Abstract
The wine bacterium Oenococcus oeni has to cope with harsh environmental conditions, including an acidic pH, a high alcoholic content, nonoptimal growth temperatures, and growth-inhibitory compounds such as fatty acids, phenolic acids, and tannins. We describe the characterization and cloning of the O. oeni ftsH gene, encoding a protease belonging to the ATP binding cassette protein superfamily. The O. oeni FtsH protein is closest in sequence similarity to the FtsH homologue of Lactococcus lactis. The O. oeni ftsH gene proved to be stress-responsive, since its expression increased at high temperatures or under osmotic shock. O. oeni FtsH protein function was tested in an Escherichia coli ftsH mutant strain, and consistent with the O. oeni ftsH gene expression pattern, the O. oeni FtsH protein provided protection for the E. coli ftsH mutant against heat shock. O. oeni and Bradyrhizobium japonicum FtsH proteins also triggered E. coli resistance to wine toxicity. Genes homologous to O. oeni ftsH were detected in many other lactic acid bacteria found in wine, suggesting that this type of gene constitutes a well-conserved stress-protective molecular device.
The wine used was red wine from the Bordeaux region, consisting of a combination of Cabernet Franc, Cabernet Sauvignon, and Merlot musts. The pH was 3.4, the ethanol content was 12%, and the sulfite content was 20 mg/liter.
Strains and growth conditions.
The O. oeni strain used was O. oeni IOEB 8406. This strain was grown at 25°C in medium adjusted to pH 5 and containing the following (per liter): yeast extract, 4 g; beef extract, 8 g; Bacto Peptone, 10 g; glucose, 10 g; fructose, 10 g; malic acid, 10 g; KH2PO4, 2 g; MgSO4 · 7H2O, 0.2 g; MnSO4 · H2O, 0.1 g; and Tween 80, 1 ml. E. coli strain XL1Blue (Stratagene) was used for cloning procedures. The ΔftsH E. coli strain AR3291 (W3110 zad220::Tn10 sfhC21 ΔftsH3::kan) (20) was used to study O. oeni FtsH protein function. The E. coli strains used were grown in Luria-Bertani (LB) medium supplemented with appropriate antibiotics at the following concentrations when necessary: ampicillin, 50 μg/ml; chloramphenicol, 10 μg/ml; and kanamycin, 50 μg/ml.
Plasmids used.
Plasmids pBluescript II KS(+) (Stratagene) and pGEM-T (Promega) were used for cloning and sequencing. Both harbor the ampicillin resistance marker. Plasmid pCR-XL-TOPO (Invitrogen) was used as an expression vector in E. coli. This plasmid harbors the kanamycin resistance marker. Plasmids pBAD18-Cm (6) and pRJ5188 (harboring the B. japonicum ftsH gene) (16) were used to test the ability of the B. japonicum ftsH gene to enhance bacterial resistance to a wine shock. Both of these plasmids harbor the chloramphenicol resistance marker.
Construction of a probe specific to the ftsH gene of O. oeni.
To obtain a probe for an O. oeni gene homologous to previously characterized bacterial ftsH genes, redundant primers were deduced from an alignment of the FtsH protein sequences from H. pylori, L. lactis, E. coli, and B. subtilis. The chosen primers were specific to the Walker A motif and the zinc binding motif, respectively. PCR was performed on O. oeni IOEB 8406 genomic DNA (100 ng) in a final volume of 100 μl with a 1 μM concentration of each primer, a 0.2 mM concentration of each of the four deoxynucleoside triphosphates (dNTPs), 8 mM MgCl2, and 5 U of Taq polymerase in the appropriate commercial buffer. The PCR program consisted of 40 cycles of 1 min at 94°C, 1 min at 45°C, and 1.5 min at 72°C, followed by 10 min at 72°C. The primers F1 (5′-CGGAATTCCGGICCICCIGGIACIGGIAA[A,G]AC-3′) and F2 (5′-CGAAGCTTGC[A,G,T]ATIGC[A,G]TGICCIGC[C,T]TC[A,G]TG [A,G]TAIGC-3′) produced an amplicon of 710 bp. This amplified product was digested with BamHI and EcoRI, since these sites were included in the design of primers F1 and F2, respectively. The digested fragment was cloned in pBluescript II KS(+) and sequenced. Similarities between this fragment and various bacterial genes homologous to ftsH confirmed that we had amplified the internal portion of a homologous gene from O. oeni. This probe was called ftsH.
Southern blot analysis.
