Abstract
Biogeochemical transformations occurring in the anoxic zones of stratified sedimentary microbial communities can profoundly influence the isotopic and organic signatures preserved in the fossil record. Accordingly, we have determined carbon isotope discrimination that is associated with both heterotrophic and lithotrophic growth of pure cultures of sulfate-reducing bacteria (SRB). For heterotrophic-growth experiments, substrate consumption was monitored to completion. Sealed vessels containing SRB cultures were harvested at different time intervals, and δ13C values were determined for gaseous CO2, organic substrates, and products such as biomass. For three of the four SRB, carbon isotope effects between the substrates, acetate or lactate and CO2, and the cell biomass were small, ranging from 0 to 2‰. However, for Desulfotomaculum acetoxidans, the carbon incorporated into biomass was isotopically heavier than the available substrates by 8 to 9‰. SRB grown lithoautotrophically consumed less than 3% of the available CO2 and exhibited substantial discrimination (calculated as isotope fractionation factors [α]), as follows: for Desulfobacterium autotrophicum, α values ranged from 1.0100 to 1.0123; for Desulfobacter hydrogenophilus, the α value was 0.0138, and for Desulfotomaculum acetoxidans, the α value was 1.0310. Mixotrophic growth of Desulfovibrio desulfuricans on acetate and CO2 resulted in biomass with a δ13C composition intermediate to that of the substrates. The extent of fractionation depended on which enzymatic pathways were used, the direction in which the pathways operated, and the growth rate, but fractionation was not dependent on the growth phase. To the extent that environmental conditions affect the availability of organic substrates (e.g., acetate) and reducing power (e.g., H2), ecological forces can also influence carbon isotope discrimination by SRB.
Sulfate-reducing bacteria (SRB) have been identified in a wide variety of anoxic environments, including microbial mats and sediments that have potential for preservation in the fossil record. SRB can account for a substantial fraction of the carbon turnover within cyanobacterial mats and thus contribute to internal biogeochemical carbon cycling, which in turn affects the sedimentary and geochemical features of the mats (5). SRB are metabolically versatile and can degrade a wide variety of organic compounds heterotrophically, while some can also grow autotrophically, fixing inorganic CO2 into central metabolic intermediates like acetyl coenzyme A (acetyl-CoA) (27). Numerous methods are now available for measuring the impact of these organisms on sulfur transformations in present and ancient microbial communities (13). However, much less is known about carbon recycling by SRB in natural habitats, where multiple substrates are available in low concentrations. One approach for determining which carbon sources are being metabolized by SRB is to track their activities based on their ability to release isotopically distinct products into their surroundings. Although labeling studies have been performed with 13C-enriched substrates (3), interpretations based on isotopic fractionation by SRB are relatively novel. To calibrate isotopic data for specific processes, species, or environmental parameters, isotope effects must be determined for different SRB growing both heterotrophically with a limiting substrate and autotrophically.
Measuring the impact of SRB on stable carbon isotopes offers a potential means of evaluating the carbon transformations carried out by these bacteria, as well as of measuring the impact of SRB on the stable carbon isotope compositions of other community members. The degree of carbon isotope fractionation for a variety of anaerobes seems to depend upon the metabolic pathways utilized, with the greatest fractionation observed for organisms using the acetyl-CoA-carbon monoxide dehydrogenase (CODH) pathway (16). Microbes can also affect the isotopic compositions of their neighbors by releasing isotopically light products or by consuming competitive substrates. For example, in environments where SRB outcompete methanogens for H2 or acetate, methanogen populations will produce less methane and the isotopic composition of this methane will change accordingly. These alterations in the quantity and isotopic composition of methane can in turn affect the isotopic signature of methanotrophs. Thus, all organisms in an environment can directly or indirectly affect the isotopic compositions of other community members.
We investigated stable carbon isotope fractionation for four mesophilic SRB. The SRB were chosen because their pathways for acetate catabolism and lithotrophic growth have been established previously and because their physiology has been characterized (see Table 1). We measured stable carbon isotope discrimination during acetate degradation and lithoautotrophic growth of Desulfobacterium autotrophicum, which uses CODH and the acetyl-CoA pathway for both the formation and cleavage of acetyl-CoA (17, 18). Similarly, the gram-positive Desulfotomaculum acetoxidans uses the acetyl-CoA pathway (22) and was included for comparisons between phyla. For contrast, we included Desulfobacter hydrogenophilus, which uses a modified tricarboxylic acid (TCA) cycle for acetate metabolism and autotrophic growth (19). We also determined the isotopic effects for the incomplete oxidizer Desulfovibrio desulfuricans grown heterotrophically with lactate or mixotrophically with hydrogen as the electron donor and acetate and CO2 for biosynthesis. With this study, we began the process of teasing apart the isotopic effects of the assimilatory and dissimilatory reactions carried out by SRB and started to decipher the effects that metabolic pathways and environmental parameters have on carbon isotope signatures for these organisms. We contrasted the isotope discrimination abilities of microorganisms that use fundamentally different pathways for carbon assimilation, each of which has a long evolutionary history that transcends these individual organisms. We compared the isotope effects for these organisms during lithoautotrophic growth under conditions in which only a small fraction of CO2 is utilized, as might be encountered in subsurface or hydrothermal systems where inorganic reduced species such as hydrogen are available. In such systems, autotrophic sulfate reducers might have played an important role as primary producers; therefore, carbon isotope studies of SRB are important for interpreting the early geologic record. We have also examined these organisms during heterotrophic growth under conditions where all organic substrate is utilized, as would be encountered in most detrital systems. By investigating fundamental parameters in pure cultures, we hoped to establish a basis for determining the modes of metabolism for SRB within their natural ecosystems.
TABLE 1.
