Abstract
A Gram-staining technique combining staining with two fluorescent stains, Oregon Green-conjugated wheat germ agglutinin (WGA) and hexidium iodide (HI) followed by flow-cytometric detection is described. WGA stains gram-positive bacteria while HI binds to the DNA of all bacteria after permeabilization by EDTA and incubation at 50°C for 15 min. For WGA to bind to gram-positive bacteria, a 3 M potassium chloride solution was found to give the highest fluorescence intensity. A total of 12 strains representing some of the predominant bacterial species in bulk tank milk and mixtures of these were stained and analyzed by flow cytometry. Overall, the staining method showed a clear differentiation between gram-positive and gram-negative bacterial populations. For stationary-stage cultures of seven gram-positive bacteria and five gram-negative bacteria, an average of 99% of the cells were correctly interpreted. The method was only slightly influenced by the growth phase of the bacteria or conditions such as freezing at −18°C for 24 h. For any of these conditions, an average of at least 95% of the cells were correctly interpreted. When stationary-stage cultures were stored at 5°C for 14 days, an average of 86% of the cells were correctly interpreted. The Gram-staining technique was applied to the flow cytometry analysis of bulk tank milk inoculated with Staphylococcus aureus and Escherichia coli. These results demonstrate that the technique is suitable for analyzing milk samples without precultivation.
Milk is normally sterile in the udder of the cow if the cow doesn't suffer from mastitis (udder infection). If the cow has mastitis, a large number of mainly gram-positive bacteria such as Streptococcus and Staphylococcus species will be present in the milk when it leaves the udder (17, 21). Furthermore, the milk will inevitably be contaminated on its way to the bulk tanks, depending on the hygienic procedures on the farm. Hygienic negligence such as improper cleaning of dirty udders, milking equipment, and the bulk tank, will increase the proportion of gram-negative bacteria in bulk tank milk (6). A method differentiating gram-positive and gram-negative bacterial populations therefore aims at providing more information on the source of the contamination than today's widely used total bacterial count (TBC) methods (15, 18, 30).
The motivation for developing rapid microbiological methods is obvious: obtaining results within minutes instead of days may identify a problem faster and the elimination process can be initiated earlier. Numerous technologies have been developed to provide faster results than cultural methods, with flow cytometry being one of them. Several flow cytometry techniques have been developed, and some are routinely applied in the dairy industry (5, 7, 23, 30). Techniques using antibodies for labeling bacteria prior to flow-cytometric analysis have been developed for Salmonella enterica serovar Typhimurium and Listeria monocytogenes in milk (7, 23). For routine measurements of the TBC of bulk tank milk, the flow cytometry-based BactoScan FC, which uses ethidium bromide to stain bacteria in milk, provides a result after 8 min (5, 30). A flow-cytometric technique for measuring TBC in raw and ultrahigh-temperature milk with the stain SYTO BC has also been described previously (16). However, these techniques are either very specific, detecting one species only, or nonspecific, detecting all bacteria. Flow-cytometric techniques for differentiation of bacteria in milk into larger groups such as gram-positive and gram-negative bacteria have not yet been described.
Gram staining was initially described in 1883 by Christian Gram's colleague Carl Friedlander, dividing bacteria into two groups, and again in 1884 by Christian Gram (12, 14). The conventional Gram-staining procedure includes the stains crystal violet and safranin. Heat-fixed cells on a slide are stained blue-violet with crystal violet, and afterwards, they are treated with ethanol. Gram-negative bacteria are decolorized by ethanol, whereas gram-positive bacteria remain stained with crystal violet. Hereafter, safranin is used to counterstain gram-negative bacteria, giving this group a pink appearance. Numerous alternative approaches to identify the Gram reaction have been reported. A method in which a colony of bacteria is subjected to 3% potassium hydroxide is widely used. The high concentration of potassium hydroxide lyses gram-negative bacteria, releasing DNA from the cells, which can be confirmed by pulling a viscous string from the colony (15, 26).
