colorimetric titration (1). Aliquots of oocysts (10,000 oocysts/ml) in 0.01 M phosphate buffer (pH 7.0) were treated with sodium hypochlorite applied at a total chlorine concentration of 2, 5 or 10 mg/liter and vortexed immediately. Samples were incubated at 22°C for between 1 and 24 h and processed by using the method described for MIOX.
In vitro culturing and infection of the cell line.
Cells from the HCT-8 (ATCC CCL244 [American Type Culture Collection]; human ileocecal adenocarcinoma) cell line were maintained with regular subculturing in RPMI 1640 growth medium with l-glutamine (Sigma-Aldrich Co., Sydney, New South Wales, Australia) and supplemented with 15 mM HEPES buffer, antibiotics (penicillin G, 100,000 U/liter; streptomycin, 0.1 g/liter), and 10% fetal calf serum adjusted to pH 7.4 (26). HCT-8 cells were incubated at 37°C with 5% (vol/vol) CO2, used to inoculate 24-well CC trays, and grown for 24 h until monolayers formed. Oocysts were prepared for cell culturing by incubation in 1 ml of acidified water (pH 2.7)-trypsin (0.025% [wt/vol]) for 20 min at 37°C with mixing by inversion (five times) every 5 min, centrifugation (10 min at 1,800 × g), and resuspension in 1 ml of maintenance medium. Maintenance medium consisted of RPMI 1640 medium with l-glutamine, 15 mM HEPES buffer, sodium bicarbonate (2 g/liter), glucose (1.0 g/liter), bovine bile (0.2 g/liter), folic acid (250 μg/liter), 4-aminobenzoic acid (1 mg/liter), calcium pantothenate (50 μg/liter), ascorbic acid (8,750 μg/liter), penicillin G (100,000 U/liter), streptomycin (100 mg/liter), lincomycin (40 mg/liter), and gentamicin (50 mg/liter) and adjusted to pH 7.4. Growth medium was removed from the monolayers by aspiration, prepared oocysts were applied to the monolayers, and the monolayers were incubated at 37°C in 5% (vol/vol) CO2 for 48 h. For disinfection experiments, control (untreated) oocysts were used to determine optimal infection, which was used to calculate log inactivation due to disinfection.
DNA extraction from CCs.
Maintenance medium was removed from the wells by aspiration and discarded, and the infected monolayers were washed three times with 500 μl of sterile Dulbecco's PBS (Sigma-Aldrich). Cell monolayers were harvested in 200 μl of 10 mM Tris-1 mM EDTA buffer (pH 8.0) (15). Resuspended cells were transferred to microcentrifuge tubes and collected by centrifugation (10 min at 8,000 × g). The supernatant was removed by aspiration, and the pellet was rinsed in an aliquot (200 μl) of GeneAmp PCR buffer II (Applied Biosystems, Melbourne, Australia). Samples were pelleted (10 min at 8,000 × g), and the supernatant was discarded. The pellet was resuspended in 50 μl of InstaGene matrix (Bio-Rad, Regents Park, New South Wales, Australia), vortexed briefly, and heated at 56°C (10 min) followed by 100°C (20 min). The sample was centrifuged (17,000 × g) to pellet the InstaGene matrix, and an aliquot (10 μl) of the supernatant was used in the Taqman PCR assay.
Real-time 18S Taqman PCR assay for detection of infectious C. parvum.
Real-time PCR was performed by using 18Si primers, previously described by Morgan et al. (27). These primers are specific to the 18S region of Cryptosporidium. The sequence of the Taqman probe was based on the conserved eukaryotic probe of Amman et al. (2) with the following sequence: 5-′-(6-FAM) ACC AGA CTT GCC CTC C (TAMRA). The probe was supplied by Genset Singapore Biotech Pty. Ltd., Singapore, Singapore. Reaction conditions were a final volume of 50 μl containing PCR buffer II (Applied Biosystems), 2.5 U of Amplitaq Gold (Applied Biosystems), 3.75 mM MgCl2, 0.5 μM each forward and reverse primers, 400 μM each deoxynucleoside triphosphate, and 0.2 μM Taqman probe. An aliquot (10 μl) of the template was used in each reaction. Cycling parameters were 10 min at 95°C followed by 50 cycles of 20 s at 94°C and 90 s at 60°C on a RotorGene 2000 system (Corbett Research, Mortlake, New South Wales, Australia). The large number of cycles was used to ensure the detection of low levels of infection. A signal was acquired either on channel 1 (dual-channel machine) or on the FAM channel (multichannel machine) (source, 470 nm; detector, 510 nm; gain set to 7). When required, PCR amplicons were visualized by UV illumination following electrophoresis in 1% (wt/vol) agarose gels containing ethidium bromide.