Evidence for growth and sulfate reduction in batch cultures of four SRB grown lithotrophically and heterotrophicallya
| Species and growth phase | Lithotrophic growth characteristic
|
Heterotrophic growth characteristic
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism characteristics | Pressure (lb/in2) | pH | OD600 | Protein concn (mg/ml) | Sulfate concn (mM) | Sulfide concn (mM) | Organism characteristics | Pressure (lb/in2) | pH | OD600 | Protein concn (mg/ml) | Sulfate concn (mM) | Sulfide concn (mM) | |
| Desulfovibrio desulfuricans | Gram-negative mixotroph with CO2-acetate | Gram-negative, incomplete oxidizer | ||||||||||||
| Initial | 15.1 | 7.09 | 0.068 ± 0.000 | 0.02 ± 0.000 | 21.3b ± 1.0 | ND | 10.0 | 6.49 | 0.049 ± 0.005 | 0.11 ± 0.01 | 19.9 ± 0.8 | 0.4 ± 0.3 | ||
| Final 1 | 8.2 | 7.45 | 0.164 ± 0.002 | 4.17 ± 0.24 | 0.0 ± 0.0 | 17.7 ± 3.7 | 10.8 | 6.72 | 0.224 ± 0.008 | 1.74 ± 0.04 | 13.9 ± 0.2 | 4.3 ± 0.1 | ||
| Final 2 | 7.7 | 7.40 | 0.167 ± 0.001 | 4.09 ± 0.13 | 0.0 ± 0.0 | 18.7 ± 0.9 | 10.9 | 6.72 | 0.229 ± 0.028 | 2.18 ± 0.28 | 14.0 ± 0.4 | 4.7 ± 0.3 | ||
| Desulfotomaculum acetoxidans | Gram-positive autotroph, acetyl-CoA pathway | Gram-positive, complete oxidizer, acetyl-CoA pathway | ||||||||||||
| Initial | 15.1 | 7.07 | 0.041 ± 0.000 | 0.03 ± 0.00 | 19.0 ± 0.3 | ND | 10.0 | 6.54 | 0.033 ± 0.003 | 0.15 ± 0.01 | 18.4 ± 0.2 | 0.3 ± 0.0 | ||
| Final 1 | 8.7 | 7.37 | 0.142 ± 0.011 | ND | 4.4 ± 1.1 | ND | 8.7 | 6.95 | 0.131 ± 0.004 | ND | 13.6 ± 0.2 | ND | ||
| Final 2 | 9.9 | 7.38 | 0.264 ± 0.016 | 3.18 ± 0.18 | 5.3 ± 1.0 | 12.0 ± 0.7 | 9.0 | 6.93 | 0.221 ± 0.010 | 1.64 ± 0.16 | 13.6 ± 0.2 | 3.4d ± 0.3 | ||
| Desulfobacter hydrogenophilus | Gram-negative autotroph, modified TCA cycle | Gram-negative, complete oxidizer, modified TCA cycle | ||||||||||||
| Initial | 15.1 | 6.96 | 0.060 ± 0.000 | 0.03 ± 0.00 | 19.2 ± 0.4 | ND | 10.0 | 6.43 | 0.097 ± 0.002 | 0.04 ± 0.00 | 18.4 ± 0.8 | 0.7 ± 0.0 | ||
| Final 1 | 7.7 | 7.39 | 0.378 ± 0.013 | 4.28 ± 0.26 | 0.1 ± 0.1 | 19.4 ± 0.2 | 9.8 | 7.07 | 0.360 ± 0.017 | 0.86 ± 0.04 | 7.8 ± 0.8 | 7.4 ± 0.5 | ||
| Final 2c | 15.2 | 7.10 | 0.037 ± 0.002 | 0.38 ± 0.28 | 18.4 ± 0.7 | 0.0 ± 0.0 | 9.8 | 7.09 | 0.381 ± 0.018 | 1.87 ± 0.08 | 8.4 ± 0.7 | 6.4 ± 0.3 | ||
| Desulfobacterium autotrophicum | Gram-negative autotroph, acetyl CoA pathway | Gram-negative, complete oxidizer, acetyl-CoA pathway | ||||||||||||
| Initial | 14.7 | 6.98 | 0.068 ± 0.000 | 0.04 ± 0.02 | 18.1 ± 0.2 | ND | 10.0 | 6.41 | 0.090 ± 0.001 | 0.27 ± 0.02 | 18.8 ± 0.4 | 0.4 ± 0.1 | ||
| Final 1 | 6.4 | 7.41 | 0.884 ± 0.001 | 4.65 ± 0.24 | 0.1 ± 0.0 | 22.0 ± 0.5 | 9.8 | 7.07 | 0.344 ± 0.008 | 2.32 ± 0.09 | 8.5 ± 1.5 | 7.8 ± 0.3 | ||
| Final 2 | 6.3 | 7.39 | 0.424 ± 0.002 | 4.66 ± 0.14 | 0.1 ± 0.0 | 20.8 ± 3.0 | 9.8 | 7.05 | 0.359 ± 0.015 | 0.72 ± 0.09 | 8.0 ± 1.4 | 7.5 ± 0.7 | ||
Sterile controls, under lithotrophic- and heterotrophic-growth conditions (respectively), were unchanged in pH (7.09 ± 0.01 and 6.51 ± 0.01), optical density (OD) (0.013 ± 0.003 and 0.014 ± 0.003), protein concentration (0.03 ± 0.00 and 0.01 ± 0.00 mg/ml), sulfate concentration (19.79 ± 0.82 and 22.53 ± 1.99 mM), sulfide concentration (0.44 ± 0.28 and 0.88 ± 0.20 mM), acetate concentration (0.0 ± 0.0 and 10.5 ± 2.6 mM), and pressure (15.0 ± 1.2 and 9.6 ± 0.2 lb/in2) over the duration of the incubation (380 and 168 h). ND, not determined.
Sample was compromised before sulfate was analyzed. Value shown is for an uninoculated control harvested at the same time.
This culture became oxidized due to stopper failure and did not grow.
Values are from a sample taken before substrate degradation was complete.
MATERIALS AND METHODS
Organisms and cultivation.