Chromosomal DNA was isolated from the O. oeni IOEB 8406 strain as follows. A 1-g cell pellet from an exponential-phase culture was washed with 10 mM EDTA-25 mM Tris-HCl (pH 8) and resuspended in 10 ml of this buffer containing 10% glycerol. Solid lysozyme (10 mg) was added, and the cells were allowed to digest overnight at 37°C. Proteinase K was added to a final concentration of 0.2 mg/ml, and incubation was continued for 12 h at 50°C. One milliliter of 10% N-laurylsarcosinate was added, and incubation was continued for an additional 12 h at 50°C. Twenty milliliters of 95% ethanol was added, and the high-molecular-weight DNA was spooled out on a glass rod. This was rinsed twice in 70% ethanol, air dried briefly, and then redissolved in 4 ml of 1 mM EDTA-10 mM Tris-HCl (pH 8). O. oeni IOEB 8406 genomic DNA was digested with appropriate restriction enzymes and transferred onto a Hybond-N+ membrane (Amersham) under vacuum. Hybridizations (overnight at 65°C) were performed with probes labeled with digoxigenin-labeled 11-dUTP by using the DIG-DNA labeling and detection kit (Roche). Detection was carried out by chemiluminescence, using an antidigoxigenin antibody and CDP-star (Roche), as recommended by the manufacturer.
Cloning of the O. oeni ftsH gene by inverse PCR.
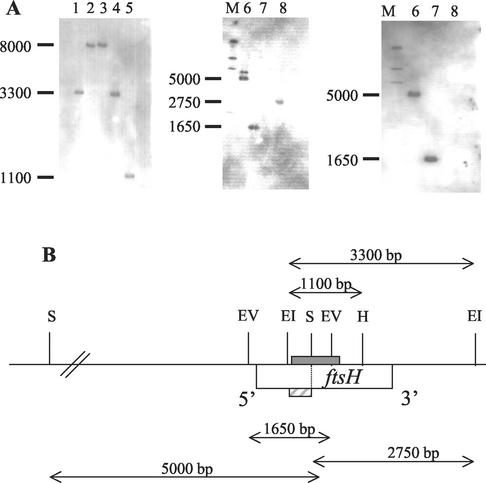
A precise restriction map was first established by hybridization of digested O. oeni DNA with the ftsH probe and a probe generated by restriction of the ftsH probe with the SalI enzyme (probe 2). Probe 2 allowed the orientation of the EcoRI and EcoRV restriction sites with respect to the SalI site, internal to the region hybridizing with the ftsH probe (Fig. 1).
FIG. 1.
Restriction mapping of the O. oeni ftsH gene. (A) Southern blots were hybridized with either the ftsH probe (left and middle panels) or probe 2 (right panel). The O. oeni genomic DNA was digested with the following enzymes: lane 1, EcoRI; lane 2, BamHI; lane 3, HindIII; lane 4, EcoRI and BamHI; lane 5, EcoRI and HindIII; lanes 6, SalI; lanes 7, EcoRV; lanes 8, EcoRI and SalI. Lanes M, molecular size markers (in base pairs). (B) Physical map of the O. oeni ftsH gene. Restriction enzyme sites: EI, EcoRI; EV, EcoRV; H, HindIII; S, SalI. The location of the ftsH probe is indicated by a grey box above the ftsH gene; the location of probe 2 is indicated by a hatched box below the ftsH gene. Distances between restriction sites are indicated.
The 5′ side of the ftsH gene was then cloned by inverse PCR (19) from genomic O. oeni DNA made of closed circular fragments generated by EcoRV enzyme restriction and ligation with T4 DNA ligase. Primers FTSH1 (5′-CGACGGCTTTAGCGAGCAAA-3′) and FTSH2 (5′-AAAAAGCTGTTACAGCAGCT-3′) were selected from the ftsH probe sequence, and the amplification of a 1,200-bp fragment was then able to take place. The polymerase used was the Expand enzyme (a blend of Taq and Pwo polymerases) (Boehringer). PCR was performed with 200 ng of DNA, prepared as described above, in a final volume of 100 μl with a 2 μM concentration of each primer, a 0.2 mM concentration of each of the four dNTPs, and 1.5 μl of Expand enzyme in the appropriate commercial buffer. The PCR program used consisted of 39 cycles of 1 min at 94°C, 1 min at 50°C, and 3 min at 72°C, followed by 10 min at 72°C. The fragment was purified and cloned in plasmid pGEM-T to yield pJPB60. The 3′ side of the ftsH gene was cloned by inverse PCR from genomic O. oeni DNA made of closed circular fragments generated by EcoRI enzyme restriction and ligation with T4 DNA ligase. PCR with the Expand enzyme was carried out with FTSH1 and FTSH2 primers, and a 2,750-bp fragment encompassing the 3′ side of the ftsH gene was amplified. This fragment was purified and cloned in plasmid pCR-XL-TOPO to yield pJPB62. The fragment inserted in pJPB62 was sequenced.