DNA standard preparation for the Taqman PCR assay.
DNA standards were prepared from fresh C. parvum oocysts following standard count determinations. An aliquot of oocysts (total, 106 oocysts) was incubated in 1 ml of acidified water (pH 2.7)-trypsin (0.025% [wt/vol]) for 20 min. Samples were centrifuged (10 min at 10,000 × g), and the supernatant was discarded. The pellet was washed in 100 μl of GeneAmp PCR buffer II and centrifuged for 10 min at 10,000 × g. The supernatant was discarded, and the pellet was resuspended in SDDW (100 μl). Samples were taken through five cycles of freezing at −180°C in liquid nitrogen (1 min) and thawing at 100°C (1 min). Samples were incubated at 100°C for 5 min and then centrifuged for 1 min at 10,000 × g for collection. Serial dilutions were made with SDDW, giving a range of standards equivalent to 105 to 10−1 oocysts/10-μl aliquot. All samples were stored at −20°C until required. All standards were used at 10 μl per 50-μl PCR.
The inclusion of DNA standards in the real-time PCR enables the generation of a standard curve (Table 1). This curve shows a line between the given concentration and the calculated concentration for the standard series and provides a line of best fit with the highest correlation. The threshold is optimized by moving the level to minimize the distance between each of the given and calculated concentrations. This process allows the quantitation of PCR products in individual unknown samples monitored on the RotorGene 2000. Calculations were based on the number of individual sporozoites added to each PCR (based on an average of four sporozoites per oocyst) for the DNA standards. This process allows the quantitation of individual life stages in infected cell monolayers. A series of negative controls were used in the assays; these included HCT-8 cells and heat-inactivated oocysts (80°C for 10 min).
TABLE 1.
Data generated from a standard curve using with RotorGene2000 systema
| Oocyst Concn | CV (%) | Ct | |
|---|---|---|---|
| Given | Calculated | ||
| 100,000 | 83,937 | 16.06 | 17.34 |
| 10,000 | 11,681 | 16.81 | 20.51 |
| 1,000 | 1,026 | 2.59 | 24.42 |
| 100 | 108 | 7.91 | 28.04 |
| 10 | 10 | 0.40 | 31.87 |
| 1 | 1 | 7.49 | 35.69 |
DNA standards consist of serial dilutions of C. parvum DNA at concentrations of 105 to 10° (one) oocysts. The given concentration is the total number of oocysts added to the PCR; the calculated concentration is the oocyst concentration calculated by using RotorGene 2000 software to find a line of best fit. CV, coefficient of variation. The line of best fit variation. The line determined for the standards had the following values: R2, 0.99691; slope, −0.259; y intercept, 9.504.
Application of the CC-PCR assay to filtering of backwash liquor.
The infectivity of C. parvum in filter backwash water (FBWW) was tested to determine the robustness of the CC-PCR assay. Several samples were obtained from the Hope Valley Water Treatment Plant (South Australia, Australia). Turbidity was determined to be 81.7 nephelometric turbidity units (ntu) for FBWW and 1.1 ntu for clarified FBWW (CFBWW). Oocysts (10,000 oocysts/ml) were suspended in the selected water types and mixed by brief vortexing. Samples (1 ml per aliquot) were taken and processed as described above. Infections in the different water types were compared by using the CC-PCR assay.
RESULTS
Microscopic enumeration of oocysts.