Desulfobacterium autotrophicum (ATCC 43914), Desulfobacter hydrogenophilus (ATCC 43915), Desulfovibrio desulfuricans (ATCC 29577), and Desulfotomaculum acetoxidans (ATCC 49208) were obtained from the American Type Culture Collection. The first two bacteria were grown in saltwater mineral medium and the last two were grown in freshwater mineral medium (26), both with resazurin redox indicator and 20 mM sulfate, and then reduced with 0.6 mM sulfide and 20 mg of dithionite per liter just prior to inoculation. The media included a bicarbonate-CO2 buffer which was necessary to promote the growth of some SRB, even heterotrophically. Cultures were prepared in sealed 2.2-liter medium bottles that were modified with severed anaerobic tubes held at the neck with butyl rubber stoppers and sealed with butyl rubber stoppers and aluminum crimp seals. Aseptic and anaerobic techniques were used for preparation of media and subsequent manipulations.
To prepare inocula for experiments, each organism was first grown to stationary phase under heterotrophic or lithotrophic conditions. For lithotrophic- or autotrophic-growth experiments, the organisms underwent at least three consecutive transfers into fresh medium before the inoculum was prepared, to ensure that the organisms were not growing with residual organic substrate. Great care was taken to ensure that the cultures were axenic, as Desulfotomaculum acetoxidans was originally described as not growing autotrophically (28). Cells were checked microscopically and then concentrated by centrifugation (10,000 × g, 20 min), and the resulting cell suspension was used as an inoculum that was equally divided among replicate cultures for the isotope fractionation experiments. The total contribution of liquid to the experimental culture vessels was thus minimized (<3%, vol/vol). All incubations were carried out at 30°C with rotary shaking (100 rpm) in the dark.
Lithotrophic versus heterotrophic growth.
Each of the four organisms was grown under both lithotrophic and heterotrophic conditions. Three replicate cultures were prepared for each of these modes of growth. One of each set of cultures was harvested immediately after inoculation to determine the initial values of substrates and the contribution of the inoculum to the final biomass, whereas the other two replicates were harvested after the substrate (organic or sulfate) was depleted. For both lithotrophic and heterotrophic conditions, duplicate sterile controls were also prepared in a fashion identical to that of freshwater medium except that no inoculum was added. In addition, experiments were performed for both autotrophic and heterotrophic growth of Desulfobacterium autotrophicum in which five identical cultures were prepared and each was sacrificed at different stages of the growth phase from initial to stationary phases.
For heterotrophic-growth experiments, 1,400 ml of medium was supplemented with 1.05 g of NaHCO3 and 10 mM sodium acetate or sodium lactate, with an N2-CO2 (80:20) gas phase with 10 lb/in2 of overpressure. For Desulfotomaculum acetoxidans, the acetate concentration was reduced to 5 mM to avoid sulfide toxicity effects (28). For lithotrophic-growth experiments, 350 ml of medium was supplemented with 2.6 g of NaHCO3 (and 10 mM acetate for the growth of Desulfovibrio desulfuricans), with an H2-CO2 (80:20) gas phase with 14.5 lb/in2 of overpressure.
Sampling and biomass collection.
Pressure was monitored frequently for all cultures. Samples of the liquid phase were taken initially and periodically thereafter for substrate analyses. Progress was monitored by determining the consumption of substrate for heterotrophic cultures and the pressure decrease in lithotrophic cultures. Cultures were harvested as they entered stationary phase unless otherwise indicated.
Each flask was harvested by first measuring the pressure in the vessel and then transferring triplicate 5-ml gas samples to evacuated 10-ml vials for measurement of δ13C in CO2. The CO2 in the gas phase was assumed to be in equilibrium with inorganic carbon in the liquid phase, which is a reasonable assumption considering the relatively low growth rates of these SRB. Duplicate 10-ml liquid samples were filtered (0.2-μm-pore-size Acrodisc filter; Gelman Sciences, Ann Arbor, Mich.) into evacuated serum vials, and then liquid samples for substrate, sulfate, and absorbance measurements were collected. The stoppers were removed from the culture vessels, and the pHs were measured immediately; the liquid volume and total vessel volume were then measured. The culture fluid was centrifuged (10,000 × g at 4°C for 30 min), and the cell pellets were pooled and resuspended in 10 ml of unamended freshwater medium. A sample of the supernatant was preserved in a sealed serum vial. The biomass was then frozen (−45°C) and lyophilized, and its dry weight was determined. All liquid and biomass samples were stored frozen until analyzed.
Isotope analyses.
The 13C/12C abundance ratio for purified CO2 samples can be measured using a mass spectrometer. For gas phase samples, copper sulfate (1 ml, 0.5 M, pH <2) was added to the sample bottles to trap sulfide, and the CO2 was cryogenically purified. CO2 was first separated from N2 or H2 by trapping it in a cold finger, and then the trap was warmed and the CO2 was purified as previously described (8) with the variable-temperature trap at −127°C. An isotopic CO2 standard was purified and collected the same way with each set of samples. The standard error of the mean for the CO2 standard was 0.28‰ (number of samples, 7) by this method, and blank samples constituted ≤1% of sample carbon yields.
The isotopic composition of the dried biomass was determined in triplicate by combustion in evacuated, sealed quartz tubes containing silver and CuO at 800°C for 2 h (9) following acidification with H3PO4 to remove inorganic carbon. The CO2 product was purified as previously described (10). The substrates (acetate and lactate) were likewise combusted but without acidification. An isotope standard, NBS-22, was processed with each batch of samples. Following purification, CO2 in the gaseous phase [CO2(g)] yields were determined and concentrations were calculated for dissolved inorganic carbon species in the liquid phase (12). The δ13C value of the NBS-22 carbon isotope standard was determined to be −29.68 ± 0.04 (mean ± standard deviation of the mean; n = 5), compared to the accepted values of −29.73 ± 0.09. Combustion blanks had carbon yields of less than 1% of the yields for final biomass values but as much as 10 to 20% of the initial biomass values, so the initial values must be interpreted with some caution.