Next, the complete O. oeni ftsH gene was cloned in pCR-XL-TOPO to take advantage of the ability to express the FtsH protein through the control of the lac promoter. PCR was carried out with the FTSH3 (5′-GTTAACCCCGATAATTTATTCATAATG-3′) and FTSH4 (5′-GCGACCAGCTAAAGTCGCGTTTTG-3′) primers. PCR was performed with 200 ng of genomic O. oeni DNA in a final volume of 100 μl with a 1 μM concentration of each primer, a 0.2 mM concentration of each of the four dNTPs, and 1.5 μl of Expand enzyme in the appropriate commercial buffer. The PCR program used consisted of 39 cycles of 1 min at 94°C, 1 min at 50°C, and 1.5 min at 72°C, followed by 10 min at 72°C. The FTSH3 primer encompasses the region from position −61 to −35 of the ftsH promoter sequence. This oligonucleotide therefore allowed cloning of the O. oeni ftsH gene with its own promoter and ribosome binding site. The FTSH4 primer represents the region from position +2229 to +2206 of the ftsH gene. Thus, its 5′ extremity ends 81 nucleotides after the ftsH stop codon. A 2,290-bp fragment was amplified, purified, and cloned in pCR-XL-TOPO to yield pJPB65. We expected to find a 50:50 distribution in the orientation of inserted fragments. However, none of the 24 positive recombinant clones that were tested contained the cloned ftsH gene in the correct orientation (under the control of the lac promoter), and all contained the ftsH gene in the incorrect orientation. This is compatible with a lac promoter-monitored toxic level of ftsH gene expression (even under noninduced conditions) and is in keeping with the observation that overproduction of the H. pylori ftsH gene considerably reduced the growth rate of E. coli host cells (5). Nevertheless, the plasmid pJPB65, containing the ftsH gene in the opposite orientation with respect to the lac promoter, allowed the study of the FtsH protein function. In this construct the ftsH gene is not expressed under its own promoter and benefits only from its own ribosome binding site. Despite this, a low level of ftsH expression was possible, as observed by reverse transcription-PCR (RT-PCR) (data not shown).
RNA isolation and RT-PCR analysis.
The mRNAs from the O. oeni IOEB 8406 strain were extracted after 3 h of incubation under various conditions. We tested the following environmental factors: heat shock (37°C), osmotic shock (0.5 M NaCl), alcohol addition (20%), sulfite addition (100 mg/liter), and ethidium bromide addition (20 μg/ml). The control culture was grown at 25°C at pH 5.0 in modified MRS medium. RNA preparation was carried out with the StrataPrep Total RNA Miniprep kit (Stratagene), which includes a DNase (RNase-free) treatment. Bacterial cells were disrupted with glass beads (0.1-mm diameter) by shaking on a vortex mixer. The quality of the RNA samples was checked on a 1% (wt/vol) agarose gel, and the concentration was determined by measuring absorbance at 260 nm. The corresponding cDNAs were synthesized by reverse transcription with murine leukemia virus reverse transcriptase and the ProSTAR first-strand RT-PCR kit (Stratagene). The reverse transcriptase reaction used random primers from the kit with 10 to 15 μg of total RNA, in a final volume of 50 μl. Three microliters of the first-strand cDNA synthesis reaction product was used as a template for PCR amplifications with specific primers. Thirty-five cycles were carried out, each consisting of 30 s of denaturation at 95°C, 30 s of annealing at 48°C, and 30 s of enzymatic primer extension at 72°C. We verified that these amplification conditions resulted in signals belonging to the exponential phase of the PCR and not to the saturation portion of the experiment. PCR fragments were visualized on a 1% (wt/vol) agarose gel. Negative controls included PCR with either water instead of reverse transcriptase mix or 3 μl of crude RNA. None of the negative controls resulted in DNA amplification. Genomic DNA was used as a positive control. Primers 5′-GGTCCTCCGGGAACCGGTAAAAC-3′ and 5′-AATTGCATGGCCGGCTTCGTGATATGC-3′ were used to amplify a 687-bp fragment specific to the O. oeni ftsH gene. They hybridize to the same regions as do the F1 and F2 primers, respectively. The O. oeni 23S rRNA gene (14) was used as a constitutive expression control, since it is a housekeeping gene which is not induced by environmental stress.
