Abstract
The prokaryote community activity and structural characteristics within marine sediment sampled across a continental shelf area located off eastern Antarctica (66°S, 143°E; depth range, 709 to 964 m) were studied. Correlations were found between microbial biomass and aminopeptidase and chitinase rates, which were used as proxies for microbial activity. Biomass and activity were maximal within the 0- to 3-cm depth range and declined rapidly with sediment depths below 5 cm. Most-probable-number counting using a dilute carbohydrate-containing medium recovered 1.7 to 3.8% of the sediment total bacterial count, with mostly facultatively anaerobic psychrophiles cultured. The median optimal growth temperature for the sediment isolates was 15°C. Many of the isolates identified belonged to genera characteristic of deep-sea habitats, although most appear to be novel species. Phospholipid fatty acid (PLFA) and isoprenoid glycerol dialkyl glycerol tetraether analyses indicated that the samples contained lipid components typical of marine sediments, with profiles varying little between samples at the same depth; however, significant differences in PLFA profiles were found between depths of 0 to 1 cm and 13 to 15 cm, reflecting the presence of a different microbial community. Denaturing gradient gel electrophoresis (DGGE) analysis of amplified bacterial 16S rRNA genes revealed that between samples and across sediment core depths of 1 to 4 cm, the community structure appeared homogenous; however, principal-component analysis of DGGE patterns revealed that at greater sediment depths, successional shifts in community structure were evident. Sequencing of DGGE bands and rRNA probe hybridization analysis revealed that the major community members belonged to delta proteobacteria, putative sulfide oxidizers of the gamma proteobacteria, Flavobacteria, Planctomycetales, and Archaea. rRNA hybridization analyses also indicated that these groups were present at similar levels in the top layer across the shelf region.
The Mertz Glacier Polynya (MGP), located off eastern Antarctica, is a major latent heat polynya in which high-salinity shelf water is formed by brine rejection during ice formation. The high-salinity shelf water exits the shelf zone through a trough to the deep sea, where it contributes about 1.5 × 106 m3 s−1 of Antarctic bottom water (5), which is about 20 to 25% of total production. Antarctic bottom water has a major influence on ocean circulation and global climate and potentially on marine biota in the mesopelagic and deep oceans. Evidence from the Ross Sea Polynya suggests that surface production tends to be rapidly exported into deep waters and sediment (16). The presence of turbidity (detected by photography [13]) indicated that sediment transport and deposition was active above MGP shelf sediments and accumulates to form the Mertz Drift (24). Seabed photography revealed the presence of extensive benthic faunal populations, including arthropods, sponges, crinoids, asteroids, urchins, and anemone (13). Benthic communities in the MGP thus appear to be benefiting from a flux of nutrient-rich particulates resulting in a relatively active ecosystem operating at low temperature (−1.8°C).
Benthic microbial communities throughout the ocean can rapidly degrade and utilize a substantial proportion of exported particulate organic matter (21), and polar benthic communities can respond to seasonal nutrient influxes as rapidly as temperate communities (32, 51). Sulfate and iron reduction, CO2 dark fixation, glucose mineralization, and amino acid uptake (30, 32, 48, 58, 62) in aphotic Antarctic and Arctic coastal, shelf, and abyssal sediments coincide with psychrophilic growth temperatures, suggesting the presence of a psychrophilic community. As the benthos is cold across much of the ocean sea floor, psychrophilic prokaryotes therefore have major roles in nutrient recycling and diagenesis (50). Various studies indicate that a complex microbial community is present, even at hadal depths, within marine sediment (27, 47), although relatively few sediment prokaryotes have been obtained in pure culture and characterized in detail. Little is also known about actual microbial community structure, for example, the heterogeneity and distributional characteristics of the community, its phylogenetic makeup, and how these correspond with major microbial processes as well as cold adaptation. The most detailed studies have been made in Arctic fjords, which indicate that an active diverse autochthonous community of bacteria dominated by Proteobacteria is present. The use of a combination of qualitative and quantitative 16S rRNA gene-based molecular approaches has provided a good understanding of diversity, structure, and function in Arctic sediments (45, 47) and allows comparison with biogeochemical data (32, 51).
In this study, we present a polyphasic examination of the community structure within surficial continental shelf sediments collected off Antarctica. The goals of the study were to determine the psychrophilic adaptations of the indigenous microbiota and corresponding community structure distribution and diversity in MGP sediments. The MGP sediment site was selected because it represented an essentially pristine ecosystem not influenced by anthropogenic or other terrigenous input and because it had a well established geology and paleochronology.
MATERIALS AND METHODS
Sampling and sample characteristics.
Seabed sampling took place within and around the George V Basin (66°S, 143°E) at water depths of 709 to 940 m during the Italian-Australian geoscience research cruise Australian Geological Survey Organization (AGSO) survey 217. By using open polypropylene tubes, a series of minicores (Table 1) were extracted from the top 15-cm surface from 4- to 6-m-long cores or from Shipek sediment grabs. Cores were obtained with a 1-metric-ton core head configured as a gravity corer with 21-cm-long minicores extracted from the center. Sediment grabs were obtained with a Shipek grabber which was deployed to obtain samples from the top sediment layer, with minicores (13 to 15 cm in length) extracted from the centers of these in which the sediment layers remained unperturbed. Core samples were stored at −20°C before processing. Portions of the surface 1 to 3 cm of the Shipek grab samples were also stored at 4°C (Table 1) and used in cultivation and enzymatic experiments. Frozen cores were cut lengthwise and subdivided by using a bandsaw, with sections intended for lipid and nucleic acid analyses stored in sterile containers at −20 or −80°C. The sediment samples investigated in this study have an established geology and paleochronology (24), with most collected within a 400-km2 shelf sediment drift deposit (Mertz Drift) located in an 800- to 870-m-deep area of the George V Basin, 80 km west of the Mertz Glacier. The top layer from which the samples were obtained was a massively bedded, ice-rafted deposit-rich muddy sand (60% mud and 40% sand) containing on average 1% (± 0.5%) total organic carbon and 39% biogenic silica (24). By using isopach map construction and 14C dating, the top 40.7-cm (±15.7 cm; n = 16) sediment depth was found to represent an oceanographically modern feature overlying three late Quaternary unit layers. Sediment accumulation rates for the time period (up to 3,500 years before present) in which the top unit formed ranged from 6 to 21 cm kiloyear−1.
TABLE 1.
Type and sampling location of marine sediment samples investigated and MPN counts for selected samples
| Sample no. | Sample type (length, cm) | Location | Coordinates | Depth (m) | MPN (107 cells g−1) in:
|
Ratio of MPN at 2°C to MPN at 25°C | Viable-cell recovery (%)b | |
|---|---|---|---|---|---|---|---|---|
| SWN | M2216a | |||||||
| 10GC01c | Core (21) | George V Basin | 66°32′S, 143°38′E | 761 | ||||
| 10GB01 | Grab | George V Basin | 66°32′S, 143°38′E | 750 | 2.50 | 0.59 | 2.8 | 2.2 |
| 13GB02C | Core (14) | Mertz Drift | 66°33′S, 143°5′E | 864 | ||||
| 13GB02 | Grab | Mertz Drift | 66°33′S, 143°4′E | 864 | 2.96 | 0.48 | 4.1 | 2.6 |
| 25GB13B | Core (13) | Mertz Drift | 66°34′S, 143°0′E | 843 | ||||
| 25GB13 | Grab | Mertz Drift | 66°34′S, 143°0′E | 843 | 0.92 | 0.08 | 1.6 | 1.7 |
| 27GB15C | Core (14) | Mertz Drift | 66°31′S, 143°23′E | 793 | ||||
| 27GB15 | Grab | Mertz Drift | 66°31′S, 143°23′E | 793 | 4.75 | 0.32 | 2.0 | 3.8 |
| 28GB16C | Core (14) | North Mertz Drift | 66°23′S, 143°19′E | 739 | ||||
| 28GB16 | Grab | North Mertz Drift | 66°23′S, 143°19′E | 739 | 1.75 | 0.35 | 2.1 | 2.1 |
| 28GB17 | Grab | North Mertz Drift | 66°24′S, 143°19′E | 739 | 2.45 | 0.27 | 2.5 | 2.9 |
| 29GB18B | Core (15) | North Mertz Drift | 66°21′S, 143°18′E | 709 | ||||
| 29GB18 | Grab | North Mertz Drift | 66°21′S, 143°18′E | 709 | 1.65 | 0.27 | 1.7 | 2.6 |
| Casey1 | Grab | O'Briens Bay | 66°16′S, 110°33′E | 25 | ||||
| Casey2 | Grab | O'Briens Bay | 66°16′S, 110°32′E | 30 | ||||
| Burton | Grab | Burton Lake, Vestfold Hills | 66°38′S, 78°8′E | 16 | ||||
M2216, M2216 marine liquid medium (diluted in an equal volume of ASW).
The total direct count used for calculating the recovery percentage is the averaged value from the top 4-cm layer of the corresponding sediment core sample.
This core was subjected to extensive clone library analysis (8) with GenBank entries AF424054 to AF424538 and AF425747 to AF425762.
Bacterial enumeration and biomass estimation.
Bacterial numbers were counted by epifluorescence with SYBR Gold (×10,000 in dimethyl sulfoxide; Molecular Probes Inc.) according to the procedure described by Weinbauer et al. (66). The filters containing stained cells were observed with an LDRMBE Leitz microscope fitted with a Leica DC300F digital camera. The average cell volume was determined from electronic images processed with a predetermined scale bar. The bacterial biovolume was then converted to carbon content by assuming 310 fg of C μm−3 (19). The sediment sample dry weight was determined by drying sediments at 60°C for 16 to 20 h.
Extracellular enzyme measurements.
Extracellular aminopeptidase and chitinase activities were determined by using the fluorigenic substrates l-leucine-4-methylcoumarinyl-7-amide and 4-methylumbelliferyl-β-d-glucosaminide dihydrate, respectively. Samples were prepared by adding approximately 200 mg of wet sediment to an equal volume of sterile artificial seawater (ASW) (Sigma sea salts, 35 g liter−1) in an Eppendorf tube (1.5 ml), to which was added substrate at 200 μM (dissolved in N,N′-dimethylformamide), which from prior experimentation was known to be below saturation levels (saturating concentrations ranged from 250 to 400 μM). The slurries were incubated in the dark at 0°C in a Ratek Instruments water bath containing diluted Castrol antifreeze concentrate for 1 to 6 h. Aminopeptidase and chitinase activities in sediment grab samples were also measured by using a temperature gradient incubator (Terratec Australia, Margate, Tasmania, Australia) set with a temperature span of 0 to 45°C. For these experiments, 500 mg of sediment slurry was suspended in 5 ml of sterile ASW in 12-ml screw cap test tubes. After incubation, sediment material was pelleted by centrifugation, and the supernatant was acidified (4) and stored frozen at −20°C. The release of fluorescent dye was measured with a Turner Designs 10AU fluorometer fitted with a UV light source (365-nm peak output) and a UV optical filter (430- to 490-nm emission range). The levels of fluorescence were compared to a standard curve generated from fluorescein (0.05 to 2.0 μM) dissolved in sterile MilliQ water by using a fluorescein optical filter. Data were normalized to sediment dry weight and expressed as micromoles of fluorescein released hour−1 gram (dry weight) of sediment−1.