An alternative Gram-staining technique with a fluorescein-conjugated lectin wheat germ agglutinin (WGA) for combined epifluorescence and a phase-contrast microscope has been described (29). WGA binds to N-acetylglucosamine in the peptidoglycan layer of the cell wall. Gram-negative bacteria have a layer of lipopolysaccharide covering the cell wall and that is why they are not stained with WGA, whereas gram-positive bacteria are stained with WGA, as they do not have a lipopolysaccharide layer. These results showed an insensitivity to the age of the culture which implied that this stain could be used directly on samples without precultivation of the sample. A modified version of the WGA technique has been described for bacteria found in a nonfood environment in which the bacteria are immobilized on a filter, washed, and then stained with WGA (11).
Some Gram-staining techniques for flow cytometry have also been proposed. One uses the fluorescent lipophilic stain rhodamine 123, which selectively stains gram-positive bacteria. The lipopolysaccharide layer of gram-negative bacteria prevents the entry of rhodamine 123 (1, 28). Another similar technique combines the two fluorescent DNA binding stains, SYTO 13 and hexidium iodide (HI). SYTO 13 is a membrane-permeable stain, and HI is blocked by the lipopolysaccharide layer of gram-negative bacteria and thus only permeable to gram-positive bacteria and gram-negative bacteria with a destabilized lipopolysaccharide layer (22). However, due to the fragile nature of the lipopolysaccharide layer, these techniques are not believed to be suitable for uncultivated samples.
The aim of the present work has been to develop a Gram-staining technique based on staining of bacteria with WGA and HI and followed by flow cytometric detection. The technique was evaluated on milk-associated bacteria (19, 20) in the stationary phase and after 6, 12, and 48 h of incubation in laboratory media. Furthermore, the method was tested on stationary-phase cultures which had been stored for 24 h at −18°C and for 14 days at 5°C. Finally, the technique was applied to bulk tank milk spiked with known bacteria. A technique for differentiation of gram-positive and gram-negative bacteria in bulk tank milk without precultivation will be the ultimate application.
MATERIALS AND METHODS
Bacterial strains and maintenance.
A total of 12 strains of bacteria were used in the experiments, including the following strains: from the BCCM/LMG bacterial collection (Ghent, Belgium), Streptococcus dysgalactiae LMG16024; from Foss Electric A/S (Hillerød, Denmark), Escherichia coli (3168); from the American Type Culture Collection (Manassas, Va.), Bacillus cereus ATCC 10796, Enterobacter cloacae ATCC 7256, Klebsiella oxytoca ATCC 8724, Lactococcus lactis ATCC 11454, Micrococcus luteus ATCC 9341, Pseudomonas fluorescens ATCC 49838, Pseudomonas putida ATCC 49128, Staphylococcus aureus ATCC 6538, Streptococcus agalactiae ATCC 27956, and Streptococcus uberis ATCC 9927.
The preparation of cultures was initiated by thawing aliquots of pure cultures that had been stored at −80°C in brain heart infusion (BHI) broth (Oxoid Ltd., Basingstoke, Hampshire, England) with 10% glycerol added, after which the cultures were streaked on to BHI agar plates. Strains were grown on BHI agar (Oxoid Ltd.) for 24 h at 25, 30, or 37°C, depending on the organism, and stored at 5°C until use.
Growth and storage conditions.
Tubes containing 10 ml of autoclaved BHI broth were inoculated with approximately 103 cells/ml and incubated at 25, 30, or 37°C, depending on the organism. To evaluate the binding of the fluorescent probes, all strains were incubated for 24 h. For testing the influence of the growth phase, the strains were incubated for 6, 12, and 48 h in parallel tubes at 25, 30, or 37°C, depending on the organism. Finally, the strains were incubated for 24 h, after which 1.0 ml of each strain was frozen at −18°C, and two vials with 1.0 ml of each strain were stored at 5°C for 14 days. The frozen samples were thawed at room temperature before staining.
Preparation of fluorescent probes.
HI (Molecular Probes, Inc., Eugene, Oreg.) was dissolved in 0.1 M NaHCO3 to give a working solution with a concentration of 200 μg/ml. The working solution was divided into aliquots and frozen at −18°C. An aliquot was thawed and sterile filtered prior to use (0.22-μm pore size).