Resistance assays.
Overnight cultures at 25°C of the ΔftsH E. coli strain AR3291 transformed with either pBAD18-Cm (control plasmid) or pRJ5188 (harboring the B. japonicum ftsH gene) were grown in LB medium supplemented with chloramphenicol. They were diluted to an optical density at 600 nm of 0.1 into fresh LB medium, supplemented with antibiotic and containing 0.1% arabinose instead of glucose to induce FtsH biosynthesis through the arabinose PBAD promoter (6), and grown for 2 h at 37°C. Next, 20 μl of cell cultures was added to 180 μl of LB medium devoid of antibiotic but containing 0.1% arabinose and supplemented with various concentrations of wine (0, 20, and 25%, making 0, 2.4, and 3% ethanol, respectively). Cultures were incubated for 3 h at 25°C. Serial 10-fold dilutions were then plated on solid LB medium, and colonies were counted on the following day. Survival ratios of CFU observed in the presence of wine to those observed without wine were calculated.
Overnight cultures at 25°C of the ΔftsH E. coli strain AR3291 transformed with either pCR-XL-TOPO (control plasmid) or pJPB65 (harboring the O. oeni ftsH gene) were grown in LB medium supplemented with kanamycin. They were diluted to an optical density at 600 nm of 0.1 into fresh LB medium, supplemented with antibiotic and containing glucose, and grown for 2 h at 37°C. Resistance assays were then carried out as described above, except that the LB medium contained glucose instead of arabinose during the wine shock.
Nucleotide sequence accession number.
The GenBank accession number for the O. oeni ftsH gene is AY196466.
RESULTS
Cloning of the O. oeni ftsH gene.
PCR with redundant primers designed from the conserved ATP binding motifs and ATPase module of several ftsH-homologous genes resulted in the amplification of an expected 710-bp DNA fragment from O. oeni IOEB 8406. Cloning and DNA sequence analysis revealed that the amplified fragment encoded a highly conserved amino acid sequence characteristic of proteins containing an ATP binding cassette (25). This fragment was used as a probe (the ftsH probe) during Southern analysis of genomic DNA of O. oeni IOEB 8406 digested with different restriction enzymes. This analysis demonstrated that the ftsH probe was represented by a single-copy gene (Fig. 1A) and that this probe was specific to a single putative ftsH gene, since no additional fragment containing other genes belonging to the AAA family could be hybridized. Using the Southern analysis, we were able to construct a restriction map (Fig. 1B) and then select appropriate restriction enzymes in order to clone the putative O. oeni ftsH gene by an inverse PCR-based approach. The cloned gene was then sequenced. Since the amino acid sequence deduced from the predicted open reading frame was highly homologous to various bacterial FtsH proteins, as described below, this open reading frame was designated the O. oeni ftsH gene.
The O. oeni ftsH gene is made of an open reading frame of 2,145 bp and encodes a polypeptide of 715 amino acids with a calculated molecular mass of 75 kDa. Comparison of O. oeni FtsH protein with members of the AAA protein family revealed a high sequence identity within an ATPase module of around 200 amino acids (2). Among these AAA proteins, the O. oeni FtsH protein displayed the highest overall sequence similarity to the bacterial FtsH proteins, most notably the L. lactis homologue (Fig. 2). The O. oeni and L. lactis FtsH proteins share 59% identical residues (as given by the Clustal program), and O. oeni FtsH exhibits 52% identity to E. coli FtsH and 51% identity to B. japonicum FtsH (Fig. 2).
FIG. 2.
Comparison of the amino acid sequences of FtsH proteins from O. oeni, L. lactis, B. japonicum, and E. coli. The first residue in each row is numbered. A asterisk above the sequences indicates a perfect consensus between the four protein sequences. A cross indicates three matching residues among the four sequences. The predicted transmembrane α-helices of FtsH proteins are underlined. The Walker A and B motifs are indicated. The SRH motif is indicated and appears in boldface. The boxed HEAGH domain is the zinc binding motif. Dashes indicate gaps introduced to optimize the alignment.
A model of the membrane topologies of the E. coli and L. lactis FtsH proteins was generated (16, 21), and according to the model, a hydropathy analysis of the O. oeni FtsH protein revealed an N-terminal domain with two putative α-helical transmembrane segments (residues 12 to 31 and 134 to 157). The cytoplasmic domain carried the ATP binding motifs (Walker A and B motifs), the conserved region of the AAA protein family, and the putative zinc binding site (Fig. 2).