MPN counting.
Surface sediment grab samples were weighed and diluted in sterile ASW, and 0.1-ml aliquots were then added to an equal volume of seawater nutrient medium (SWN) or half-strength marine 2216 broth (Difco Laboratories, Detroit, Mich.) diluted with an equal volume of ASW in the first 8-well row in sterile 96-well titer trays. From the initial dilution, 11 1:5 dilution steps were made with a multichannel pipettor. Eight replicates were used for each sample tested. All media and diluents used were prechilled to 2°C before use. Titer trays were incubated for up to 6 weeks at 2 and 25°C. Most-probable-number (MPN) counts were then computed from the numbers of wells showing positive growth at maximum dilution by using a modified Gauss-Newton algorithm (29). The ratio of MPN values at 2 and 25°C was used as a relative scale for the psychrophily of the cultured population. The SWN consisted of 0.05 g or yeast extract, 0.05 g of tryptone, 0.05 g of bacteriological peptone, 0.05 g of d-glucose, 0.05 g of soluble starch, and 0.02 g of sodium pyruvate dissolved in 1,000 ml of natural seawater or ASW. The solution was heated sufficiently to dissolve the starch. After cooling, 0.1 ml of 1 M sodium phosphate buffer (pH 7.0) was added, and the medium pH was adjusted to about 7.3 to 7.5. The solution was then filtered with 0.2-μm-pore-size disposable filters, and 0.1 ml of a sterile vitamin solution (2) was added.
Bacterial isolation.
Chemoheterotrophic bacteria present in the MGP sediment samples were isolated from the highest growth-positive dilutions of the MPN trays. Sediment grab samples were also diluted in SWN (102 to 103) prechilled at 2°C, enriched at 1 to 2°C for 24 h, and then directly plated onto prechilled SWN solidified with 1.2% agar and incubated at 2 or 10°C for up to 6 weeks in the dark aerobically. SWN agar supplemented with 0.1 g of l-cystine liter−1, 0.25 g of sodium thioglycolate liter−1, 0.25 g of sodium formaldehyde sulfoxylate liter−1, and 2 mg of methylene blue liter−1 was also used for isolation at 10°C, with plates incubated in anaerobic jars containing anaerobic GasPaks (Oxoid) which were replaced once every 2 weeks. SWN agar was prepared by autoclaving 2.5% agar in ASW, cooling the molten agar to 50°C, and mixing the agar with an equal volume of double-strength liquid SWN preheated to 50°C in a water bath. Plates were then immediately poured, allowed to solidify, and then cooled to 2 to 4°C before use.
Bacterial colonies were selected on the basis of differing morphology, transferred to fresh SWN agar, and incubated at 2°C. Purification was conducted on marine 2216 agar, as growth was more abundant and more stable. The growth of bacterial isolates was tested in marine 2216 liquid medium at a range of temperatures to determine if they were psychrophilic or psychrotolerant as previously defined by Morita (37).
PLFA and tetraether lipid profiling.
Sediment samples were extracted quantitatively by the modified one-phase chloroform-methanol Bligh-Dyer method (67). After phase separation, the lipids were recovered from the lower chloroform layer. Phospholipid fatty acid (PLFA) was obtained by elution from a silicic acid chromatography column with methanol. Fatty acids were liberated from the phospholipid fractions by saponification and were converted to the corresponding fatty acids methyl esters (FAMEs) by treatment with 3 ml of methanol-chloroform-concentrated HCl (10:1:1 by volume; 100°C, 60 min). The FAMEs were extracted into hexane-chloroform (1:1) and stored at −20°C. The Archaea-derived isoprenoid glycerol dialkyl glycerol tetraethers (GDGTs) were eluted from the silicic acid chromatography column with acetone. The samples were treated with acid at 100°C to decompose the phospholipid. The GDGTs were then extracted into hexane-chloroform (1:1) and stored at −20°C. All samples were treated with bis(trimethylsilyl)-trifluoroacetamide prior to chromatography to convert hydroxy groups in the FAMEs or GDGTs to the corresponding O-trimethylsilylethers. Gas chromatographic analysis of the FAMEs was performed with a Hewlett-Packard 5890 gas chromatograph-mass spectrometer equipped with a 50-m by 0.32-mm (inner diameter) cross-linked methyl silicone fused silica HP2 capillary column (0.17-μm film thickness) and flame ionization detector. The carrier gas was helium. Standard gas chromatography conditions included samples being injected at 50°C in splitless mode. After 1 min, the oven temperature was increased to 150°C at a rate of 30°C/min, then to 250°C at 2°C/min, and finally to 300°C at 5°C/min, at which the oven temperature was maintained for 15 min. Two FAMEs, 19:0 and 23:0, were added as internal standards. Analysis of GDGTs used the same gas chromatography hardware with a 2.5-m by 0.25-mm (inner diameter) BPX5 capillary column with a film thickness of 0.17 μm (SGE Chromatography Products, Melbourne, Australia). Samples were injected via an on-column inlet at 50°C. After 2 min, the temperature was increased to 350°C at a rate of 15°C/min and then to 380°C at 1°C/min. After the end of the run, the oven temperature was decreased at 20°C/min to preclude damage to the column resulting from rapid cooling. 1,2-Di-O-hexadecyl-rac-glycerol (Sigma) was added as an internal standard. The GDGTs eluted as broad but well-separated peaks.
DGGE.
DNA was extracted from sediment sections by using a modified version of the method of Rochelle et al. (49). Briefly, about 0.5 to 1 g of wet sediment was suspended in 2 ml of extraction buffer, which consisted of 0.15 M NaCl, 0.1 M disodium EDTA, 4% sodium dodecyl sulfate, 10 mg of polyvinylpyrrolidone, and 30 mg of lysozyme (pH 8.0). The mixture was incubated successively three times at 55°C for 10 min and at −80°C for 30 min. The mixture was then extracted once each with 1 volume of Tris-equilibrated phenol and then 1 volume of phenol-chloroform-isoamyl alcohol (25:24:1). DNA in the aqueous phase was precipitated with 0.7 volume of isopropanol at room temperature for 1 h and centrifuged at 21,000 × g for 30 min at 4°C. The pellet was air dried and dissolved in up to 100 μl of sterile MilliQ water. The DNA samples were further purified with Chroma Spin+TE-1000 columns (Clontech) and stored at −20°C before use. DNA yields were generally 50 to 70% higher (with at least equal purity) than what was found if a bead beating protocol (9) was used. Primers used for all denaturing gradient gel electrophoresis (DGGE) analyses included 341Fclamp (5′-CGC CCG CCG CGC CCC GCG CCC GCC CGC CGC CCC CGC CCC CCT ACG GGA GGC AGC AG-3′) and 907R (5′-CCG TCA ATT CCT TTG AGT TT-3′). These primers are capable of amplifying most bacteria (but not Archaea) from the sediment samples. The PCR consisted of a touchdown thermal cycling program with the following steps: an initial denaturing step at 94°C for 5 min; 10 cycles of 94°C for 1 min, 65°C for 1 min (decreasing by 1°C each cycle), and 72°C for 3 min; 20 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min; and a final extension at 72°C for 4 min. Half of the resultant PCR products were run on 6% acrylamide gels with a denaturing gradient of 30 to 65% (where 100% denaturant is 7 M urea and 40% formamide), using the Bio-Rad D-Code detection system. Gels were run at 80 V for 16 h at 60°C in a buffer consisting of 40 mM Tris, 20 mM glacial acetic acid, and 10 mM disodium EDTA (pH 8.0). Following electrophoresis, gels were stained in a 1:1,000 SYBR Gold solution in the dark with gentle shaking for approximately 20 min. They were then briefly washed with deionized water and destained with deionized water for 20 min before being viewed with UV transillumination. Image analysis of the gels was performed with ImageTool software (University of Texas Health Science Center, San Antonio) to convert banding patterns into a presence-absence matrix, and the most prominent bands from the resulting DGGE profiles were compared by taking into account differences in the migration of DGGE bands from control bacterial strains (Psychrobacter glacincola and Gelidibacter algens). Analysis of DGGE patterns included principal-coordinate analysis and Jaccard similarity analysis with the Taxon program package (Entomology Division, Commonwealth Scientific and Industrial Research Organisation, Sydney, New South Wales, Australia).
DGGE bands were excised with sterile scalpels and incubated in 100 μl of MilliQ water for 15 min to elute gel denaturants. The gel slice was then freeze-thawed twice in a fresh 100-μl aliquot of sterile MilliQ water at 56°C and at −20°C. Eluted DNA was then amplified by using the Qiagen HotStart reaction mix kit with 341Fclamp and 907R as the primers. The reamplified DNA was then run on a DGGE gel (as described above) and compared against the original sample. If a single band which matched the position was obtained, it was excised from the DNA and was reamplified by using unclamped primers. If multiple bands were formed, the individual bands were also excised from the gel, purified, and amplified by using unclamped primers. For the DNA sequencing reactions, 100 ng of reamplified DGGE band PCR product and 5 pmol of unclamped primer were used. Sequencing reactions were carried out with the DCTS Quickstart sequencing kit (Beckman) according to the manufacturer's protocols. Individual base positions were resolved and recorded with a Beckman CEQ2000XL capillary automated sequencer. In some cases, band DNA had to be cloned, and this was done by procedures described previously (9).
rRNA probing with 32P-labeled oligonucleotides.