All oocyst stocks were enumerated and adjusted as necessary to give equal numbers of oocysts. Samples were counted in triplicate and gave 100 ± 8 oocysts per aliquot when 100 oocysts were expected.
Sensitivity of the Taqman assay.
The Taqman assay was initially assessed with DNA extracted from purified oocysts to determine the level of sensitivity and evaluate the reliability of the assay. The results demonstrated that DNA from a single oocyst (diluted from a DNA extraction of 10 oocysts) could be detected 100% of the time (10 of 10 samples) (Fig. 1). With the RotorGene 2000 system, the reliable detection limit of the single-round PCR assay was found to be a single oocyst when serial dilutions of DNA extracts were used. When challenged with the 0.1 oocyst dilution, the PCR was successful for 62.5% of the reactions (five of eight samples). Detection at this lower limit was possible because there are 40 copies of the target rRNA gene in a single oocyst (10 per diploid sporozoite) and this assay is capable of detecting as few as 4 copies. Thus, low levels of infection can be detected with this assay.
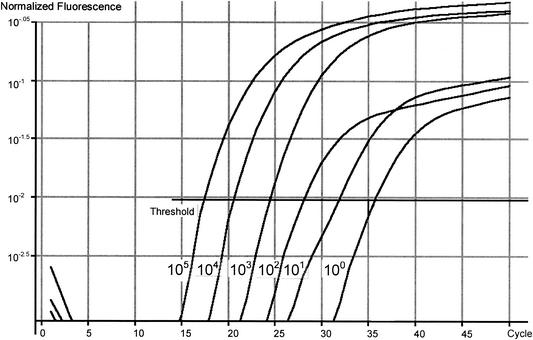
FIG. 1.
Real time PCR log graph of an 18Si Taqman PCR assay conducted with oocyst DNA extracts containing from 100,000 oocysts to a single oocyst by using the RotorGene 2000. Normalized fluorescence was used to determine the average background for each sample by using the first five cycles and fluorescence as an indicator for the background level for each sample. All data points were then divided by this value to normalize the data and were converted to a log scale. The number of PCR cycles is indicated on the x axis. Ct values determined for individual standards were as follows: 100,000 oocysts, 18.2; 10,000 oocysts, 20.72; 1,000 oocysts, 24.67; 100 oocysts, 29.0; 10 oocysts, 33.5; and 1 oocyst, 41.1.
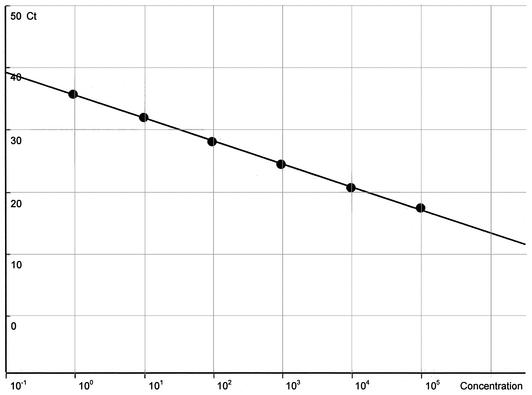
The Ct value is defined as the cycle number at which amplification starts and is proportional to the amount of starting DNA in the PCR. By setting a threshold line and calculating the intersection with each of the sample curves, the Ct value for each sample can be established. The Ct values were 18.2 and 41.1 for 105 oocysts and 1 oocyst, respectively (Fig. 1). A standard curve was generated by using the data generated for DNA standards (Fig. 2) and a line of best fit. The HCT-8 cell extract failed to produce a detectable signal in the PCR, indicating no amplification of the host cell DNA with the 18Si primers (Fig. 1).
FIG. 2.
Standard curve for serially diluted Cryptosporidium DNA standards produced by using RotorGene 2000 software.
Validation of Taqman assay sensitivity with CCs.