The O. oeni ftsH gene is a stress-responsive gene.
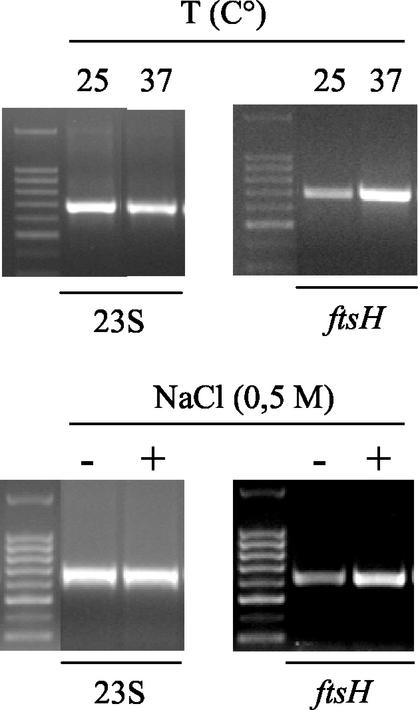
The influence of typical wine stresses upon O. oeni ftsH gene expression was tested. We did not observe any significant changes in ftsH expression when O. oeni was subjected to alcohol, sulfite, or ethidium bromide addition. In contrast, increased ftsH gene expression was observed at high temperatures and under osmotic shock (Fig. 3).
FIG. 3.
The O. oeni ftsH gene is a stress-responsive gene. The induction of O. oeni ftsH gene expression after heat or osmotic shocks was analyzed by RT-PCR. The RT-PCR patterns were obtained with cDNA from the RNA of O. oeni IOEB 8406 extracted after 3 h of application of the indicated thermal or osmotic stress. The gene-specific primers that were used are indicated on the bottoms of the panels.
The O. oeni and B. japonicum FtsH proteins protect bacteria against wine stress.
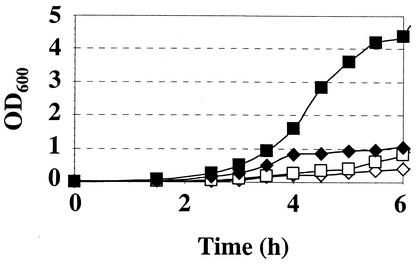
Since there is no known transformation method for O. oeni, knockout constructions and complementation tests are impossible. Therefore, the function of O. oeni FtsH was explored in the ΔftsH E. coli strain AR3291. This strain is a viable ΔftsH mutant, due to a suppressor mutation in sfhC (fabZ) that allows cells to survive although showing slowed growth at 37°C (20). The same strain had been used to study the function of B. japonicum FtsH (15). When E. coli AR3291/pCR-XL-TOPO was grown at 37°C, it showed a clear growth defect, whereas the expression of O. oeni FtsH significantly improved the growth of E. coli AR3291/pJPB65 at 37°C (Fig. 4). Thus, O. oeni FtsH is able to confer a growth advantage upon the E. coli ftsH mutant, indicating that the heterologous protein can compensate for the loss of at least some of the important FtsH functions in E. coli AR3291.
FIG. 4.
Growth complementation of an E. coli ΔftsH mutant by the O. oeni ftsH gene. The E. coli ΔftsH strain AR3291 transformed with pCR-XL-TOPO (diamonds) or with pJPB65 (squares) was grown in LB medium at either 25°C (open symbols) or 37°C (closed symbols). OD600, optical density at 600 nm.
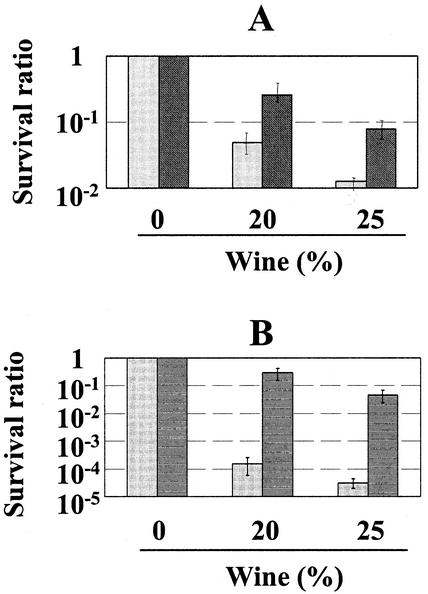
We then analyzed whether the O. oeni and B. japonicum ftsH genes could confer protection on bacteria when grown in a wine-containing medium. When E. coli ΔftsH cells harboring pJPB65 (O. oeni ftsH) or vector without ftsH (pCR-XL-TOPO) were exposed to wine in liquid medium, it was readily apparent that O. oeni FtsH confers resistance to this toxic medium (Fig. 5). The survival ratios were significantly higher when cells cultured with wine harbored the O. oeni and B. japonicum ftsH expression plasmids. The expression of O. oeni ftsH resulted in a 10- to 100-fold relative resistance, and that of B. japonicum ftsH resulted in a 100- to 10,000-fold relative resistance. We define relative resistance as the ratio of the survival rate of the bacteria transformed with O. oeni or B. japonicum ftsH to that of the bacteria transformed with the corresponding vectors in a given environmental condition.