Cryogenically purified CO2 was analyzed with a Nuclide 6-60 mass spectrometer (Nuclide Corporation, State College, Pa.). The measured isotopic composition was expressed as δ13C, which can be defined as follows:
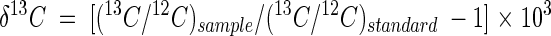 |
(1) |
where (13C/12C)sample is the 13C/12C abundance ratio for a sample and (13C/12C)standard is the 13C/12C abundance ratio for the Peedee Belemnite carbonate standard. Values of δ13C therefore represent differences, in parts per thousand (per mile), between the 13C/12C value of a sample and that of the standard.
In the lithotrophic-growth experiments, carbon isotopic fractionation for the conversion of substrates to products is expressed as
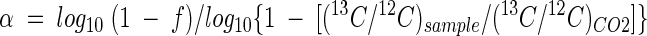 |
(2) |
where f is the amount of C in harvested biomass divided by the amount of C in inorganic carbon at the beginning of the incubation and (13C/12C)CO2 represents the CO2 at the beginning of the incubation. For heterotrophic-growth experiments, carbon isotope differences between substrates and products (biomass) were expressed as the change in δ13C notations (Δδ13C), indicating the deviation of 13C content of the cell biomass from that of the CO2 or organic substrate.
The total inorganic carbon (TIC) in the culture flasks was distributed in several forms, not all of which could be measured directly. Therefore, for mass balance calculations, the mole fractions of the various forms of inorganic carbon were calculated from the amounts of CO2(g) measured in headspace samples from each flask and the measured pH according to the equations and from the equilibrium and solubility constants of Stumm and Morgan (23). The isotopic compositions were calculated from the measured δ13C value of CO2(g) with correction for relevant equilibrium isotope fractionation effects (7) as described by Gelwicks et al. (12). For each flask, both the amount and the isotopic composition of the TIC pool were thus calculated. The isotopic composition of the TIC released from the degradation of organic substrates was determined by a mass balance calculation, such that
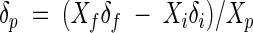 |
(3) |
where δp and Xp represent the isotopic composition and mole fraction of TIC released from the substrate and δf, δi, Xf, and Xi represent the isotopic compositions and mole fractions from the final and initial inorganic carbon pools, respectively (Xi + Xp = Xf = 1).
Analytical methods.
Pressure in the vessels was measured with a handheld pressure gauge (Cole-Parmer, Vernon Hills, Ill.). Cell culture fluids optical density was measured at 600 nm with a Cary spectrophotometer (Varian, Palo Alto, Calif.). Protein was determined with a BCA-200 protein assay kit with bovine serum albumin used as a standard (Pierce Chemical Co., Rockford, Ill.). Sulfate was analyzed by ion chromatography with an IC system, an AS4A column, and a CD20 conductivity detector (all from Dionex, Sunnyvale, Calif.). The eluant was 1.8 mM sodium carbonate-1.7 mM sodium bicarbonate, with elution at 2 ml/min. Sulfide was measured colorimetrically by a modified methylene blue assay as previously described (6). Acetate and lactate were analyzed by high-performance liquid chromatography with an Aminex HPX-87 organic acid column (Bio-Rad Laboratories, Richmond, Calif.) and a System Gold high-performance liquid chromatograph (Beckman Instruments Inc., Berkeley, Calif.). The eluant was 0.01 M H2SO4, with elution at 0.9 ml/min with UV detection (wavelength, 204 to 212 nm) with a photodiode array detector. Concentrations of protein, sulfide, sulfate, lactate, and acetate were determined by comparison of peak areas to external standards. All samples were analyzed in triplicate (n = 3) except that duplicate analyses were performed for the sulfide assay.
RESULTS
All four SRB grew under both lithotrophic- and heterotrophic-growth conditions. During lithotrophic growth, the consumption of hydrogen was indicated by a pressure decrease. Metabolism usually ceased when sulfate was depleted (Table 1). Heterotrophic growth proceeded until the organic substrates were consumed. Changes in pressure, pH, optical density, and protein, sulfate, and sulfide concentrations are shown in Table 1.
Lithotrophic growth of the four SRB permitted a relatively straightforward interpretation of carbon flow and isotope effects in these systems. Closed-system isotope effects were not encountered during lithoautotrophic growth, as evidenced by the fact that the carbon isotope composition of the remaining CO2 was unchanged and because less than 3% of the total TIC pool was incorporated into biomass (data not shown). Likewise, when Desulfovibrio desulfuricans was grown mixotrophically, acetate for biosynthesis was supplied in excess, and because an incorporation level of less than 10% was measured during this growth experiment, the effect on the isotopic composition of the acetate pool was minimal. Patterns of isotopic discrimination associated with lithotrophic growth are shown in Fig. 1. Isotope fractionation factors (α) were calculated between cell biomass and gaseous CO2 to facilitate comparison with previously published values, although the CO2 was in equilibrium with bicarbonate and carbonate ions and the actual inorganic carbon substrates used by the enzymes of these four organisms have not been determined. The contribution of biomass from the inoculum (initial) did not substantially alter the isotopic values of the final products because the inoculum represented a small percentage of the final harvested biomass and because the isotopic compositions of the inoculum and final biomass were similar (data not shown).
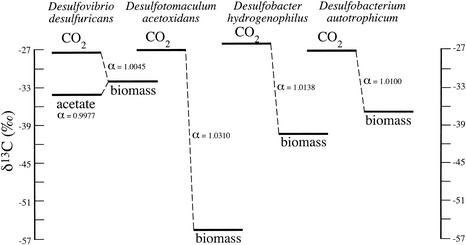
FIG. 1.
Stable carbon isotope values for substrates and biomass for four SRB grown lithotrophically with excess HCO3− and CO2. Each bar represents the mean isotopic value of the substrate (CO2 or acetate) or biomass expressed relative to PDB (‰). Fractionation (α) is noted for the conversion of CO2(g) and acetate into biomass, as indicated by the dashed lines.
A wide range in the magnitude of isotope discrimination was observed for the four organisms grown lithotrophically (Fig. 1). For Desulfovibrio desulfuricans, fractionation factors were small, as calculated from both CO2 and acetate. Isotope fractionation values (α) for Desulfobacter hydrogenophilus and Desulfobacterium autotrophicum were similar, being 1.0138 and 1.0100, respectively. Desulfotomaculum acetoxidans yielded the largest fractionation, with an α value of 1.0310 (Fig. 1). Desulfobacterium autotrophicum and Desulfotomaculum acetoxidans both excreted approximately 1 to 3 mM acetate into the medium when grown lithoautotrophically.