The sensitivity of the CC-Taqman PCR assay was assessed by using C. parvum oocysts taken through the CC process. Monolayers were seeded with serial dilutions of oocysts from 105 to a single oocyst. DNA was extracted, and one-fifth of the DNA extract was used as a template in each real-time PCR. The Taqman assay could reliably detect infection in the monolayers from an inoculum of 10 oocysts 93% of the time (14 of 15 samples). When lower numbers were used, infection was established 20% of the time (3 of 15 samples). These data may reflect the fact that not all oocysts are infective. Experiments with 10 replicate wells for both untreated and UV-treated (3 mJ/cm2) oocysts were compared to evaluate the reproducibility of the CC-Taqman PCR assay. The results demonstrated that the assay was highly reproducible, with a mean Ct value of 22.35 (standard deviation, ±0.178; n = 10) for untreated oocysts. When UV treatment was performed, the Ct value increased to 27.911 (standard deviation, ±2.08; n = 10). This increase in Ct was due to a decrease in the number of infective oocysts present in the sample after UV treatment. There was also an increase in standard deviation with this treatment, most likely due to the inaccuracies of the UV dose applied to the oocysts. Heat-inactivated oocysts were included in the CC-Taqman PCR assay and, when tested, were not detected, indicating successful removal of oocysts that had not undergone excystation and cell debris from the monolayers before extraction of the DNA.
Validation of water types with the CC-PCR assay.
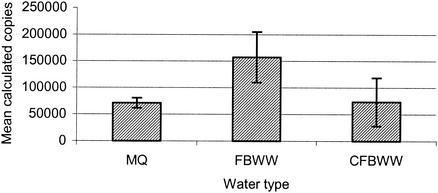
The infectivities of C. parvum in MQ water, FBWW, and CFBWW samples spiked with 10,000 oocysts/ml were compared by using the CC-PCR assay (Fig. 3). The presence of contaminants in FBWW in no way inhibited infection in the CC-PCR assay. Interestingly, a significant increase in the level of infection was observed for FBWW compared to both MQ water and CFBWW when a single-factor analysis of variance was used (P = 0.00951, F critical (Fcrit) = 5.59166, n = 10, and P = 0.021109, Fcrit = 5.3176, n = 10, respectively). MilliQ (MQ) water and CFBWW showed no difference in the level of infection (P = 0.923, Fcrit = 5.5915, n = 10).
FIG. 3.
Measurement of infection with C. parvum in a range of water types, including MQ, FBWW (81.7 ntu), and CFBWW (1.1 ntu). Infectivity was measured by using the CC-PCR assay. Error bars indicate standard deviations.
Inactivation of C. parvum with disinfectants. (i) UV light disinfection experiments.
UV light successfully inactivated C. parvum oocysts in CC-Taqman PCR assays. Table 2 shows the results for CC infectivity assays of C. parvum to determine inactivation by a range of LP-UV doses. Complete inactivation was achieved at a UV dose of 1,000 mJ/cm; this dose was selected to give an absolute end point for the assay. Increased inactivation was achieved with increasing UV dose. Significant inactivation of C. parvum oocysts was observed at low doses of UV ranging from 3 to 30 mJ/cm2, and this result was reproducible, with 3.75 log10 units of inactivation being achieved with 500 mJ/cm2 (n = 5).
(ii) Ozone disinfection experiments.
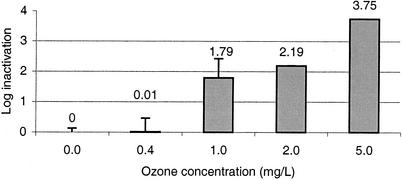
Figure 4 shows the results for CC-Taqman PCR infectivity assays of C. parvum to determine inactivation after disinfection with ozone at doses of 1.0, 2.0, and 5.0 mg/liter for 10 min. Significant inactivation (1.79 log10 units) was achieved with ozone at 1.0 mg/liter (n = 3). When ozone levels were increased, the CC-Taqman PCR assay was able to detect up to 3.75 log10 units of inactivation (the limit of the system, based on oocyst recoveries from reaction vessels) (Fig. 3). When ozone was applied at 0.2 mg/liter, no inactivation was observed; this result was due to the ozone demand of the solution.
FIG. 4.
Inactivation of C. parvum oocysts by ozone in the CC-Taqman PCR assay (n = 4). Error bars indicate standard deviations.