FIG. 5.
The O. oeni and B. japonicum ftsH genes protect E. coli from wine toxicity. The E. coli ΔftsH strain AR3291 was transformed with control plasmids (pCR-XL-TOPO [A] or pBAD18-Cm [B]) (light-gray bars) and O. oeni or B. japonicum ftsH gene-containing plasmids (pJPB65 [A] and pRJ5188 [B], respectively) (dark-gray bars). After 3 h of incubation at 25°C with the indicated concentrations of wine, the bacterial cultures were serially diluted and plated. Colonies were counted, and the survival ratios (ratios of CFU observed at a given concentration of wine to those observed without added toxic compounds) were calculated.
Homologous genes in other lactic acid bacteria.
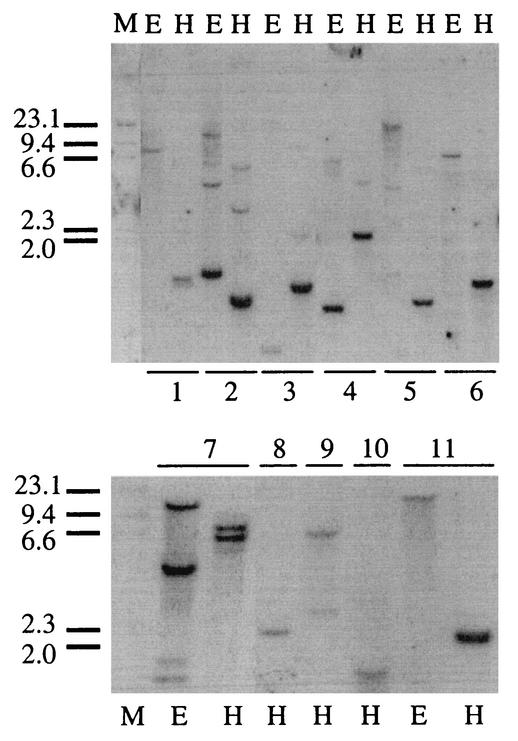
The ftsH probe (Fig. 1) was used in Southern hybridization analysis with either EcoRI- or HindIII-digested chromosomal DNAs from various wine lactic acid bacteria (Fig. 6). Single chromosomal bands could be detected from the bacteria Lactobacillus fructivorans, Pediococcus pentosaceus, Pediococcus dextrinicus, Lactobacillus plantarum, Pediococcus parvulus, Lactobacillus buchneri, Pediococcus acidilactici, Pediococcus damnosus, Lactobacillus hilgardii, Lactobacillus brevis, Lactobacillus delbrueckii, and Leuconostoc mesenteroides.
FIG. 6.
ftsH homologues in other wine lactic acid bacteria. Southern hybridization of the ftsH probe to chromosomal DNAs from various bacteria is shown. The genomic DNAs were digested before transfer with either the EcoRI (lanes E) or HindIII (lanes H) enzymes. Lanes M, molecular size markers (in kilobase pairs). Lanes: 1, Lactobacillus fructivorans ATCC 8288; 2, P. pentosaceus ATCC 33326; 3, P. dextrinicus ATCC 33087; 4, Lactobacillus plantarum IOEB 9106; 5, P. parvulus ATCC 19371; 6, Lactobacillus buchneri ATCC 11305; 7, P. damnosus ATCC 25248; 8, Lactobacillus hilgardii IOEB 9101; 9, Lactobacillus brevis ATCC 14869; 10, Lactobacillus delbrueckii ATCC 9649; 11, Leuconostoc mesenteroides IOEB 8293.
The FtsH1 and FtsH2 primers, which allow amplification of the O. oeni ftsH probe, were used in an attempt to amplify sequences that would be homologous to this probe. All of the genomic DNAs tested from the bacteria mentioned above generated a 710-bp PCR fragment that proved to be homologous to the ABC and SRH (second region of homology) domains of the previously known FtsH proteins (Fig. 7).