RNA was extracted from MGP sediment core sections by using a procedure adapted from that of Hurt et al. (28). Unless otherwise specified, all water and buffers used were treated with diethylpyrocarbonate. About 2 to 3 g (wet weight) of sediment was ground three successive times with 2 g of acid-washed sand and 1 ml of denaturation buffer (4 M guanidinium isothiocyanate, 10 mM Tris-HCl [pH 7.0], 1 mM disodium EDTA, 0.5% β-mercaptoethanol) in liquid nitrogen with a mortar and pestle. The slurry was then extracted with 4 ml of extraction buffer (100 mM Tris-HCl, 100 mM disodium EDTA, 1.5 M NaCl, 1% cetyldimethylethylammonium bromide, and 2% sodium dodecyl sulfate in 100 mM sodium phosphate buffer, pH 7.0), mixed gently, and incubated at 65°C for 30 min. Sediment was removed by centrifugation (4,000 × g, 5 min) and reextracted with 2 ml of extraction buffer with incubation at 65°C for 10 min. Sediment was removed from the combined extracts by centrifugation, and the supernatant was then further treated by extraction with 1 volume of 24:1 chloroform-isoamyl alcohol. RNA was precipitated from the aqueous phase by the addition of 0.6 volume of isopropanol at room temperature. RNA was pelleted by centrifugation (21,000 × g for 20 min), dissolved in 1 ml of sterile water, treated with DNase-free RNase, and further purified by using the Qiagen RNeasy RNA extraction kit according to the manufacturer's instructions. RNA was quantified by spectrophotometry with a Bio-Rad SmartSpec 3000. For the hybridization experiments, RNA was denatured in 3 volumes of 2% glutaraldehyde for 10 min at a concentration of about 0.5 μg ml−1 and applied by filtration to premoistened GeneScreen Plus membranes (NEN catalog no. NEF976) by filtration within a Bio-Rad slot blotter. The RNA was cross-linked to the membrane by using a UV cross-linker. Oligonucleotide probes (Table 2) (5 pmol) were end labeled with 100 μCi of [γ-32P]ATP by using T4 polynucleotide kinase. Unincorporated radiolabel was removed by using G-25 microspin columns (Amersham). Membranes containing cross-linked RNA were prehybridized for 4 to 6 h at 40°C in hybridization buffer (0.9 M NaCl, 50 mM NaPO4, 5 mM disodium EDTA in filter-sterilized 10× Denhardt's solution) to which had been added 3 mg of polyadenylic acid (Sigma catalog no. P 9403) ml−1 just prior to use. Enough radiolabeled probe was added to obtain approximately a Geiger count of 100 at a distance of 5 cm and then allowed to hybridize for 16 to 18 h at 40°C. The hybridization buffer was then removed, and the membranes were washed four successive times (twice for 2 min vigorously and twice for 30 min gently) in 100 ml of washing buffer (Table 1) at the temperatures listed in Table 1 to remove unbound probe. The blots were then exposed to Kodak Biomax MS film for 10 min to 3 h, depending on the probe used. Control RNAs for the various probes were extracted from the following species: Halorubrum lacusprofundi (Arch915), Escherichia coli (EUB338 and GB23S42a), Pisum sativum (EUK1379), Thiothrix eikelboomii (GAM660 and DSS658a), G. algens (CFB319a), Pirellula staleyi (PLA927), and Arthrobacter agilis (GB1199). Probe signal intensity was calculated by comparing unknown samples to the universal control probe by using a Bio-Rad GS800 imaging densitometer, with slot band intensity calculated by using the PDQuest and Quantity-1 (Bio-Rad) software packages. Control strain RNA signals were used to correct for differences in binding efficiency. RNA levels for each probe are given as the normalized percent proportion of the universal probe signal.
TABLE 2.
Group-specific rRNA ologinucleotide probes and corresponding washing conditions used in this study
| Target group | Probe namea | Sequence (5′→3′) | Td (°C)b |
|---|---|---|---|
| Universal | UNI1390 | GACGGGCGGTGTGACAA | 44 |
| Bacteria | EUB338 | GCTGCCTCCCGTAGGAGT | 54 |
| Archaea | ARC915 | GTGCTCCCCCGCCAATTCCT | 56 |
| Eukaryotes | EUK1379 | TACAAAGGGCAGGGAC | 42 |
| Gamma/beta proteo- bacteria | BG23S42 | GCCTTCCCACWTCGTTT | 60 |
| S oxidizers (gamma proteobacteria) | GAM660 | TCCACTTCCCTCTAC | 52 |
| Desulfosarcina group | DSS658a | TCCACTTCCCTCTCCCAT | 58 |
| Flavobacteria | CFB319a | TGGTCCGTGTCTCAGTAC | 56 |
| Planctomycetales | PLA927 | CACCGCTTGTGTGAGCCC | 50 |
| Gram-positive bacteria | GB1199 | AGGGGGCATGATG | 34 |
Probe sources are from reference 45. Probe GP1199 is specific to most but not all members of the Actinobacteria and Firmicutes; probe PLA927 was designed in this study.
All probes were washed at the dissociation temperature (Td) in 1× SSC buffer (0.15 M NaCl, 0.015 M trisodium citrate [pH 7.0] containing 1% sodium dodecyl sulfate, except for probes CF319a and DSS658a which were washed with 1× SSC containing 0.1% sodium dodecyl sulfate.
Bacterial identification by 16S rRNA gene sequence analysis.
Small amounts of biomass from selected strains were suspended in 0.4 ml of 0.1 M NaCl-0.1 M disodium EDTA (pH 8.1) buffer and pretreated with 2 mg of lysozyme ml−1 at 37°C for 1 to 24 h before lysis by the addition of 40 μl of 10% sodium dodecyl sulfate. The lysate was extracted with a single volume of 24:1 chloroform-isoamyl alcohol at 4°C and centrifuged at 21,000 × g for 2 min. DNA in the aqueous phase was purified by using the Prep-A-Gene kit (Bio-Rad) according to the manufacturer's instructions. The 16S rRNA gene was then amplified from the DNA (7) and purified by using the Prep-a-Gene kit, and sequencing reactions were prepared by using the DCTS Quickstart sequencing kit (Beckman) according to the manufacturer's protocols. Individual base positions were then resolved and recorded with a Beckman CEQ2000XL automated capillary sequencer.
Sequence analysis.
Sequence data were verified by using Sequencher version 4.1, compared to the GenBank nucleotide database by using BLAST searches (1), and aligned in BioEdit (23) to reference sequences downloaded from GenBank. The aligned data were then analyzed with PHYLIP software (18) through BioEdit by using the maximum-likelihood algorithm to calculate sequence similarity and neighbor joining to create phylogenetic trees.
Nucleotide sequence accession numbers.
The sequences were deposited under GenBank accession numbers AF530092 to AF530158.
RESULTS
Biomass and enzyme activity.
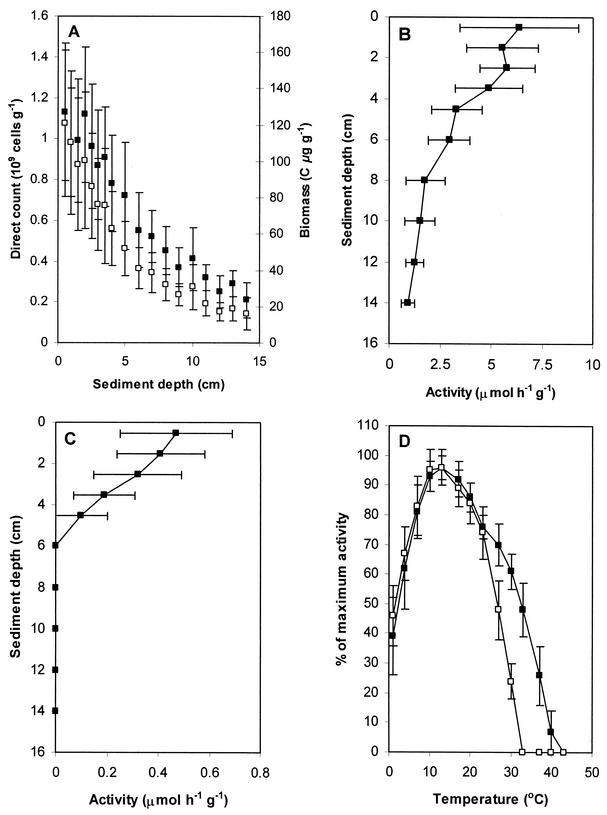
Biomass and extracellular enzyme activity were calculated to determine the variation in bacterial populations and biomass across the sampling site. Overall, it was found that both cell number (range, 0.09 × 109 to 1.43 × 109 cells g−1) and biomass (range, 9 to 153 μg of C g−1) correlated (r2 = 0.95; P < 0.01) in five different MGP sediment cores and declined with sediment core depth (r2 = 0.89 to 0.94; P < 0.01) (Fig. 1A). Aminopeptidase activity exhibited a gradual decline with depth, with activity ranging from 2.95 to 8.30 μmol h−1 g−1 in the 0- to 1-cm layer and declining to 0.36 to 1.49 μmol h−1 g−1 at a depth of 13 to 15 cm (Fig. 1B). Chitinase activity exhibited considerable sample-to-sample variability; however, activity was generally strongest in the 0- to 1-cm surface layer (0.16 to 0.71 μmol h−1 g−1) and declined to levels below reliable detectability (<0.01 μmol h−1 g−1) at depths of greater than 3 to 5 cm (Fig. 1C). In sediment grab samples, aminopeptidase and chitinase activities varied between 3.96 and 12.53 μmol h−1 g−1 and between <0.01 and 0.26 μmol h−1 g−1, respectively. Optimal catalytic activity occurred at between 10 and 17°C for both enzymes, with chitinase activity exhibiting more thermolability than aminopeptidase activity (maximum temperature for growth, 31 and 39°C, respectively) (Fig. 1D).
FIG. 1.
(A) Cell biomass (▪) and bacterial direct count (□) estimates for MGP sediment cores at different depths. (B) Aminopeptidase (substrate, l-leucine-4-methylcoumarinyl-7-amide) activity in MGP sediment cores at different depths. (C) Chitinase (substrate, 4-methylumbelliferyl-β-d-glucosaminide dihydrate) activity in MGP sediment cores at different depths. (D) Temperature range for aminopeptidase (▪) and chitinase (□) activities in MGP sediment grabs. All values are averages and standard deviations from five sediment samples.
Enumeration and identification of psychrophilic bacterial isolates.