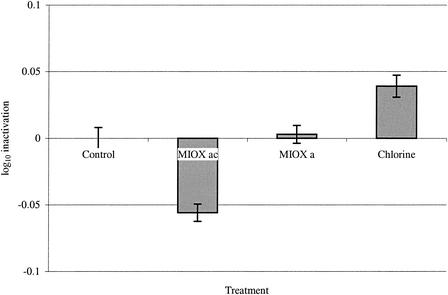
(iii) MIOX and sodium hypochlorite disinfection experiments.
MIOX and sodium hypochlorite were both shown to be ineffective in the inactivation of C. parvum oocysts (Fig. 5). The results showed no significant inactivation of oocysts compared to untreated controls in the CC-Taqman PCR assay. The log10 inactivation rates observed for the treatments were as follows: MIOXa, +0.05; MIOXac, −0.004; and sodium hypochlorite, −0.04. The results for the CC-Taqman PCR assay determined that MIOX or chlorine used at a dose of 10 mg of total chlorine/liter for 1 h resulted in less than 0.1 log10 unit of inactivation. Tests were performed with various concentrations of total chlorine of up to 5 mg/liter and incubation times of up to 24 h, with no significant inactivation being detected by this assay (data not shown).
FIG. 5.
Inactivation of C. parvum oocysts with MIOXa or sodium hypochlorite at 10 mg/liter in 0.01 M phosphate buffer (pH 7.0) (n = 5). Error bars indicate standard deviations.
DISCUSSION
The CC-Taqman PCR assay was demonstrated to be successful at assessing the inactivation of C. parvum oocysts following exposure to a range of disinfectants that act on oocysts in different ways; these included UV light, ozone, chlorine, and MIOX. The results from the UV studies indicated that the CC assay is a useful tool for determining oocyst inactivation and, when combined with the Taqman PCR, allows reliable and rapid quantitation of the inactivation. Previous studies demonstrated effective inactivation of Cryptosporidium with UV light; Rochelle et al. (32) showed 1.6 ± 0.2 log10 units (mean ± standard deviation) of inactivation in HCT-8 cells at 4 mJ/cm2. These results compare favorably with the results achieved in this study. However, the results achieved in this study are lower than those reported by Craik et al. (13), who showed 2 log10 units of inactivation with 10 mJ/cm2 and 3 log10 units of inactivation with 25 mJ/cm2.
When inactivation of fresh oocysts was tested with the CC-Taqman PCR assay, variabilities between experiments and for replicates within experiments were very small (Ct value [mean and standard deviation], 21.09 ± 0.35). This finding was also demonstrated when either MIOX or chlorine disinfection was used (Ct values, 21.64 ± 0.299 for MIOXac, 21.91 ± 0.308 for MIOXa, and 22.07 ± 0.364 for chlorine). Following UV treatment, the Ct value increased to 26.7 ± 1.022. The UV cabinet used in these experiments was not a quasi-collimated beam apparatus, and the dose may not have been delivered with the same accuracy as with a quasi-collimated beam. In addition, the time required to deliver the lower doses was very short (2 to 5 s for 3 mJ/cm2), much shorter than that used with a quasi-collimated beam (approximately 30 s for 3 mJ/cm2), and may not have allowed the lamp to sufficiently warm up due to the manipulative constraints of the UV cabinet. All other disinfection treatments resulted in small standard deviations and high levels of reproducibility. Reduced effects may also have been due to shading of oocysts, as the assays were performed in a static system. By incorporation of continuous slow mixing, the shading effects would be limited.
Ozone inactivation of C. parvum (2.19 log10 units) was achieved in this study with ozone at 2.0 mg/liter for 10 min. These results compare favorably with those of Rochelle et al. (32), who achieved 2.2 log10 units of inactivation with 16 mg/liter/min in the CC assay. Renneker et al. (30) achieved greater inactivation (3 log10 units with 7.73 mg/liter/min) with an ozone-demand-free diluent. The fact that our system did not incorporate demand-free buffer may account for the discrepancy.