FIG.7.
PCR amplification of sequences from various lactic acid bacteria, homologous to the ABC domain of ftsH. The amplified sequences were aligned and compared. oeni, O. oeni; mesen, Leuconostoc mesenteroides; delbru, Lactobacillus delbrueckii; buch, Lactobacillus buchneri; hilg, Lactobacillus hilgardii; acidi, P. acidilactici ATCC 8042; pento, P. pentosaceus; damno, P. damnosus; fruct, Lactobacillus fructivorans; plant, Lactobacillus plantarum; saliv, Lactobacillus salivarius ATCC 11740; dextri, P. dextrinicus; lactis, L. lactis. An asterisk below the sequences indicates a perfect consensus between the protein sequences. Dots indicate conservative substitutions. The Walker B motif and the SRH domain are indicated. The consensus SRH sequence is outlined above the black bar. Dashes indicate gaps introduced to optimize the alignment.
DISCUSSION
The ftsH gene of O. oeni was cloned, sequenced, and shown to functionally replace the ftsH requirement in an E. coli ΔftsH mutant when grown at 37°C. The salt- and temperature-induced ftsH gene expression in O. oeni is in keeping with the reported observation that in L. lactis most of the salt-induced proteins were also induced by heat shock (10). We have shown here that the ftsH gene is one of the molecular devices in O. oeni that might enable this species to cope better with high fermentation temperatures. In addition, the O. oeni FtsH protein conferred protection against wine toxicity to the E. coli ΔftsH mutant. This ability is shared not only by the O. oeni homologue but also by the B. japonicum counterpart. Since this ability was tested in a foreign host, E. coli, one can speculate that it is likely to be shared by the FtsH proteins of many other species.
FtsH proteins belong to the AAA protein family (11), which constitutes a distinct subfamily of the Walker-type ATPases. In addition to the two consensus motifs, Walker A and B, in the Walker-type ATPases, AAA proteins have another highly conserved amino acid sequence within their ATPase domain, the SRH. The SRH plays an important role in ATP hydrolysis, and some highly conserved amino acid residues within the SRH were found to be essential for the in vivo protease activity of FtsH (9).
Alignment of the 13 sequences of the FtsH ATPase domains from various lactic acid bacteria defined an SRH consensus (Fig. 7). This consensus differs in only two positions from the SRH consensus obtained by comparison of 54 representative AAA proteins (9). However, these two differences, I297 M and P303S (the numbering is that of the E. coli FtsH protein), affect only poorly conserved residues of the SRH, which are not essential for FtsH activity. Indeed, the activity of a P303A mutant of the E. coli FtsH protein is almost the same as that of a wild-type FtsH protein (9). The highly conserved residues N301, D307, L310, R312, and R315, on the other hand, which are critical for E. coli FtsH activity, belong to the lactic acid bacterial consensus sequence. In conclusion, the lactic acid bacterial sequences collected are likely to be part of authentic and active FtsH proteins. Thus, FtsH is probably a universal molecular device, at least throughout the prokaryotic kingdom.
Acknowledgments
We thank Franz Narberhaus for the gifts of plasmid pRJ5188 and the E. coli ΔftsH strain AR3291. We also thank Amélie Vallet for technical assistance.