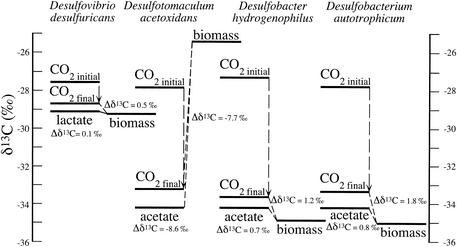
The interpretation of isotope discrimination during heterotrophic growth of these organisms is more complicated. Heterotrophic growth of all four SRB strains in these experiments resulted in complete conversion of the organic substrates to inorganic carbon and biomass. Therefore, in interpreting our results, the isotope discrimination of the process of selective uptake of acetate and CO2 for biosynthesis (assimilation) and that of the process of release of CO2 from degradation (dissimilation) must be distinguished. Furthermore, the CO2 released from acetate (or lactate) degradation mixes with the CO2 present in the culture vessels initially. Consequently, the isotopic value of the total CO2 pool can change over the course of the reaction. The carbon released by organic substrate degradation was usually isotopically lighter than that of the initial inorganic carbon pool, resulting in a net lowering of the 13C composition of the CO2(g) (Fig. 2). The TIC pool increased by approximately 10 to 20 mmol, depending upon how much organic carbon was added initially. Differences in isotopic compositions (Δδ13C) (Fig. 2) were calculated based on the δ13C values of the final CO2 pools, which consisted of the initial CO2 in the vessel and the CO2 released from acetate. All four organisms use both acetyl-CoA and CO2 for biosynthesis; therefore, the total isotope fractionation exhibited during growth includes the effects of enzymatic isotope discrimination and carbon flows at branch points in the reaction network associated with the incorporation of acetate and CO2 into biomass. The situation is further complicated by the exchange between the carboxyl group of acetate and CO2 for organisms with CODH (17). For simplicity, it is most useful to evaluate the overall isotope discrimination associated with the conversion of organic substrates to biomass. Such overall discrimination is typically small, less than 1‰ for Desulfovibrio desulfuricans, Desulfobacter hydrogenophilus, and Desulfobacterium autotrophicum (Fig. 2). The unusual exception was Desulfotomaculum acetoxidans, for which the biomass was isotopically heavier than either potential carbon source, acetate or CO2. This result suggests that, at a critical branching point, this organism must selectively release 12C as CO2 and the remaining 13C-enriched acetyl-CoA is incorporated into biomass. If this explanation prevails, then the CO2 released should be isotopically lighter than the CO2 released by the other acetate degraders. However, the amount of acetate incorporated into biomass is small relative to the amount of dissimilated CO2 added to the TIC pool. Thus, this discrimination would be difficult to estimate precisely by measuring (small) changes in the isotopic composition of the TIC pool.
FIG. 2.
Stable carbon isotope values for substrates and products from four SRB grown heterotrophically. Each bar represents the mean isotopic value of the substrate (acetate or lactate and CO2) or products (biomass and CO2) expressed relative to PDB (‰). Both the initial and final δ13C values for CO2(g) are indicated, with a dashed arrow indicating the trend towards lower values. Fractionation (Δδ13C) (dashed lines) is indicated for the conversion of acetate or lactate and of the final CO2 pool into biomass.
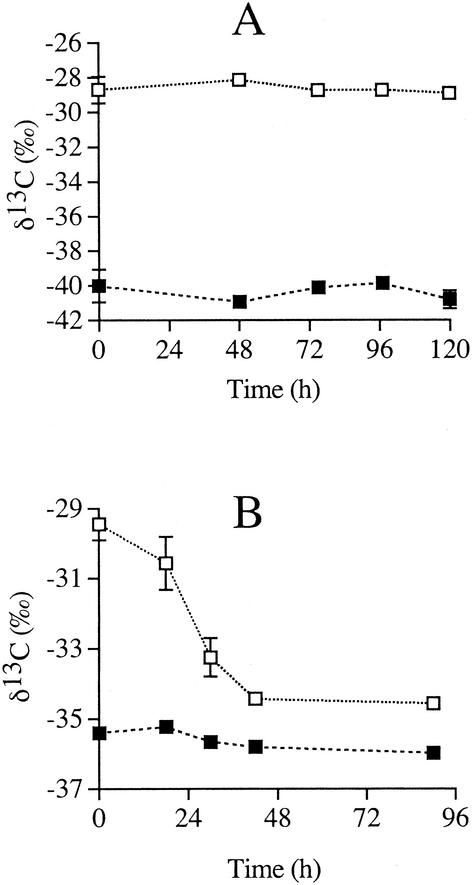
In order to distinguish between the effects of substrate uptake and subsequent reactions in assimilatory pathways, isotopic discrimination was monitored for Desulfobacterium autotrophicum over a time course during heterotrophic and lithoautotrophic growth. As before, lithotrophic growth was accompanied by a decrease in pressure, a depletion of sulfate, and an increase in pH (data not shown). However, there was no change in the isotopic composition of the CO2(g) and the biomass in cultures harvested during different stages of the growth phase (Fig. 3). Therefore, the observed isotope discrimination was not complicated by growth phase effects, and closed-system effects were not encountered during lithotrophic growth. The fractionation factor α, estimated from the average CO2 and biomass δ13C values, was 1.0123, slightly higher than that found in the previous experiment (Fig. 1). Heterotrophic growth of Desulfobacterium autotrophicum with acetate did result in changes to the isotopic composition of the TIC pool as product CO2 was released from acetate degradation as discussed previously. As acetate was consumed, the biomass became slightly lighter isotopically, as the effect of a slightly heavier inoculum was diluted by new growth (Fig. 3). However, the consistent isotopic difference between biomass product and acetate substrate during growth indicates that little overall isotopic discrimination occurred during acetate uptake by this organism. The isotope fractionation between final CO2 and biomass, Δδ13C, was 1.3‰, and that between acetate and biomass was 1.6‰; these values were similar to the values measured previously (Fig. 2).