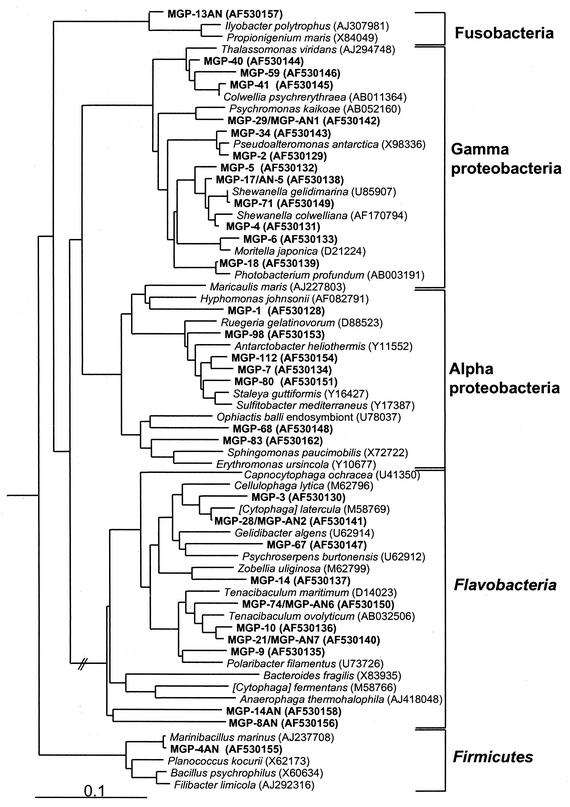
MPN counting with the SWN medium recovered viable counts of 0.9 × 107 to 4.7 × 107 cells g−1, which represented 1.7 to 3.8% of the averaged direct count for the top 4-cm layer (Table 1). By comparison, viable counts obtained with half-strength marine 2216 broth were on average almost an order of magnitude lower (average of 3.8 × 106 cells g−1). The average MPN ratio determined for trays incubated at 2 and 25°C was 2.4 (range, 1.6 to 4.1), indicating that psychrophilic bacteria (which are unable to grow at 25°C) were mostly counted. Isolates obtained from the highest dilutions of the 2°C MPN trays belonged to the family Alteromonadaceae (including species of Shewanella, Colwellia, and Moritella), Photobacterium profundum, and the genus Tenacibaculum (Fig. 2). Isolates obtained from the 25°C MPN trays grouped mostly in the genus Pseudoalteromonas. A diverse range of isolates was obtained on SWN agar plates incubated at 2°C, and these were identified as Alteromonadaceae (as described above but also including Psychromonas strains), Flavobacteria, and alpha proteobacteria (Fig. 2). Most taxa isolated on agar plates were psychrophilic. On SWN plates incubated under anaerobic conditions, facultatively anaerobic gram-negative bacteria also isolated under aerobic conditions were mostly recovered, including strains of Psychromonas, Shewanella, and novel Flavobacteria strains. In addition to these, gram-positive sporulating fermentative strains, which belonged to the species Marinibacillus marinus, and nonsporulating gram-positive anaerobes related to the Fusobacteria were also obtained (Fig. 2). The optimal temperatures for growth of the sediment isolates ranged from 10 to 26°C, with a median of 15°C.
FIG. 2.
Phylogram based on 16S rRNA gene sequences for MGP isolates (in boldface) obtained from MPN trays and from samples directly plated onto SWN agar. Strains isolated from samples incubated under anaerobic conditions are indicated by the suffix AN. GenBank accession numbers are indicated in parentheses after the sequence name.
PLFA and GDGT lipid profiles.
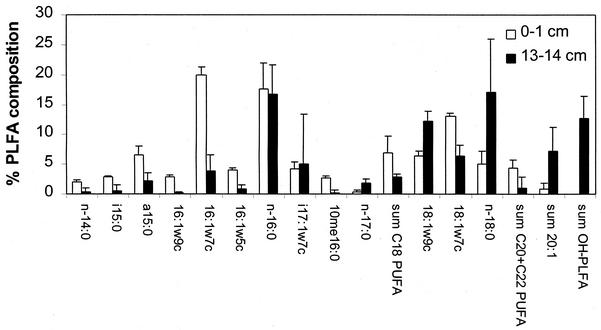
PLFA and GDGT profiles were obtained from the surfaces (0- to 2-cm layer) and bases (13- to 15-cm layer) of five MGP sediment cores to assess biomass and community characteristics within and between sediment samples. The total PLFA concentration averaged 16.0 ± 9.8 μg g (dry weight)−1 in the surface layer, decreasing to 2.6 ± 1.8 μg g (dry weight)−1 in the 13- to 15-cm layer. Although PLFA profiles were quite similar at the same depth, profiles differed substantially between the two depths (Fig. 3). The most obvious difference was the presence of several hydroxy fatty acid components in the deeper sediment layer, making up 9.3 to 17.7% of total PLFA (Fig. 3). Mostly β-hydroxy components were detected and are probably derived from bacterial lipopolysaccharide (65). The total C20 plus C22 polyunsaturated fatty acids (PUFA), which is an indicator of labile freshly sedimented organic material (54), averaged 0.7 μg g−1 in the surface layer but was near or below detection limits (<0.1 μg g−1) in most of the deeper samples. The relatively high C20/C22 PUFA ratio (approximately 4:1) in the surface samples suggested that diatom biomass dominated deposited organic matter (63), which concurs with the high biogenic silica content (39% by weight) of the sediment. The abundance of bacterium-specific PLFA markers, represented by the sum of odd-numbered carbon chain length and branched-chain fatty acids, did not vary significantly with depth (0- to 1-cm, 18.3 ± 4.2%; 13- to 15-cm, 14.2 ± 8.6%). Analysis of GDGTs indicated that concentrations averaged only 0.5 ± 0.2 μg g−1 in the surface layer, increasing to 7.4 ± 6.7 μg g−1 at the 13- to 15-cm depth. GDGT components included calderoarchaeol (GDGT I), GDGTs with either one or two monocyclic diphytanes (GDGTs III and IV), and a GDGT component which was comprised of a dicyclic and a dicyclic cyclohexyl diphytane (GDGT VIII) (Table 3). The detected GDGTs occurred in relatively similar proportions across the MGP site (Table 3) and correspond to components that predominate in cold marine sediments (53).
FIG. 3.
Comparison of PLFA profiles in MGP sediment cores (n = 5) at depths of 0 to 1 and 13 to 14 cm. Error bars indicate standard deviations.
TABLE 3.
GDGT abundance and composition in different MGP sediment layers
| Sediment sample | Depth (cm) | Conc (μg g−1) | % of total GDGTa
|
|||
|---|---|---|---|---|---|---|
| GDGT Ia | GDGT III | GDGT IV | GDGT VIII | |||
| 13GB02C | 0-1 | 0.6 | 63.0 | 12.4 | 4.6 | 20.1 |
| 13GB02C | 13-14 | 16.5 | 43.6 | 17.2 | 14.3 | 24.9 |
| 25GB13B | 0-1 | 0.2 | 49.0 | 19.2 | 8.8 | 23.1 |
| 25GB13B | 13-14 | 0.6 | 76.8 | 14.8 | 2.5 | 5.9 |
| 27GB15C | 0-1 | 0.8 | 63.1 | 10.7 | 6.3 | 19.9 |
| 27GB15C | 12-13 | 11.1 | 40.7 | 28.8 | 7.1 | 23.4 |
| 28GB16C | 0-1 | 0.8 | 61.0 | 13.2 | 4.3 | 21.5 |
| 28GB16C | 13-14 | 7.6 | 46.0 | 25.2 | 8.2 | 20.6 |
| 29GB18B | 0-1 | 0.3 | 68.8 | 19.9 | 2.2 | 9.9 |
| 29GB18B | 14-15 | 1.4 | 58.1 | 17.4 | 6.5 | 18.1 |
The chemical nomenclature for GDGT carbon skeleton moities is based on the scheme described by Schouten et al. (53).
DGGE profiles.
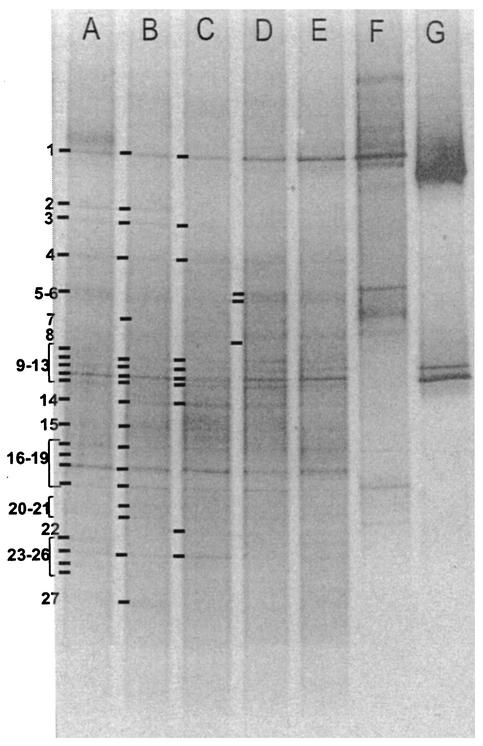
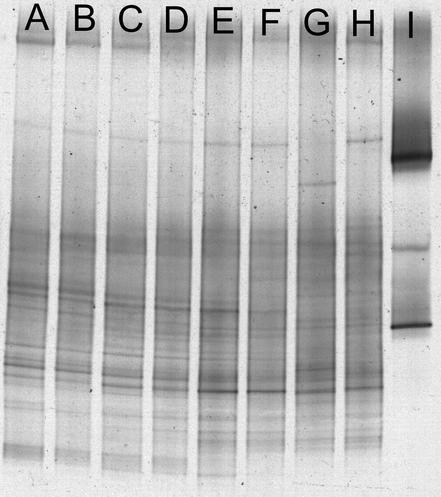
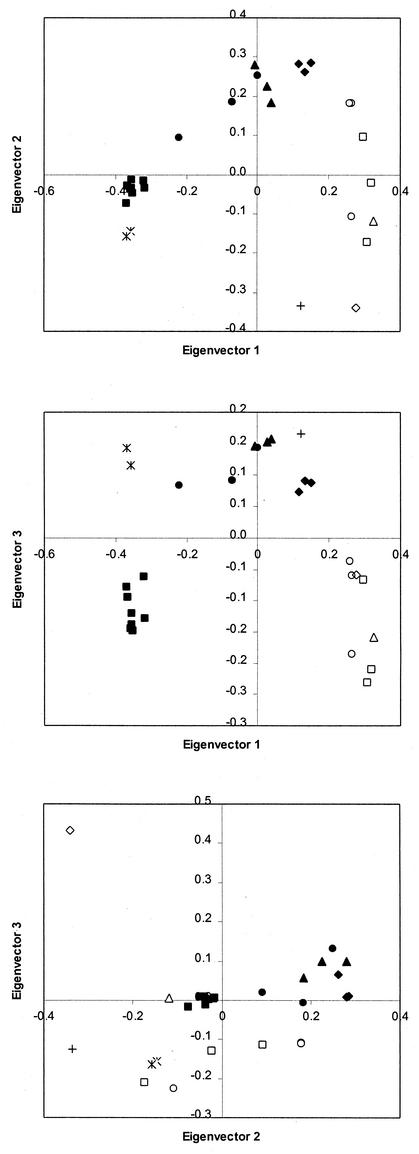
DGGE analysis was performed to assess bacterial community heterogeneity and structural shifts vertically and stratigraphically in MGP sediment. Stratigraphic DGGE profiles with bacterium-specific primers were obtained from sediment slices of five sediment cores (13BG02C, 25GB13B, 27GB15C, 28GB16C, and 29GB18B) at a depth of 1 to 2 cm. Vertical DGGE profiles were also obtained from three cores (10GC01, 27GB15C, and 29GB18B), with sediment depths of 2 to 4, 4 to 6, 6 to 8, 8 to 10, 10 to 12, and 12 to 14 cm investigated in cores 27GB15C and 29GB18B and additional depths of 14 to 16 and 18 to 20 cm investigated in core 10GC01. All DGGE bands from the horizontal (Fig. 4) and vertical (Fig. 5) profiles as well as profiles of geographically distant samples (Table 1) were pooled and subjected to principal coordinates analysis (PCA) and Jaccard analysis for differentiation of individual samples. High congruencies between DGGE profiles were obtained from depths of 1 to 2 cm (Fig. 4) and of 2 to 4 cm (Fig. 5). This is supported along all six calculated eigenvectors, incorporating 79% of the total variance of the data. On the PCA plots in which the first three eigenvectors (Fig. 6) are compared, 1- to 2-cm and 2- to 4-cm samples always tightly clustered. The similarity for these when calculated directly from Jaccard coefficients ranged from 82.6 to 96.2%, with 76% of sampled DGGE bands present in all of the samples taken from both the 1- to 2-cm and 2- to 4-cm depth ranges. At greater depths DGGE patterns became increasingly less similar, with the changes appearing to occur in a gradual and progressive fashion. At each depth range sampled, the DGGE pattern similarity was supported by the first three or four eigenvectors, which cover 49 to 57% of the variance (Fig. 6), and for samples between 6 and 14 cm, Jaccard similarity averaged from 69.8% ± 14.6% to 81.9% ± 3.7%. At depths of between 5 and 9 cm the similarity to the 1- to 4-cm samples was still relatively high, ranging from 48.5 to 81%, but at depths of 15 cm and below the similarity was less than 30%. Comparisons were also made with two in-shore sediment samples from O'Briens Bay (Table 1; Fig. 4 and 6), collected by scuba divers at a water depth of 20 to 30 m, with the top 5 cm sampled. A relative high similarity was found between these samples and the MGP sediment 1- to 4-cm depth range cores, ranging from 79.2 to 85.6%. A very low level of similarity (3.4 to 19.2%), however, was found with a sediment grab collected from the anoxic zone of Burton Lake (a meromictic, seasonally mixed marine basin) (Table 1; Fig. 4 and 6). The number of prominent DGGE bands used in the analyses ranged from 16 to 30 for each MGP sample. There was no significant reduction in prominent band numbers between depths of 4 and 21 cm (r2 = 0.31; P = 0.24), although there were consistently higher number of prominent bands within the 1- to 4-cm depth range compared to the deeper samples (r2 = 0.79; P = 0.03).