REFERENCES
- 1.Akiyama, Y., T. Ogura, and K. Ito. 1994. Involvement of FtsH in protein assembly into and through the membrane. I. Mutations that reduce retention efficiency of a cytoplasmic reporter. J. Biol. Chem. 269:5218-5224. [PubMed] [Google Scholar]
- 2.Confalonieri, F., and M. Duguet. 1995. A 200-amino acid ATPase module in search of a basic function. Bioessays 17:639-650. [DOI] [PubMed] [Google Scholar]
- 3.Deuerling, E., B. Paeslack, and W. Schumann. 1995. The ftsH gene of Bacillus subtilis is transiently induced after osmotic and temperature upshift. J. Bacteriol. 177:4105-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards, C. G., R. B. Beelman, C. E. Bartley, and A. McConnell. 1990. Production of decanoic acid and other volatile compounds and the growth of yeast and malolactic bacteria during vinification. Am. J. Enol. Vitic. 41:48-56. [Google Scholar]
- 5.Ge, Z., and D. E. Taylor. 1996. Sequencing, expression, and genetic characterization of the Helicobacter pylori ftsH gene encoding a protein homologous to members of a novel putative ATPase family. J. Bacteriol. 178:6151-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzzo, J., M.-P. Jobin, and C. Diviès. 1998. Increase of sulfite tolerance in Oenococcus oeni by means of acidic adaptation. FEMS Microbiol. Lett. 160:43-47. [Google Scholar]
- 8.Herman, C., D. Thévenet, R. D'Ari, and P. Bouloc. 1995. Degradation of σ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. USA 92:3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karata, K., T. Inagawa, A. J. Wilkinson, T. Tatsuta, and T. Ogura. 1999. Dissecting the role of a conserved motif (the second region of homology) in the AAA family of ATPases. J. Biol. Chem. 274:26225-26232. [DOI] [PubMed] [Google Scholar]
- 10.Kilstrup, M., S. Jacobsen, K. Hammer, and F. K. Vogensen. 1997. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl. Environ. Microbiol. 63:1826-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer, T. 2000. AAA proteases: cellular machines for degrading membrane proteins. Trends Biochem. Sci. 25:247-251. [DOI] [PubMed] [Google Scholar]
- 12.Lonvaud-Funel, A., A. Joyeux, and C. Desens. 1988. Inhibition of malolactic fermentation of wines by products of yeast metabolism. J. Sci. Food Agric. 44:183-191. [Google Scholar]
- 13.Lonvaud-Funel, A. 1998. Le développement des bactéries lactiques dans le vin, p. 197-224. In P. Ribéreau-Gayon, D. Dubourdieu, B. Donèche, and A. Lonvaud-Funel (ed.), Traité d’oenologie, vol. 1. Microbiology du vin. Vinification. Dunod, Paris, France.
- 14.Lonvaud-Funel, A. 1999. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Leeuwenhoek 76:317-331. [PubMed] [Google Scholar]
- 15.Martinez-Murcia, A. J., N. M. Harland, and M. D. Collins. 1993. Phylogenetic analysis of some leuconostocs and related organisms as determined from large-subunit rRNA gene sequences: assessment of congruence of small- and large-subunit rRNA derived trees. J. Appl. Bacteriol. 74:532-541. [PubMed] [Google Scholar]
- 16.Narberhaus, F., C. Urech, and H. Hennecke. 1999. Characterization of the Bradyrhizobium japonicum ftsH gene and its product. J. Bacteriol. 181:7394-7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson, D., A. A. Lauridsen, T. Tomoyasu, and T. Ogura. 1994. A Lactococcus lactis gene encodes a membrane protein with putative ATPase activity that is homologous to the essential Escherichia coli gene product. Microbiology 140:2601-2610. [DOI] [PubMed] [Google Scholar]
- 18.Schumann, W. 1999. FtsH a single-chain charonin. FEMS Microbiol. Rev. 23:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Silver, J. 1992. Inverse polymerase chain reaction, p. 137-146. In M. J. McPherson, P. Quirke, and G. R. Taylor (ed.), PCR: a practical approach. IRL Press, Oxford, United Kingdom.
- 20.Tatsuta, T., T. Tomoyasu, B. Bukau, M. Kitagawa, H. Mori, K. Karata, and T. Ogura. 1998. Heat shock regulation in the ftsH null mutant of Escherichia coli: dissection of stability and activity control of σ32 in vivo. Mol. Microbiol. 50:583-593. [DOI] [PubMed] [Google Scholar]
- 21.Tomoyasu, T., K. Yamanaka, K. Murata, T. Suzaki, P. Bouloc, A. Kato, H. Niki, S. Hiraga, and T. Ogura. 1993. Topology and subcellular localization of FtsH protein in Escherichia coli. J. Bacteriol. 175:1352-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomoyasu, T., T. Yuki, S. Morimura, H. Mori, K. Yamanaka, H. Niki, S. Hiraga, and T. Ogura. 1993. The Escherichia coli FtsH protein is a procaryotic member of a protein family ATPases involved in membrane functions, cell cycle control, and gene expression. J. Bacteriol. 175:1344-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomoyasu, T., J. Gamer, B. Bukau, M. Kanemori, H. Mori, A. J. Rutman, A. B. Oppenheim, T. Yura, K. Yamanaka, H. Niki, S. Hiraga, and T. Ogura. 1995. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor σ32. EMBO J. 14:2551-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivas, N., A. Lonvaud-Funel, and Y. Glories. 1997. Effect of phenolic acids and anthocyanins on growth, viability and malolactic activity of a lactic acid bacterium. Food Microbiol. 14:291-300. [Google Scholar]
- 25.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]