FIG. 3.
Stable carbon isotope compositions for Desulfobacterium autotrophicum grown lithotrophically (A) and heterotrophically (B). The δ13C values for CO2 (▪) and biomass (□) are shown with error bars indicating ± 1 standard deviation.
DISCUSSION
Stable carbon isotope fractionation differed considerably for the four SRB studied. The greatest fractionation was observed when the SRB were grown autotrophically, the same conditions under which acetogens have been shown to have large fractionation factors (16). A modest isotope effect was measured for Desulfobacter hydrogenophilus, which uses a modified reductive citric acid cycle, whereas the two organisms using the acetyl-CoA synthetase pathway, Desulfotomaculum acetoxidans and Desulfobacterium autotrophicum, exhibited widely different fractionations. One obvious conclusion from this study, and from other studies with a variety of bacteria (see reference 16 for examples), is that isotope effects depend on much more than the pathways utilized and that generalizations about isotopic discrimination by specific enzymes such as CODH across different species cannot easily be made.
Stable carbon isotope fractionation depended on the mode of growth of Desulfobacterium autotrophicum. Grown heterotrophically on acetate, the biomass was only slightly lighter isotopically than the substrate, whereas values for 1,000 ln α of 10.0 to 12.2 were observed for autotrophic growth. Thus, even though the same pathway and central enzyme (CODH) are used for acetate synthesis and degradation in this organism, isotopic discrimination is very different depending on the direction in which the pathway operates. This could be due to the use of different isoforms of CODH, which has been suggested as a means for regulating the direction in which the pathway operates (18). In addition to the incorporation of two carbon atoms into acetyl-CoA by this enzyme, the organism also employs carboxylation reactions for the synthesis of metabolic intermediates. Approximately one-third of its biomass derives from CO2 even when the organism is growing on acetate. The isotopic discrimination observed during heterotrophic growth of Desulfobacterium autotrophicum was likely due to either acetyl-CoA being more isotopically selective for biosynthesis (assimilation) than for catabolism for energy (dissimilation) or to the selective incorporation of 12CO2 for carboxylation reactions. However, the subtle differences required to establish the dominant explanation could not be distinguished in our system.
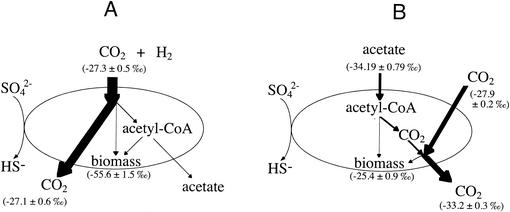
Desulfotomaculum acetoxidans also uses CODH and the acetyl-CoA pathway, yet the isotope effects for both lithotrophic and heterotrophic growth were remarkably different from those of Desulfobacterium autotrophicum. Indeed, of all the organisms studied here, Desulfotomaculum acetoxidans exhibited the greatest potential difference between biomass isotope values for autotrophic and heterotrophic growth. Desulfotomaculum acetoxidans uses an oxidative acetyl-CoA/CODH pathway with tetrahydrofolate as the methyl carrier, just like the acetogen Acetobacterium woodii, whereas Desulfobacterium autotrophicum requires a tetrahydropterin carrier. Substantial carbon isotope discrimination (ɛ, ∼59‰) has been measured for the synthesis of acetate from CO2 and H2 by this organism (12). Acetyl-CoA can be either incorporated into biomass or converted to acetate, yielding CO2 and energy (Fig. 4). There may be an isotope effect associated with this branching point, so that in Desulfotomaculum acetoxidans, the CODH may select [12C]acetyl-CoA for cleavage just as the enzyme does for the reverse reaction in acetogens. If this is the case, then 13C-enriched acetyl-CoA would remain for incorporation into biomass, explaining why the biomass for Desulfotomaculum acetoxidans was so isotopically heavy when grown on acetate. To our knowledge, the isotope value for A. woodii growing heterotrophically on acetate has not been determined; therefore, this potential mechanism can be neither confirmed nor denied as the source of the isotopic fractionation. Fuchs et al. (11) observed a similar phenomenon for a methanogen grown autotrophically at low gassing rates, such that the completeness of substrate usage and the balance between carbon flowing along the dissimilation and assimilation pathways created biomass that was isotopically heavier than the carbon source. Additional slight differences in the enzymes between organisms may contribute to isotopic variability. Although the CODH in the gram-positive Desulfotomaculum acetoxidans may catalyze the same reactions as its gram-negative counterpart, Desulfobacterium autotrophicum, the reaction centers and kinetics may be substantially different.
FIG. 4.
Schematic representations of metabolic pathways for lithotrophic (A) and heterotrophic (B) growth of Desulfotomaculum acetoxidans by using the acetyl-CoA/CODH pathway. Line thickness corresponds to millimoles of carbon.
Desulfobacter hydrogenophilus utilizes the modified citric acid cycle for autotrophic growth. During autotrophic growth, isotope discrimination observed in the present study (α = 1.0138; 1,000 ln α = 13.7‰) was similar to that reported previously by Preuß et al. (16) (Δδ13C range, −8.4‰ to −13.3‰). In the heterotrophic-growth experiments, the biomass was depleted by approximately 2‰ in 13C relative to the final CO2. This small difference resembles the small difference observed for Desulfobacterium autotrophicum, suggesting that the carbon isotopic discrimination during biosynthesis was relatively minor for both organisms.