FIG. 4.
DGGE gel showing distribution of bacterial 16S rRNA gene fragments in a variety of Antarctic sediment samples. Lanes A to E, MGP samples at a 1- to 2-cm depth; lane F, Burton Lake, Vestfold Hills; lane G, control DNA. Black bars at the left of DGGE bands indicate which bands were sequenced. Bands sequenced are enumerated in descending order and correspond to the MGP band numbers shown in Fig. 7.
FIG. 5.
DGGE gel showing distribution of bacterial 16S rRNA gene fragments within a MGP sediment core (10GC01). Lane designations are by sample depth: A, 2 to 4 cm; B, 4 to 6 cm; C, 6 to 8 cm; D, 8 to 10 cm; E, 10 to 12 cm; F, 14 to 16 cm; G, 16 to 18 cm; H, 18 to 20 cm. Lane I, control.
FIG. 6.
Principal-coordinate plots showing the similarity of patterns of prominent DGGE bands in MGP sediment at various depths and in comparison to other Antarctic sediment samples. The threeseparate plots show different combinations of the first three eigenvectors. Symbols: ▪, 2- to 4-cm depth; •, 4 to 6 cm; ▴, 6 to 8 cm; ⧫, 8 to 10 cm; □, 10 to 12 cm; ○, 12 to 14 cm; ▵, 14 to 16 cm; ◊, 18 to 20 cm; +, Burton Lake; ✻, O'Briens Bay.
DGGE band sequence analysis.
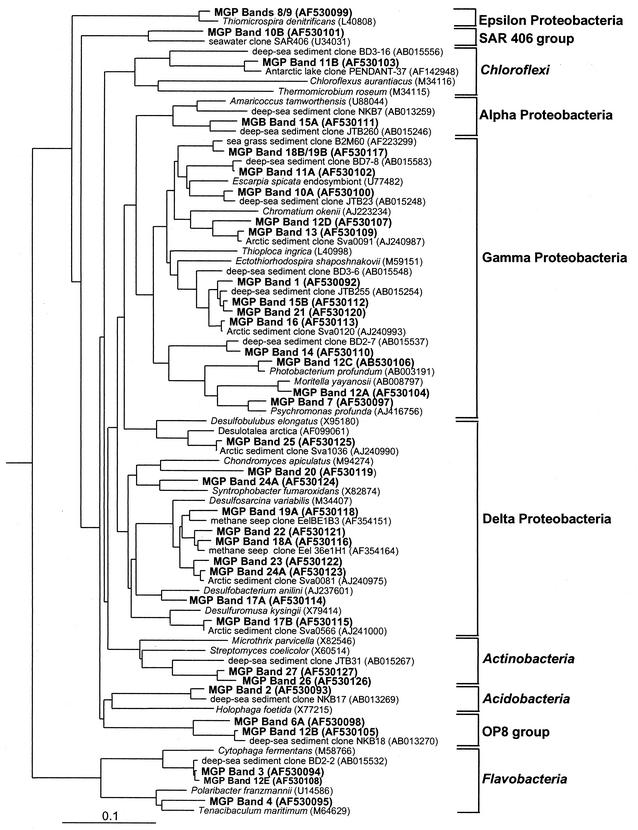
Sequences were obtained from prominent DGGE bands (Fig. 4) to determine major bacterial community members in the MGP sediments at a depth of 1 to 2 cm. This was done to confirm the impression that community structure variation between the samples was low. DGGE bands from different sediment samples which migrated the same distance on the gel contained the same or a very similar sequences, except for bands 10 to 12, 15, 18, 19, and 24, which contained multiple 16S rRNA gene fragments which carried between samples. Certain sequences were also detected in well-separated bands (for example band sequences 8 and 9) (Fig. 7). The lack of gel denaturant resolution, different DNA melting conformations, interoperon sequence variation, and high community diversity give rise to these effects (14, 42).
FIG. 7.
Phylogenetic tree based on 16S rRNA gene sequences of DGGE fragments from MGP sediments. The band numbers correspond to bands indicated by black bars in Fig. 4. GenBank accession numbers are indicated in parentheses after the sequence name.
16S rRNA gene sequence comparisons revealed that most 1- to 2-cm-depth DGGE bands represented Proteobacteria (Fig. 7), in particular, gamma and delta proteobacteria. The gamma proteobacteria detected formed two major groups, the first represented by the family Alteromonadaceae, including bands which contained sequences grouping closely with various deep-sea and sea-ice derived psychrophilic species (Fig. 7). The second group was the largest and most diverse and included several lineages that grouped among sulfur-oxidizing and phototrophic bacteria (Fig. 7). Several of the DGGE bands were very similar to clones detected in Arctic fjord sediment (47) and in deep-sea sediment (34, 35). The presence of delta proteobacteria in MGP sediments was indicated by several DGGE bands, most of which grouped among Desulfosarcina and their relatives (Fig. 7). The presence of microaerobic sulfur-reducing bacteria (SRB) within the sediment was suggested by DGGE band sequences (numbers 8 and 9) grouping close to the species Thiomicrospira denitrificans (epsilon proteobacteria) (Fig. 7). Several DGGE band sequences grouped in lineages that contain few if any cultured strains, including the OP8 and SAR406 (22) green nonsulfur bacteria and Acidobacteria groups (Fig. 7). The function of bacteria within these groups is essentially unknown, but they have been found to be well represented in molecular studies of marine sediment and seawater (3, 8, 59). Several DGGE bands occurring throughout the MGP sediment cores grouped in a distinct branch of Actinobacteria along with other marine sediment clones; similarly, several DGGE bands grouped within the Flavobacteria (Fig. 7).
rRNA hybridization.
The relative abundance of microbiota in the MGP sediments was assessed by using group-specific oligonucleotide probes targeting rRNA. Total extracted RNA concentrations from sections of five MGP cores declined with sample depth from a level of 900 to 1,850 ng g (wet weight) of sediment−1 at 0 to 1 cm to 40 to 100 ng g−1 at 4 to 7 cm. Although there was more than a twofold variation in RNA yields between sediment cores, differences in the binding of the universal probe (UNI1390) were minimal. The combined signal for bacterium (EUB338)-, archaeon (ARC915)-, and eukaryote (EU1379)-specific probes averaged about 90% after normalization, with the bacterial 16S rRNA signal increasing with depth while the archaeal signal was pronouncedly higher in the surface sediment layer (0 to 1 cm) but declined abruptly between 0 to 1 and 1 to 4 cm (Table 4). The abundance of putative sulfur-oxidizing bacteria detected by using a probe specific to the subset of the gamma proteobacteria including both free-living and endosymbiotic taxa (45) was high, representing 16 to 22% of the 16S rRNA pool at the 0- to 4-cm depth (Table 4). The combined abundance of gamma and beta proteobacteria detected with the GB23S42a probe was 23.9% ± 3.4% (Table 4), while the abundance of some SRB was ascertained with a probe specific to the Desulfosarcina group. Various studies indicate that this group dominates marine sediment SRB populations (44, 46), and in the MGP it was the dominant delta proteobacteria group according to clone library analysis (8). In this study the abundance of the Desulfosarcina group significantly increased in all samples between the 0- to 1-cm and 2- to 4-cm depths, comprising up to 13% of the 16S rRNA at 2 to 4 cm (Table 4). The abundance of Planctomycetales was assessed by using a new 16S rRNA probe which replaced an existing planctomycete-specific probe which cross-hybridizes heavily with benthic eukaryotic 18S rRNA (45). Only very weak levels of cross-hybridization (<3% of the PLA927 control signal) were found for the new probe with the tested nonplanctomycetes, and the probe will potentially bind to all planctomycetes according to GenBank BLAST analysis. Applying the probe to the MGP sediment, the relative abundance of the planctomycetes ranged from 2 to 5% of the extracted rRNA (Table 4). The abundance of the class Flavobacteria was quite high (10 to 12% of rRNA at 0 to 4 cm).
TABLE 4.
rRNA hybridization values for different MGP sediment layers
| Oligonucleotide probe | % Range of universal rRNA (normalized) at a sediment core depth range (cm) of:
|
% Clone abundance at a sediment depth of 0-1 and 1.5-2.5 cmc | |
|---|---|---|---|
| 0-1a | 1-4b | ||
| EUB338 | 54.3 (9.0) | 74.2 (7.1) | ND |
| EUK1379 | 20.6 (6.8) | 10.4 (2.6) | ND |
| ARC915 | 6.2 (1.2) | 1.1 (0.4) | 18.2, 5.1 |
| GB23S42a | 23.4 (6.7) | 27.5 (7.5) | 41.8, 42.9− |
| GAM660 | 26.8 (7.1) | 22.1 (5.7) | 16.5, 14.7− |
| DSS658a | 1.9 (0.8) | 8.8 (2.3) | 1.1, 7.3 |
| FLA319a | 10.4 (3.9) | 12.0 (11.4) | 14.7, 5.7 |
| PLA927 | 1.9 (1.5) | 3.9 (3.5) | 1.5, 4.6 |
Sediment cores (13GB02C, 27GB15C).