It is well known that sodium hypochlorite is not an effective disinfectant for Cryptosporidium (oocysts were still infective in an animal model after exposure to 5.25% chlorine [16]); therefore, the lack of inactivation was anticipated. The failure of MIOXa to inactivate Cryptosporidium was unexpected, as it was previously demonstrated to be extremely effective at oocyst inactivation, generating 3.0 log10 units of inactivation at a dose of 5 mg/liter for 4 h and 4 log10 units of inactivation at a dose of 5 mg/liter for 8 to 12 h in a mouse infectivity assay (40). Further reports have indicated that MIOX are effective in wastewater, achieving 3 log10 units of inactivation with a 90-min contact time and 10 to 13 mg/liter, although the oxidant demand was increased (6), and 3 log10 units of inactivation with 4 mg/liter for 4 h in oxidant-demand-free water. In this study, a direct comparison between MIOX and sodium hypochlorite showed minimal inactivation at 10 mg/liter after 1 h. Further experiments were performed with a contact time of up to 24 h and 10 mg of total chlorine/liter and resulted in less than 2 log10 units of inactivation for both MIOX and sodium hypochlorite (data not shown). These results suggest that the solution produced by the MIOX BPS system is not capable of inactivating C. parvum, as previously reported. Replication of the previously published results has proven to be difficult for the original authors (6, 40; M. Sobsey, personal communication), suggesting that the results obtained here are valid. This point is of particular concern, as this technology is being applied at water treatment facilities for disinfection of Cryptosporidium.
The CC-Taqman PCR assay presented here was demonstrated to be quantitative, sensitive, and reproducible. It omits the need for laborious immunofluorescence microscopy or reverse transcriptase PCR and allows direct quantitation of DNA from infected monolayers. The method was demonstrated to be effective for assessing a range of disinfectants relevant to the water industry. Our results confirm the efficacy of UV and ozone as disinfectants and question the use of MIOX as disinfectants for Cryptosporidium.
Acknowledgments
We acknowledge the financial support received from the Co-operative Research Centre for Water Quality and Treatment, Australian Water Quality Centre, and South Australian Water Corporation.
Thanks are due to Sam Brooke for the production of ozonated water at the Australian Water Quality Centre.
REFERENCES
- 1.American Public Health Association. 1995. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, D.C.
- 2.Amman, R. R., B. J. Binder, R. J. Olsen, S. W. Chisholm, R. Devereaux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barer, M. R., and A. E. Wright. 1990. A review: Cryptosporidium in water. Lett. Appl. Microbiol. 11:271-277. [Google Scholar]
- 4.Belosevic, M., S. A. Craik, J. L. Stafford, N. F. Neumann, J. Kruithof, and D. W. Smith. 2001. Studies on the resistance/reactivation of Giardia muris cysts and Cryptosporidium parvum oocysts exposed to medium-pressure ultraviolet radiation. FEMS Microbiol. Lett. 204:197-203. [DOI] [PubMed] [Google Scholar]
- 5.Bukhari, Z., M. M. Marshall, D. G. Korich, C. R. Fricker, H. V. Smith, J. Rosen, and J. L. Clancy. 2000. Comparison of Cryptosporidium parvum viability and infectivity assays following ozone treatment of oocysts. Appl. Environ. Microbiol. 66:2972-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casteel, M. J., M. D. Sobsey, and M. J. Arrowood. 2000. Inactivation of Cryptosporidium parvum oocysts and other microbes in water and wastewater by electrochemically generated mixed oxidants. Water Sci. Technol. 41:127-134. [Google Scholar]
- 7.Chauret, C. P., K. Nolan, P. Chen, S. Springthorpe, and S. Sattar. 1998. Ageing of Cryptosporidium parvum oocysts in river water and their susceptibility to disinfection by chlorine and monochloramine. Can. J. Microbiol. 44:1154-1160. [DOI] [PubMed] [Google Scholar]