Previous studies with 14CO2 have shown that Desulfovibrio desulfuricans incorporates CO2 and acetate in a 1:1 ratio (C ratio, 1:2) (4). In our experiments, the isotopic composition of the biomass was consistent with the incorporation of carbon in a 1:2 ratio from these sources (Fig. 3). Thus, actual discrimination, or selective incorporation of 12C from either lactate or the CO2 or acetate sources, was insignificant. This was not due to closed-system isotope effects, because for mixotrophic growth the substrates were supplied in excess and for heterotrophic growth no change in biomass δ13C values was observed over the course of a separate growth phase experiment (data not shown). No attempt was made to determine intramolecular isotope effects for the decarboxylation of lactate, so this possibility cannot be ruled out, although such effects would have to have been small. Due to differences in experimental design, it is not possible to compare our results directly to those of the previous studies of Kaplan and Rittenberg (15) with Desulfovibrio desulfuricans, in which biomass and the CO2 release by decarboxylation of lactate were isotopically lighter than the carboxyl group of lactate by 6.0‰ and 5.5 to 7.8‰, respectively. Others (21) have reported that a Desulfovibrio gigas organism grown on lactate released CO2 that was depleted in 13C by 8 to 16‰ compared to that of the initial substrate, with the isotope effect proportional to the rate of CO2 release. Therefore, Desulfovibrio spp. apparently can release isotopically light CO2 from lactate, although we did not observe this in our study.
Despite the metabolic diversity of the group of SRB examined in this study, the overall isotopic discrimination during heterotrophic growth at the expense of lactate or acetate is typically minimal, with the notable exception of that for Desulfotomaculum acetoxidans as discussed above. Fractionation effects associated with other forms of heterotrophic growth of microorganisms are generally small, less than 3‰ (1, 14). To our knowledge, this is the first report of carbon isotope fractionation associated with the oxidation of acetate by either the acetyl-CoA or modified tricarboxylic acid cycle in anaerobes. These results will be very useful for assessing the effects of SRB on carbon isotope distributions in various environments. For example, our results support the contention of Blair and Carter (2) that in sediments of Cape Lookout Bight, acetate utilization by SRB would have little effect on carbon isotope values. However, caution must be used to not extrapolate this reasoning to other environments, as our results from the autotrophic-growth experiments demonstrate.
In our heterotrophic-growth experiments, SRB did not usually change the carbon isotope composition of the CO2 pools except when the organic substrate was isotopically different from the initial CO2 supplied. In some photosynthetic ecosystems, however, TIC would not be as abundant as it was in our experiments, and therefore it may become limiting. In such cases, autotrophic growth of SRB may affect the isotopic composition of inorganic carbon pools by competing for and thus depleting those pools and thus may indirectly affect isotope values of other organisms in microbial consortia. When SRB compete with other organisms such as methanogens that do discriminate isotopically with respect to common substrates such as acetate (24), the rate of acetate consumption by SRB may indirectly affect the isotopic compositions of their neighbors. The similarity in the isotopic compositions of SRB and their organic substrates also implies that it may be possible to correlate SRB with the source of organic substrate in biodegradation studies.
The large difference in isotope discrimination observed between heterotrophic and lithotrophic growth of SRB may provide a means for distinguishing which mode of growth SRB are using in natural environments. In habitats such as marine sediments or microbial mats, the isotopic values of acetate or other organic substrates will likely reflect their origin as breakdown products from photosynthetic organisms. Thus, in typical marine sediments, acetate would be expected to be approximately −15 to −25‰, and measurement of pore water acetate in sediments has in fact revealed values of −17.6‰ in sulfate-reducing zones (2). By using the isotope fractionation factors from this study and the marine δ13C values for CO2 of 0 to −25‰ (25), one could expect autotrophic SRB biomass to be as low as −55‰. In other environments, such as basaltic aquifers, deep-ocean sediments, and hydrothermal vents, where CO2 δ13C values tend to be lighter than surface marine values and the rates of metabolism are low, the biomass of SRB growing autotrophically could be even lighter. Of course, one cannot measure pure SRB biomass in these environments, but the isotopic composition of SRB biomass should correlate with the isotopic composition of the cellular constituents of SRB, including lipid components such as fatty acids.
These laboratory studies of isotopic discrimination by SRB provide useful background information for ecological interpretations of the isotope ratios observed in natural systems. Such work also helps to identify the physiological and biochemical factors that create the greater carbon isotope fractionation that is characteristic of autotrophy. However, care must be taken in extrapolating the results of a limited number of pure culture studies to complex ecosystems in which environmental parameters and microbial interactions may have profound effects on isotopic discrimination. Further investigations are still needed to distinguish between the effects of growth rate and metabolic pathways and to relate the isotopic signatures of cellular constituents such as lipid fatty acids to bulk biomass measurements. This will help to determine, for example, the relative importance of discrimination by particular microbial species versus the effects of growth rate and environmental parameters in creating the carbon isotope patterns observed in natural environments.
Strict anaerobes probably dominated Earth's earliest biosphere; therefore, an understanding of carbon isotopic discrimination associated with the growth and metabolism of modern anaerobes will facilitate the interpretation of the geologic isotopic record of early life on Earth. It has been proposed that the isotopically light carbon typically observed in well-preserved Archean sedimentary rocks was principally due to isotopic discrimination by the enzyme ribulose-5-bisphosphate carboxylase (RubisCO) (for examples, see reference 20). However, given the broad diversity of pathways utilized by anaerobes for inorganic carbon assimilation, the Calvin cycle was probably not the dominant autotrophic pathway in the early biosphere as it is today. As pointed out by Preuß et al. (16), and supported by this study, carbon isotope discrimination by autotrophic anaerobes is more than sufficient to explain these ancient isotopic patterns. Although sulfate reduction might not have been dominant in the Archean eon (13), anaerobes using the acetyl-CoA synthase pathway, and CODH in particular, certainly might have been principal constituents in the ancestral pool of organisms from which modern life was derived. Indeed, the fact that CODH has been found in both the Archaea and the Bacteria, even in deeply branching groups (by 16S rRNA phylogeny) (27), suggests that this enzyme has a long history and may strongly resemble one of the first carbon-fixing enzymes.
Acknowledgments
This work was supported by a grant from the National Aeronautics and Space Administration (NASA) Exobiology Program and by the NASA Astrobiology Institute. Londry was supported by a research associateship from the National Research Council.
We thank A. Tharpe, L. Crumbliss, M. Discipulo, and D. Garcia for technical assistance, G. Cooper for the use of his ion chromatography system, and L. Jahnke, T. Hoehler, and R. Summons for helpful discussions and suggestions for the manuscript.