Sediment cores (25GB13B, 28GB16C, 29GB18B).
From MGP sediment 10GC01 over depths of 0 to 0.4 cm and 1.5 to 2.5 cm. Data are from reference 8. ND, not determined.
DISCUSSION
MGP sediments were studied to obtain a working knowledge of the prokaryotic community by using a variety of different techniques complemented with extensive 16S rRNA gene clone library analysis published elsewhere (8). From this it was clearly observed that MGP sediments were active, inhabited by a rich diversity of cold-active prokaryotes that exhibited a definable community structure.
Biomass and enzyme activity.
Initial analyses were conducted to observe the heterogeneity of the site, and from bacterial direct counts, unnormalized enzyme activity, and lipid geochemistry data, the MGP sediments showed two- to threefold variations in biomass levels and the biomass also declined at approximately the same degree with depth throughout the study site. Aminopeptidase rates (Fig. 1B) were in a range similar to those reported for Ross Sea sediments (17) and Atlantic Ocean sediments (43). Aminopeptidase activity and bacterial direct count strongly correlated (+0.98) within the MGP sediment sample set, suggesting that aminopeptidase-producing microorganisms make up a large proportion of the community in all samples. Normalizing aminopeptidase activity with biomass data revealed that specific activity generally declined with depth (r2 = 0.66), suggesting that either a less active community was present or a lower proportion of microorganisms produced aminopeptidase. In addition to examining aminopeptidase activity, the capacity for the sediment community to decompose complex organic matter was also investigated, with chitin chosen owing to its high abundance (1011 metric tons produced globally each year) and its importance in the oceans as a source of carbon and nitrogen (25). The chitinase activity measured in this study was similar to rates of other glycosidic enzymes such as β-glucosidase (17), with no activity detected below 3 to 5 cm. This suggests that most of the active polysaccharide degradation appears to take place in the surface sediment layers, which usually undergo active physical and biological mixing (60). Chitin degradation has been reported to occur most intensely in marine sediments, although much degradation of chitin occurs as it migrates down the water column within particulate material (11). Chitinase activity generally correlated (+0.77) with biomass. Chitin degradation in MGP sediments could be mediated by facultative anaerobes, such as psychrophilic strains of Colwellia and Shewanella (Fig. 2), some of which have been shown to be able grow aerobically and anaerobically on chitin as sole carbon and/or nitrogen sources (6).
Psychrophily and heterotrophic cultivated diversity of MGP sediments.
Owing to the low in situ temperature of the MGP sediments, it was interesting to determine the psychrophilic adaptations of the MGP microbial communities and the types of easily cultured microbes present. Temperature profiles for both aminopeptidase and chitinase activities were similar, with a linear decline in activity occurring below 10°C (r2 = 0.98). At the MGP site in situ temperature, the extrapolated enzyme activities were estimated to be about 32 to 37% and 23 to 30% of aminopeptidase and chitinase maximal activities, respectively. This relatively high proportion of activity suggests that although operating under suboptimal conditions, growth and degradative processes are still relatively efficient in Antarctic shelf sediments. On the basis of MPN data, a high proportion of the readily cultured heterotrophic microbial community was psychrophilic. MPN count ratios between incubation temperatures of 2 and 25°C provide a rough guide to the predominance of psychrophiles in a community. In sea-ice algal assemblages (chlorophyll a > 250 μg m−2), the rations between MPNs at 2 and 25°C ranged from 3.5 to 9, indicating that a majority of the cultured population could be considered psychrophilic, which is supported by cultivation and characterization studies (6, 7, 10). In the MGP sediments the ratio, although somewhat lower (Table 1), still suggests that the habitat contains a high proportion of psychrophilic bacteria, with the median optimal growth temperature for cultivated strains being about 15°C. High populations of psychrophilic bacteria in marine sediment had been demonstrated in several earlier studies by direct cultivation (see, e.g., references 49 and 68). It must be noted that cultivation-based studies do not take into account uncultivated species present within the sediment, and thus the prevalence of psychrophily within the sediment community could be underestimated.
Most of the heterotrophic isolates obtained were psychrophilic, with many grouping in genera previously isolated from sea-ice and various deep-sea habitats. The strains found, however, appear in most cases to constitute novel species. 16S rRNA gene sequences closely matching (>98 to 100% similarity) several of the isolates were detected in clone libraries created from core 10GC01, mostly from the surface layer (0 to 0.4 cm deep) (8). These included Shewanella gelidimarina, P. profundum, Moritella spp., Colwellia spp., Psychromonas spp., and Tenacibaculum spp. all of which are facultatively anaerobic psychrophiles. Some of these were also detected with a different primer set in the DGGE analysis (Fig. 7). This clearly suggests that these species are highly abundant in MGP surface sediments (mostly >107 cells g−1), where they could be contributing significantly to carbon degradative processes, including active decomposition of protein and polysaccharides, and may supply essential omega-3 fatty acids (see below). Many other isolates were obtained; however, these were not detected in either the clone libraries or by DGGE, suggesting that they are likely to have a relatively low abundance in the sediment.
Lipid geochemistry.
Analysis of PLFA and GDGTs was carried out to construct a picture of biomass distribution in the sediment and also to compare MGP sediments with other open oceanic regions. Indeed, PLFA profiles of the MGP sediment surface layer (0- to 1-cm depth range) were qualitatively similar to those of open ocean and estuarine sediments (20, 57, 69), with the same PLFA components present. A significant shift in the PLFA profile at 13 to 15 cm occurred (Fig. 3), emphasized by the increased prevalence of hydroxy fatty acids and greater abundance of C17 to C20 chain length fatty acids. This difference does not appear due to partial oxidation of fatty acid components, as PLFA is quite labile and PLFA levels correlate with viable biomass levels (67); instead, the different profiles suggest that a significantly different community occurs in the MGP site compared to the sediment surface layer.
MGP surface sediment contained significant levels of eicosapentaenoic acid (20:5ω3) and docosahexanoaenoic acid (22:6ω3), which are found in algae and psychrophilic bacteria. Although it is normally accepted that microeukaryotes contribute most PUFA to sediments, there is a possibility that bacteria may also contribute to the C20 to C22 PUFA signal, as many isolates obtained from the MGP sediment grabs were able to produce 20:5ω3 and 22:6ω3. PUFA-producing isolates grouped in the gamma proteobacteria and included Colwellia, Shewanella, Psychromonas, and Photobacterium strains (56). PUFA-producing bacteria have also been found to be common in permanently cold habitats, including the deep-sea (15), and in sea ice (40). Based on MPN culture data and clone abundance data (8), these bacteria may collectively represent up to 1 to 5% of the bacterial population in the surficial sediment layer. As the strains can contain up to 20% of their PLFA in the form of PUFA (15, 40), this indicates that these bacteria could potentially contribute a substantial proportion of the C20 to C22 PUFA level. Colonization of PUFA-synthesizing bacteria within and on epibenthic metazoa may represent an important source of these vital nutrients to high trophic levels, which are unable to synthesize essential omega-3 fatty acids de novo (39). Clearly, more knowledge of the significance and functional contributions of sediment psychrophiles to the food chain is important and could allow for more accurate assessments of the fate of organic carbon in the benthos by using geochemical procedures.
The Archaea-derived GDGTs detected in the sediment cores are typical of open-ocean sediments (53). The component concentrations and ratios were also found to be relatively similar, suggesting that archaeal populations may have constant population levels and possibly constant species composition across the site. GDGT VIII, which is derived from nonthermophilic Crenarchaeota, is of interest, as members of marine group I appear to make up a large proportion of mesopelagic bacterioplankton (31). Clone library data from core 10GC01 (8) and rRNA hybridization data (Table 4) indicate that Archaea are populous in the MGP sediment cores, making up 1 to 7% of the extracted rRNA. In other studies the abundance of Archaea measured by rRNA hybridization ranges from <1.0 to 8% (52, 61). The relative archaeal abundance appeared to be in good agreement with the GDGT concentration at the 0- to 1-cm depth, since GDGTs occur in only a low ratio to PLFA levels. GDGT levels also are relatively low per cell compared with PLFA content in most bacteria (41). The increased GDGT levels in the 13- to 15-cm layer did not correlate with archaeal RNA abundance (5.2% of 16S rRNA), and GDGT concentrations (Table 3) were greater than PLFA concentrations at this depth. GDGTs thus appear to be preserved in the deeper anoxic layers. This is consistent with data from ancient marine sediments, in which high concentrations of GDGTs have been observed (33).
Molecular analysis of community structure.
Some molecular evidence (12, 36) indicates that the microbial community structure in the top layer of surficial marine sediment appears to be relatively homogenous even though its crosses a steep decline in redox potential. The community homogeneity appears to occur in the sediment mixed layer and is probably the result of active bioturbation (21), which introduces fresh nutrients from the seawater-sediment interface, encourages reoxidation of compounds involved in anaerobic respiration, and physically redistributes the microbial community (60). In this study similar results are shown by DGGE analysis, in which DGGE patterns in three core were very similar between depths of 1 and 4 cm. Although the mixed layer was not analyzed specifically, seabed photography (13) at the sampling sites indicated the presence of diverse epibenthic fauna, including many invertebrate species, which should have a physical impact on the sediment. Energetic bottom currents (24) could also have a measurable impact on the surface mixing of Mertz Drift sediments. At depths of 6 cm and below there was an apparent gradual and apparently successional community shift, as suggested by PCA analysis of DGGE patterns (Fig. 6). The gradual nature of the change combined with lower biomass and activity at these depths suggests that sediment burial and exhaustion of labile nutrients and useable electron sinks (26) could be responsible for shifts in community structure, which become increasingly disparate at the surface 1- to 4-cm layer. These changes are also in concordance with PLFA data, which suggest the presence of a different community at a depth of 13 to 15 cm. The overall community shifts also seem to be consistent with depth, with results from separate cores showing high similarity in band patterns. This suggests that variations in organic matter deposition into the sediment, which constrain biomass levels (17), did not affect community structure; rather, it is likely that the deposited organic matter was of the same type and quality, resulting in homogenous populations in the surface layer. The thickness of the mixed layer appears to be controlled by organic matter flux (55), and thus the mixing layer depth is not invariant and could, like biomass, be dependent on local surface productivity. The lack of coupling between structure and functionality between 1 and 4 cm could be due to prokaryotes present being able to grow efficiently over a broad range of anaerobic redox conditions, perhaps by using different electron acceptors (e.g., from nitrate to iron to sulfate) or by growing by fermentation; many are probably capable of facultative anaerobic and/or microaerophilic growth.