- 8.Chauret, C. P., C. Z. Radziminski, M. Lepuil, R. Creason, and R. C. Andrews. 2001. Chlorine dioxide inactivation of Cryptosporidium parvum oocysts and bacterial spore indicators. Appl. Environ. Microbiol. 67:2993-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou, C. F., B. J. Marinas, and J. Q. Adams. 1995. Modified indigo method for gaseous and aqueous ozone analyses. Ozone Sci. Eng. 17:329-344. [Google Scholar]
- 10.Clancy, J. L., T. M. Hargy, M. M. Marshall, and J. E. Dyksen. 1998. UV light inactivation of Cryptosporidium oocysts. J. Am. Water Works Assoc. 90:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy, J. L., Z. Bukhari, T. M. Hargy, J. Bolton, B. Dussert, and M. M. Marshall. 2000. Using UV to inactivate Cryptosporidium. J. Am. Water Works Assoc. 92:97-104. [Google Scholar]
- 12.Corona Vasquez, B., J. L. Rennecker, A. M. Driedger, and Marinas, B. J. 2002. Sequential inactivation of Cryptosporidium parvum oocysts with chlorine dioxide followed by free chlorine or monochloramine. Water Res. 36:178-188. [DOI] [PubMed] [Google Scholar]
- 13.Craik, S. A., D. Weldon, G. R. Finch, J. R. Bolton, and M. Belosevic. 2001. Inactivation of Cryptosporidium parvum oocysts using medium- and low-pressure ultraviolet radiation. Water Res. 35:1387-1398. [DOI] [PubMed] [Google Scholar]
- 14.Current, W. L., and T. B. Haynes. 1984. Complete development of Cryptosporidium in cell culture. Science 224:603-605. [DOI] [PubMed] [Google Scholar]
- 15.Di Giovanni, G. D., H. Hashemi, N. J. Shaw, F. A. Abrams, M. W. LeChevallier, and M. Abbaszadegan. 1999. Detection of infectious Cryptosporidium parvum oocysts in surface and filter backwash water samples by immunomagnetic separation and integrated cell culture-PCR. Appl. Environ. Microbiol. 65:3427-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fayer, R. 1995. Effect of sodium hypochlorite exposure on infectivity of Cryptosporidium parvum oocysts for neonatal BALB/c mice. Appl. Environ. Microbiol. 61:844-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1315-1322. [DOI] [PubMed] [Google Scholar]
- 18.Finch, G. R., E. K. Black, L. Gyurek, and M. Belosevic. 1993. Ozone inactivation of Cryptosporidium parvum in demand-free phosphate buffer determined by in vitro excystation and animal infectivity. Appl. Environ. Microbiol. 59:4203-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, J. A., R. Fayer, J. M. Trout, L. Xiao, A. A. Lal, S. Kerby, and M. C. Jenkins. 2001. Real-time PCR for the detection of Cryptosporidium parvum. J. Microbiol. Methods 47:323-337. [DOI] [PubMed] [Google Scholar]
- 20.Hijjawi, N. S., B. P. Meloni, U. M. Morgan, and R. C. A. Thompson. 2001. Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. Int. J. Parasitol. 31:1048-1055. [DOI] [PubMed] [Google Scholar]
- 21.Hunter, P. R., and Q. Syed. 2001. Community surveys of self reported diarrhoea can dramatically overestimate the size of outbreaks of waterborne cryptosporidiosis. Water Sci. Technol. 43:27-30. [PubMed] [Google Scholar]
- 22.Korich, D. G., J. R. Mead, M. S. Madore, N. A. Sinclair, and C. R. Sterling. 1990. Effects of ozone, chlorine dioxide, chlorine, and chloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 56:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden, K. G., G. Shim, and M. D. Sobsey. 2001. Comparative effectiveness of UV wavelengths for the inactivation of Cryptosporidium parvum oocysts in water. Water Sci. Technol. 43:171-174. [PubMed] [Google Scholar]
- 24.MacDonald, L. M., K. Sargent, A. Armson, R. C. A. Thompson, and J. A. Reynolds. 2002. The development of a real-time quantitative PCR method for characterisation of a Cryptosporidium parvum in vitro culturing system and assessment of drug efficacy. Mol. Biochem. Parasitol. 121:279-282. [DOI] [PubMed] [Google Scholar]
- 25.Mackenzie, W., N. M. Hoxie, M. E. Proctor, M. S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. G. Addiss, K. R. Fox, J. R. Rose, and J. P. Davis. 1995. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331:161-167. [DOI] [PubMed] [Google Scholar]