REFERENCES
- 1.Abraham, W.-R., C. Hesse, and O. Pelz. 1998. Ratios of carbon isotopes in microbial lipids as an indicator of substrate usage. Appl. Environ. Microbiol. 64:4202-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair, N. E., and W. D. Carter, Jr. 1992. The carbon isotope biogeochemistry of acetate from a methanogenic marine sediment. Geochim. Cosmochim. Acta 56:1247-1258. [Google Scholar]
- 3.Boschker, H. T. S., S. C. Nold, P. Wellsbury, D. Bos, W. de Graaf, R. Pel, R. J. Parkes, and T. E. Cappenberg. 1998. Direct linking of microbial populations to specific biogeochemical processes by 13C-labeling of biomarkers. Nature 392:801-805. [Google Scholar]
- 4.Brysch, K., C. Schneider, G. Fuchs, and F. Widdel. 1987. Lithoautotrophic growth of sulfate-reducing bacteria, and description of Desulfobacterium autotrophicum gen. nov., sp. nov. Arch. Microbiol. 148:264-274. [Google Scholar]
- 5.Canfield, D. E., and D. J. Des Marais. 1993. Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochim. Cosmochim. Acta 57:3971-3984. [DOI] [PubMed] [Google Scholar]
- 6.Cline, J. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 7.Deines, P., D. Langmuir, and R. S. Harmon. 1974. Stable carbon isotope ratios and the existence of a gas phase in the evolution of carbonate ground waters. Geochim. Cosmochim. Acta 38:1147-1164. [Google Scholar]
- 8.Des Marais, D. 1978. Variable-temperature cryogenic trap for the separation of gas mixtures. Anal. Chem. 50:1405-1406. [Google Scholar]
- 9.Frazer, J. 1962. Simultaneous determination of carbon, hydrogen and nitrogen. II. An improved method for solid organic compounds. Mikrochim. Acta 1962:993-999.
- 10.Frazer, J., and R. Crawford. 1963. Modifications in the simultaneous determination of carbon, hydrogen and nitrogen. Mikrochim. Acta 1963:561-566. [Google Scholar]
- 11.Fuchs, G., R. Thauer, H. Ziegler, and W. Stichler. 1979. Carbon isotope fractionation by Methanobacterium thermoautotrophicum. Arch. Microbiol. 120:135-139. [Google Scholar]
- 12.Gelwicks, J. T., J. B. Risatti, and J. M. Hayes. 1989. Carbon isotope effects associated with autotrophic acetogenesis. Org. Geochem. 14:441-446. [DOI] [PubMed] [Google Scholar]
- 13.Habicht, K. S., and D. E. Canfield. 1996. Sulphur isotope fractionation in modern microbial mats and the evolution of the sulphur cycle. Nature 382:342-343. [Google Scholar]
- 14.Hayes, J. M. 1993. Factors controlling 13C contents of sedimentary organic compounds: principles and evidence. Mar. Geol. 113:111-125. [Google Scholar]
- 15.Kaplan, I. R., and S. C. Rittenberg. 1964. Carbon isotope fractionation during metabolism of lactate by Desulfovibrio desulfuricans. J. Gen. Microbiol. 34:213-217. [DOI] [PubMed] [Google Scholar]
- 16.Preuß, A., R. Schauder, and G. Fuchs. 1989. Carbon isotope fractionation by autotrophic bacteria with three different CO2 fixation pathways. Z. Naturforsch. 44c:397-402. [Google Scholar]
- 17.Schauder, R., B. Eikmanns, R. K. Thauer, F. Widdel, and G. Fuchs. 1986. Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric acid cycle. Arch. Microbiol. 145:162-172. [Google Scholar]
- 18.Schauder, R., A. Preuß, M. Jetten, and G. Fuchs. 1989. Oxidative and reductive acetyl CoA/carbon monoxide dehydrogenase pathway in Desulfobacterium autotrophicum. 2. Demonstration of the enzymes of the pathway and comparison of CO dehydrogenase. Arch. Microbiol. 151:84-89. [Google Scholar]
- 19.Schauder, R., F. Widdel, and G. Fuchs. 1987. Carbon assimilation pathways in sulfate-reducing bacteria. II. Enzymes of a reductive citric acid cycle in the autotrophic Desulfobacter hydrogenophilus. Arch. Microbiol. 148:218-225. [Google Scholar]
- 20.Schidlowski, M. 1988. A 3,800-million-year isotopic record of life from carbon in sedimentary rocks. Nature 333:313-318.
- 21.Smejkal, V., F. D. Cook, and H. R. Krouse. 1971. Studies of sulfur and carbon isotope fractionation with microorganisms isolated from springs of Western Canada. Geochim. Cosmochim. Acta 35:787-800. [Google Scholar]
- 22.Spormann, A. M., and R. K. Thauer. 1988. Anaerobic acetate oxidation to CO2 by Desulfotomaculum acetoxidans. Demonstration of enzymes required for the operation of an oxidative acetyl-CoA/carbon monoxide dehydrogenase pathway. Arch. Microbiol. 150:374-380. [Google Scholar]
- 23.Stumm, W., and J. Morgan. 1981. Aquatic chemistry. John Wiley & Sons, New York, N.Y.
- 24.Summons, R. E., P. D. Franzmann, and P. D. Nichols. 1998. Carbon isotopic fractionation associated with methylotrophic methanogenesis. Org. Geochem. 28:465-475. [Google Scholar]
- 25.Whiticar, M. J., E. Faber, and M. Schoell. 1986. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—isotope evidence. Geochim. Cosmochim. Acta 50:693-709. [Google Scholar]
- 26.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, New York, N.Y.
- 27.Widdel, F., and T. A. Hansen. 1992. The dissimilatory sulfate- and sulfur-reducing bacteria, p. 583-624. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, New York, N.Y.
- 28.Widdel, F., and N. Pfennig. 1977. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch. Microbiol. 112:119-122. [DOI] [PubMed] [Google Scholar]