The DGGE bands analyzed at each depth usually represent the more populous species in the community, although PCR bias (64) makes this uncertain to some degree. However, in this investigation the level of any PCR bias would have been evenly distributed, as the PCR-DGGE was conducted simultaneously for whole sample sets under nearly the exact same conditions. Another limitation of DGGE analysis is that it can clearly resolve only a small proportion of the diversity within complex communities. In the case of the MGP sediment samples, about 4 to 8% of 16S rRNA gene phylotypes present were actually detected with PCR-DGGE, as species richness calculations suggest that the sediments contain 440 to >1,130 16 rRNA gene phylotypes, depending on the sample depth (8). In general, most sediment species present are not sufficiently abundant to exhibit an obvious band, and these merge to form an unresolved background blur. Thus, it is possible that significant underlying variation that is not resolvable by using DGGE analysis may exist between sediment samples. However, combined with RNA data, the DGGE analysis proved to be reliable, in that prokaryotic groups making up large proportions of extracted RNA were also well represented among prominent DGGE bands. RNA hybridization with the GAM660 probe revealed that putative sulfur- or sulfide-oxidizing gamma proteobacteria were a dominant bacterial group present in the MGP sediments, with an average abundance ranging from 16.3 to 22.1% of extracted rRNA across five cores (Table 4). This supports the DGGE sequence data, which indicated that several prominent bands (from the 1- to 2-cm depth sample) observed in DGGE gels belonged to this group. The GAM660 probe hybridization level in MGP sediments was higher than that found in Svalbard, Arctic Ocean, sediment (9.3 to 22.2% of prokaryotic rRNA) (45), and the diversity of GAM660-hybridizing phylotypes was exceptionally high, with three major phylotype groups (represented by sediment clones JTB255/BD3-6, BD7-8, and sva0091) delineated (8). The GAM660 probe does not hybridize to all phylotypes in these groups, and thus the probe may underestimate the presence of putative sulfide oxidizers.
In this study the distribution of SRB was estimated only with the probe DSS658a, which is specific for the Desulfosarcina group. This group is probably the most common SRB group in marine sediment (44, 46), although other sulfate- and sulfur- or iron-reducing delta proteobacteria are also prominent (Fig. 7). By using the DSS658a probe as a proxy, it was evident that SRB are common in the MGP sediments, representing a major component of the 16S rRNA pool (Table 4). A clear zonation was observed, with a relatively low DSS658a signal (<3%) found in the top 1 cm. Although oxygen penetration was not measured for the MGP sediments, muddy shelf sediments typically have limited O2 penetration, usually less than 15 mm (38). The abundant levels of SRB and putative sulfide oxidizers suggest that the MGP sediments have an active sulfate-sulfide oxidation-reduction cycle. DGGE analysis also indicated the Desulfosarcina group and other SRB-related taxa were prominent members of the MGP sediment community (Fig. 7).
Other major bacterial groups detected by both DGGE and RNA hybridization included Flavobacteria (5.7 to 12.0% of extracted 16S rRNA) and Planctomycetales (1.9 to 6.1%) (Table 4). The level of Flavobacteria detected is comparable to that in surficial sediment collected off Svalbard (2.7 to 6.1% [45]) and in the Wadden Sea (6.2 to 18.1% [36]).
DGGE analysis indicated that most detected gram-positive bacteria formed a deep-branching lineage within the Actinobacteria, which from comparisons of GenBank sequences includes clones from both deep and shallow marine sediment samples. The new planctomycete rRNA probe indicated that this group was also relatively abundant in the sediment, with an increase in abundance occurring with depth (from 2 to 6%). No DGGE bands typed as belonging to the planctomycetes, due to nucleotide mismatches within the 341Fclamp DGGE primer. Overall, most DGGE sequences were closely related to sequences detected in other marine or marine-derived sediments of widely varying depths, suggesting that they may represent groups widely distributed in the marine environment. Most DGGE bands found were also sampled within clone library data (8).
Conclusions.
By using a variety of procedures, MGP sediments were shown to be a metabolically active environment at low in situ temperatures, the culturable bacterial population of which is clearly and predominantly psychrophilic. As shown by DGGE analysis, the community structural variability of the sediments was lower than expected, with a definably homogenous community existing at a depth of 1 to 4 cm. This homogeneity occurred even though there were two- to threefold variations in biomass levels and variations in enzyme activity. A possible hypothesis is that homogeneity in community structure could be produced by the lack of physical variation in the sediment at the site, the chemical similarity of exported organic matter (which is dominated by sea-ice phytoplankton), and functional redundancy among the indigenous microorganisms. Only at greater depths were significant qualitative and quantitative changes in the community structure observed, which had distinctly different PLFA and DGGE profiles. Lipid geochemistry indicates that the sediment is readily comparable to other open-ocean sediments, suggesting a hypothesis that structural and functional features demonstrated in the MGP could be a typical feature of shelf sediments. This is suggested by many MGP sediment 16S rRNA gene phylotypes being quite similar to other cloned sequences obtained from marine sediments collected from different depths (8). With analysis of additional samples from geographically dispersed locations and with more specific targeting of groups common in the benthos, an improved understanding of prokaryotic activity and functionality could be obtained for marine sediment in general.
Acknowledgments
This research was supported by Australian Research Council grants A09905709 and F09905711 and Antarctic Science Advisory Committee grant 1165.
We thank the crew of the Tangaroa (National Institute of Water and Atmosphere, Auckland, New Zealand) and Peter Harris and the paleogeology science team for assistance in sample collection.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch, W. E., and R. S. Wolfe. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptosulfonic acid (HSCoM)-dependent growth of Methanobacterium ruminatium. Appl. Environ. Microbiol. 32:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bano, N., and J. T. Hollibaugh. 2002. Phylogenetic composition of bacterioplankton assemblages from the Arctic Ocean. Appl. Environ. Microbiol. 68:505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belanger, C., B. DesRosiers, and K. Lee. 1997. Microbial extracellular enzyme activity in marine sediments—extreme pH to terminate reaction and sample storage. Aquat. Microb. Ecol. 13:187-196. [Google Scholar]
- 5.Bindoff, N. L., G. D. Williams, and I. Allison. 2000. Sea-ice growth and water mass modification in the Mertz Glacier Polynya during winter. Ann. Glaciol. 33:399-406.
- 6.Bowman, J. P., M. V. Brown, and D. S. Nichols. 1997. Biodiversity and ecophysiology of bacteria associated with Antarctic sea ice. Antarc. Sci. 9:134-142. [Google Scholar]
- 7.Bowman, J. P., S. A. McCammon, M. V. Brown, D. S. Nichols, and T. A. McMeekin. 1997. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 63:3068-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowman, J. P., and R. D. McCuaig. 2003. Biodiversity, community structural shifts, and biogeography of prokaryotes within Antarctic continental shelf sediment. Appl. Environ. Microbiol. 69:2463-2483. [DOI] [PMC free article] [PubMed]
- 9.Bowman, J. P., S. M. Rea, S. A. McCammon, and T. A. McMeekin. 2000. Diversity and community structure within anoxic sediment from marine salinity meromictic lakes and a coastal meromictic marine basin, Vestfold Hills, Eastern Antarctica. Environ. Microbiol. 2:227-237. [DOI] [PubMed] [Google Scholar]
- 10.Bowman, J. P., S. M. Rea, M. V. Brown, S. A. McCammon, M. C. Smith, and T. S. McMeekin. 2000. Community structure and psychrophily in Antarctic microbial ecosystems, p. 287-292. In C. R. Bell, M. Brylinsky, and M Johnson-Green (ed.), Microbial biosystems: new frontiers. Proceedings of the 8th International Symposium on Microbial Ecology, vol. 1. Atlantic Canada Society for Microbial Ecology, Kentville, Nova Scotia, Canada.
- 11.Boyer, J. N. 1994. Aerobic and anaerobic degradation and mineralization of 14C-chitin by water column and sediment inocula of the York River estuary, Virginia. Appl. Environ. Microbiol. 60:174-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braker, G., H. C. Ayala del Río, A. H. Devol, A. Fesefeldt, and J. M. Teidje. 2001. Community structure of denitrifiers, Bacteria, and Archaea along redox gradients in Pacific Northwest marine sediments by terminal restriction fragment length polymorphism analysis of amplified nitrite reductase (nirS) and 16S rRNA genes. Appl. Environ. Microbiol. 67:1893-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brancolini, G., and P. Harris. 2000. Post-cruise report AGSO survey 217: joint Italian/Australian marine geoscience expedition aboard the R.V. Tangaroa to the George Vth Land region of East Antarctica during February-March, 2000. AGSCO record no. 2000/38. AGSO, Canberra, Australia.
- 14.Buchholz-Cleven, B. E. E., B. Rattunde, and K. L. Straub. 1997. Screening for genetic diversity of isolates of anaerobic Fe(II)-oxidizing bacteria using DGGE and whole-cell hybridization. Syst. Appl. Microbiol. 20:301-309. [Google Scholar]
- 15.DeLong, E. F., D. G. Franks, and A. A. Yayanos. 1997. Evolutionary relationships of cultivated psychrophilic and barophilic deep-sea psychrophilic bacteria. Appl. Environ. Microbiol. 63:2105-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiTullio, G. R., J. M. Grebmeier, K. R. Arrigo, M. P. Lizotte, D. H. Robinson, A. Leventer, J. B. Barry, M. L. VanWoert, and R. B. Dunbar. 2000. Rapid and early export of Phaeocystis antarctica blooms in the Ross Sea, Antarctica. Nature 404:595-598. [DOI] [PubMed] [Google Scholar]
- 17.Fabiano, M., and R. Danovaro. 1998. Enzymatic activity, bacterial distribution, and organic matter composition in sediments of the Ross Sea (Antarctica). Appl. Environ. Microbiol. 64:3838-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felsenstein, J. 1993. PHYLIP v. 3.57. University of Washington, Seattle.