- 26.Meloni, B. P., and R. C. A. Thompson. 1996. Simplified method for obtaining purified oocysts from mice and growing Cryptosporidium parvum in vitro. J. Parasitol. 82:757-762. [PubMed] [Google Scholar]
- 27.Morgan, U. M., C. C. Constantine, D. A. Forbes, and R. C. A. Thompson. 1997. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J. Parasitol. 83:825-830. [PubMed] [Google Scholar]
- 28.Ong, C. S. L., D. L. Eisler, A. Alikhani, V. W. K. Wong, J. Tomblin, W. R. Bowie, and J. L. Isaac-Renton. 2002. Novel Cryptosporidium genotypes in sporadic cryptosporidiosis cases. First report of human infections with cervine genotype. Emerg. Infect. Dis. 8:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeters, J. E., E. A. Mazas, W. J. Masschelein, I. Villacorta Martinez di Maturana, and E. Debacker. 1989. Effect of disinfection of drinking water with ozone or chlorine dioxide on survival of Cryptosporidium parvum. Appl. Environ. Microbiol. 55:1519-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennecker, J. L., B. Corona Vasquez, A. M. Diuedger, S. A. Rubin, and B. J. Marinas. 2001. Inactivation of Cryptosporidium parvum oocysts with sequential application of ozone and combined chlorine. Water Sci. Technol. 43:167-170. [PubMed] [Google Scholar]
- 31.Rochelle, P. A., D. M. Ferguson, T. J. Handojo, R. De Leon, M. H. Stewart, and R. L. Wolfe. 1996. Development of a rapid detection procedure for Cryptosporidium, using in vitro cell culture combined with PCR. J. Eukaryot. Microbiol. 43:72S. [DOI] [PubMed]
- 32.Rochelle, P. A., M. M. Marshall, J. R. Mead, A. M. Johnson, D. G. Korich, J. S. Rosen, and R. deLeon. 2002. Comparison of in vitro cell culture and a mouse assay for measuring infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 68:3809-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochelle, P. A., D. M. Ferguson, T. J. Handojo, R. de Leon, M. H. Stewart, and R. L. Wolfe. 1997. An assay combining cell culture with reverse transcriptase PCR to detect and determine the infectivity of waterborne Cryptosporidium parvum. Appl. Environ. Microbiol. 63:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruffnel, K. M., J. L. Rennecker, and B. J. Marinas. 2000. Inactivation of Cryptosporidium parvum oocysts with chlorine dioxide. Water Res. 34:868-876. [DOI] [PubMed] [Google Scholar]
- 35.Shim, G.-A., K. G. Linden, M. J. Arrowood, and M. D. Sobsey. 2001. Low-pressure UV inactivation and DNA repair potential of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 67:3029-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slifko, T. R., D. Friedman, J. B. Rose, and W. Jakubowski. 1997. An in vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl. Environ. Microbiol. 63:3669-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theodos, C. M., J. K. Griffiths, J. D'Onfro, A. Fairfield, and S. Tzipori. 1998. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 42:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tillet, H. E., J. deLouvois, and P. G. Wall. 1998. Surveillance of outbreaks of waterborne infectious disease: categorizing levels of evidence. Epidemiol. Infect. 120:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Upton, S. J., M. Tilley, and D. B. Brillhart. 1994. Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J. Clin. Microbiol. 33:371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venczel, L. V., M. Arrowood, M. Hurd, and M. D. Sobsey. 1997. Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Appl. Environ. Microbiol. 63:1598-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widmer, G., D. Akiyoshi, M. A. Buckholt, X. Feng, S. M. Rich, K. M. Deary, C. A. Bowman, P. Xu, Y. Wang, X. Wang, G. A. Buck, and S. Tzipori. 2000. Animal propagation and genomic survey of a genotype 1 isolate of Cryptosporidium parvum. Mol. Biochem. Parasitol. 108:187-197. [DOI] [PubMed] [Google Scholar]