- 19.Fichez, R. 1991. Composition and fate of organic matter in submarine cave sediments: implications for the biogeochemical cycling of organic carbon. Oceanol. Acta 14:369-377. [Google Scholar]
- 20.Gong, C., and D. J. Hollander. 1997. Differential contribution of bacteria to sedimentary organic matter in oxic and anoxic environments, Santa Monica Basin, California. Org. Geochem. 26:545-563. [Google Scholar]
- 21.Gooday, A. J., and C. M. Turley. 1990. Responses by benthic organisms to inputs of organic material to the ocean floor: a review. Phil. Trans. R. Soc. London A 331:119-138. [Google Scholar]
- 22.Gordon, D. A., and S. J. Giovannoni. 1996. Detection of stratified microbial populations related to Chlorobium and Fibrobacter species in the Atlantic and Pacific oceans. Appl. Environ. Microbiol. 62:1171-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall, T. A. 1999. A user-friendly biological sequence alignment editor for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 24.Harris, P. T., G. Bancolini, L. Armand, M. Busetti, R. J. Beaman, G. Giorgetti, M. Presti, and F. Trincardi. 2001. Continental shelf drift deposit indicates non-steady state Antarctic bottom water production in the Holocene. Mar. Geol. 179:1-8. [Google Scholar]
- 25.Herwig, R. P., N. B. Pellerin, R. L. Irgens, J. S. Maki, and J. T. Staley. 1988. Chitinolytic bacteria and chitin mineralization in the marine waters and sediments along the Antarctic Peninsula. FEMS Microbiol. Ecol. 53:101-112. [Google Scholar]
- 26.Hoehler, T. M., M. C. Alperin, D. B. Albert, and C. S. Martens. 2000. Apparent minimum free energy requirements for methanogenic Archaea and sulfate-reducing bacteria in an anoxic marine sediment. FEMS Microbiol. Ecol. 38:33-41. [Google Scholar]
- 27.Horikoshi, K., and K. Tsuji (ed.). 1999. Extremophiles in deep-sea environments. Springer, Tokyo, Japan.
- 28.Hurt, R. A., X. Qiu, L. Wu, Y. Roh, A. V. Palumbo, J. M. Tiedje, and J. Zhou. 2001. Simultaneous recovery of RNA and DNA from soils and sediments. Appl. Environ. Microbiol. 67:4495-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irwin, P., S. Tu, W. Damert, and J. Phillips. 2000. A modified Gauss-Newton algorithm and ninety-six well micro-technique for calculating MPN using Excel spreadsheets. J. Rapid Methods Autom. Microbiol. 8:171-191. [Google Scholar]
- 30.Isaksen, M. F., and B. B. Jorgensen. 1996. Adaptation of psychrophilic and psychrotrophic sulfate-reducing bacteria to permanently cold marine environments. Appl. Environ. Microbiol. 62:408-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507-510. [DOI] [PubMed] [Google Scholar]
- 32.Kostka, J. E., B. Thamdrup, R. N. Glud, and D. E. Canfield. 1999. Rates and pathways of carbon oxidation in permanently cold Arctic sediments. Mar. Ecol. Prog. Ser. 180:7-21. [Google Scholar]
- 33.Kuypers, M. M. M., P. Blokker, J. Erbacher, H. Kinkel, R. D. Pancost, S. Schouten, and J. S. Sinninghe Damsté. 2001. Massive expansion of marine Archaea during a mid-Cretaceous oceanic anoxic event. Science 293:92-94. [DOI] [PubMed] [Google Scholar]
- 34.Li, L., C. Kato, and K. Horikoshi. 1999. Bacterial diversity in deep-sea sediments from different depths. Biodivers. Conserv. 8:659-677. [Google Scholar]
- 35.Li, L., C. Kato, and K. Horikoshi. 1999. Microbial diversity in sediments collected from the deepest cold-seep area, the Japan Trench. Mar. Biotechnol. 1:391-400. [DOI] [PubMed] [Google Scholar]
- 36.Llobet-Brossa, E., R. Roselló-Mora, and R. Amann. 1998. Microbial community composition of Wadden Sea sediments as revealed by fluorescence in situ hybridization. Appl. Environ. Microbiol. 64:2691-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita, R. Y. 1975. Psychrophilic bacteria. Bacteriol. Rev. 39:144-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mucci, A., B. Sundby, M. Gehlen, T. Arakaki, S. Zhong, and N. Silverberg. 2000. The fate of carbon in continental shelf sediments of eastern Canada: a case study. Deep-Sea Res. II 47:733-760. [Google Scholar]
- 39.Müller-Navarra, D. C., M. T. Brett, A. M. Liston, and C. R. Goldman. 2000. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403:74-77. [DOI] [PubMed] [Google Scholar]
- 40.Nichols, D. S., P. D. Nichols, and T. A. McMeekin. 1995. Ecology and physiology of psychrophilic bacteria from Antarctic saline lakes and sea-ice. Sci. Prog. 78:311-347. [Google Scholar]
- 41.Nichols, P. D., C. A. Mancuso, and D. C. White. 1987. Measurement of methanotroph and methanogen signature phospholipids for use in assessment of biomass and community structure in model systems. Org. Geochem. 11:451-461. [Google Scholar]
- 42.Nubel, U., M. M. Bateson, M. T. Madigan, M. Kuhl, and D. M. Ward. 2001. Diversity and distribution in hypersaline microbial mats of bacteria related to Chloroflexus spp. Appl. Environ. Microbiol. 67:4365-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poremba, K. 1995. Hydrolytic enzymatic activity in deep-sea sediments. FEMS Microbiol. Ecol. 16:213-222. [Google Scholar]
- 44.Purdy, K. J., D. B. Nedwell, T. M. Embley, and S. Takii. 2001. Use of 16S rRNA-target oligonucleotide probes to investigate the distribution of sulfate-reducing bacteria in esturaine sediments. FEMS Microbiol. Ecol. 36:165-168. [DOI] [PubMed] [Google Scholar]
- 45.Ravenschlag, K., K. Sahm, and R. Amann. 2001. Quantitative molecular analysis of the microbial community in marine Arctic sediments (Svalbard). Appl. Environ. Microbiol. 67:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravenschlag, K., K. Sahm, C. Knoblauch, B. B. Jørgensen, and R. Amann. 2000. Community structure, cellular rRNA content, and activity of sulfate-reducing bacteria in marine Arctic sediments. Appl. Environ. Microbiol. 66:3592-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravenschlag, K., K. Sahm, J. Pernthaler, and R. Amann. 1999. High bacterial diversity in permanently cold marine sediments. Appl. Environ. Microbiol. 65:3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichardt, W. 1987. Differential temperature effects on the efficiency of carbon pathways in Antarctic marine benthos. Mar. Ecol. Prog. Ser. 40:127-135. [Google Scholar]
- 49.Rochelle, P. A., B. A. Cragg, J. C. Fry, R. J. Parkes, and A. J. Weightman. 1994. Effect of sample handling on estimation of bacterial diversity in marine sediments by 16S rRNA gene sequence analysis. FEMS Microbiol. Ecol. 15:215-226. [Google Scholar]
- 50.Rüger, H. J. 1989. Benthic studies of the northwest African upwelling region: psychrophilic and psychrotrophic bacterial communities from areas with different upwelling intensities. Mar. Ecol. Prog. Ser. 57:45-52. [Google Scholar]
- 51.Rysgaard, S., B. Thamdrup, N. Risgaard-Petersen, H. Fossing, P. Berg, P. B. Christensen, and T. Dalsgaard. 1999. Seasonal carbon and nutrient mineralization in a high-Arctic coastal marine sediment, Young Sound, Northeast Greenland. Mar. Ecol. Prog. Ser. 175:261-276. [Google Scholar]
- 52.Sahm, K., and U. G. Berninger. 1998. Abundance, vertical distribution, and community structure of benthic prokaryotes from permanently cold marine sediments (Svalbard, Arctic Ocean). Mar. Ecol. Prog. Ser. 165:71-80. [Google Scholar]
- 53.Schouten, S., E. C. Hopman, R. C. Pancost, and J. S. Sinninghe Damsté. 2000. Widespread occurrence of structurally diverse tetraether membrane lipids: evidence for the ubiquitous oresence of low-temperature relatives of hyperthermophiles. Proc. Natl. Acad. Sci. USA 97:14421-14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw, P. M., and R. B. Johns. 1985. Organic geochemical studies of a recent Inner Great Barrier Reed sediment. I. Assessment of input sources. Org. Geochem. 8:147-156. [Google Scholar]
- 55.Smith, C. R., and C. Rabouille. 2002. What controls the mixed-layer depth in deep-sea sediments? The importance of POC flux. Limnol. Oceanogr. 47:418-426. [Google Scholar]
- 56.Smith, M. C. 2002. Molecular genetics of polyunsaturated fatty acid biosynthesis in Antarctic bacteria. Ph.D. thesis. University of Tasmania, Hobart, Tasmania, Australia.
- 57.Steward, C. C., S. C. Nold, D. B. Ringleberg, D. C. White, and C. R. Lovell. 1996. Microbial biomass and community structures in the burrows of bromophenol producing and non-producing marine worms and surrounding sediments. Mar. Ecol. Prog. Ser. 133:149-165. [Google Scholar]
- 58.Tan, T. L., and H. J. Rüger. 1989. Benthic studies of the Northwest African upwelling region: bacteria standing stock and ETS-activity, ATP-biomass and adenylate energy charge. Mar. Ecol. Prog. Ser. 51:167-176. [Google Scholar]
- 59.Teske, A., K. U. Hinrichs, V. Edgcomb, A. de Vera Gomez, D. Kysela. S. P. Sylva, M. L. Sogin, and H. W. Jannasch. 2002. Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl. Environ. Microbiol. 68:1994-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turley, C. 2000. Bacteria in the cold deep-sea boundary layer and sediment-water interface of the NE Atlantic. FEMS Microbiol. Ecol. 33:89-99. [DOI] [PubMed] [Google Scholar]
- 61.Vetriani, C., H. W. Jannasch, B. J. MacGregor, D. A. Stahl, and A. L. Reysenbach. 1999. Population structure and phylogenetic characterization of marine benthic Archaea in deep-sea sediments. Appl. Environ. Microbiol. 65:4375-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vetter, Y. A., and J. W. Deming. 1994. Extracellular enzyme activity in the Arctic Northeast Water Polynya. Mar. Ecol. Prog. Ser. 114:23-34. [Google Scholar]
- 63.Viso, A. C., and J. C. Marty. 1993. Fatty acids from 28 marine microalgae. Phytochemistry 34:1521-1533. [Google Scholar]
- 64.von Wintzingerode, F., U. B. Göbel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]
- 65.Wakeham, S. G. 1999. Monocarboxylic, dicarboxylic and hydroxy acids released by sequential treatments of suspended particles and sediments of the Black Sea. Org. Geochem. 30:1059-1074. [Google Scholar]
- 66.Weinbauer, M. G., C. Beckmann, and M. G. Höfle. 1998. Utility of green fluorescent nucleic acid dyes and aluminum oxide membrane filters for rapid epifluorescence enumeration of soil and sediment bacteria. Appl. Environ. Microbiol. 64:5000-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White, D. C., W. M. Davis, J. S. Nichols, J. D. King, and R. J. Bobbie. 1979. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51-62. [DOI] [PubMed] [Google Scholar]
- 68.Xiao, C., P. Zhou, and D. Wang. 1990. Identification and growth temperature characteristics of psychrophiles from Antarctic sediments. Acta Microbiol. Sin. 30:239-242. [Google Scholar]
- 69.Zimmerman, A. R., and E. A. Canuel. 2001. Bulk organic matter and lipid biomarker composition of Chesapeake Bay surficial sediments as indicators of environmental processes. Estuar. Coast. Shelf. Sci. 53:319-341. [Google Scholar]