Oregon Green-conjugated WGA (Molecular Probes, Inc.) was dissolved in 0.1 M NaHCO3 to give a stock solution with a concentration of 1 mg/ml. The stock solution was divided into aliquots and frozen at −18°C. An aliquot was thawed and further diluted with deionized water, to give a working solution with a concentration of 200 μg/ml and sterile filtered prior to use (0.22-μm pore size).
Optimizing WGA binding.
Cultures (24 h) of M. luteus and S. uberis were diluted 1:100 with solutions containing 0, 1, and 3 M potassium chloride (KCl). Of these, 100 μl was stained with 10 μl of WGA working solution and allowed to react for 4 min at room temperature. Before flow-cytometric analysis, the samples were diluted with the adequate KCl solution to give a final volume of 1.0 ml without changing the KCl concentration.
A flow cytometer (PAS IIIi; Partec GmbH, Münster, Germany) equipped with an argon laser (488 nm) was used for analysis. The forward and side scatter signals and the green fluorescent signal (520 nm) were acquired. The detectors of the flow cytometer were all set to logarithmic amplification, and gain voltages were 200 for the forward scatter detector, 300 for the side scatter detector, and 550 for the green fluorescence detector. The dynamic range of the instrument was 4 decades on all parameters, with a measurement resolution of 1,024 channels. Standard beads (standard C; Partec GmbH) were used to ensure consistent performance of the flow cytometer during all experiments by checking the green fluorescent intensity of the beads. The green fluorescence signal was used as a trigger with channel 200 as the threshold. Azide-free diluent (Mallinckrodt Baker BV, Deventer, The Netherlands) was used as the sheath liquid. Samples were analyzed with a flow rate of 12 μl/min for 1 min.
Fluorescence microscopy.
An Axioskop epifluorescence microscope (Carl Zeiss, Göttingen, Germany) equipped with a 100-W Hg vapor arc lamp was used to observe the binding of the fluorescent probes. Aliquots of 5 μl were placed on glass slides under coverslips and observed through a 100× oil immersion objective lens (excitation, 450 to 490 nm; emission, >520 nm). Photographs were obtained with a CoolSnap charge-coupled device camera (Roper Scientific GmbH, Ottobrunn, Germany).
Gram-staining technique for bacterial cultures.
Cultures were diluted 1:100 with a 3 M KCl solution (3 M KCl, 0.035 M EDTA [pH 7.0]). Of the sample, 100 μl was stained with 20 μl of HI working solution at 50°C for 15 min. Thereafter, 10 μl of WGA working solution was added and allowed to react for 4 min at room temperature. Before flow-cytometric analysis, the sample was diluted with the 3 M KCl solution to give a final volume of 1.0 ml (final concentration of cells, ∼105 cells/ml).
Samples were measured immediately after staining with a FACScan flow cytometer (BD Immunocytometry, San Jose, Calif.) equipped with an argon laser (488 nm). Besides forward and side scatter signals, the green fluorescent signal (530 nm), the orange fluorescent signal (585 nm), and the red fluorescent signal (>650 nm) were acquired. The detectors of the flow cytometer were all set to logarithmic amplification, and gain voltage settings were E01 for the forward scatter detector, 570 for the side scatter detector, 752 for the green fluorescence detector, 613 for the orange fluorescence detector, and 650 for the red fluorescence detector. The dynamic range of the instrument was 4 decades on all parameters, with a measurement resolution of 1,024 channels. Standard beads (particle control sample, Foss Electric A/S) were used to ensure consistent performance of the flow cytometer during all experiments by checking the fluorescent intensities (channel numbers) of the beads. Due to fluorescence crossover between the orange and green signals, the orange fluorescence signals were compensated by 30% of the green signals. The orange fluorescence signal was used as a trigger, with channel 200 as the threshold. However, of these events, only events with red fluorescent intensities above channel 256 (first decade) were interpreted as bacteria. Distilled and sterile-filtered water was used as the sheath liquid. Samples were analyzed with a flow rate of 12 μl/min for 5 min or a maximum of 10,000 events.
Gram-staining technique for spiked milk samples.
Ten microliters of a stationary-phase culture (∼109 cells/ml) was added to 1 ml of a bulk tank milk sample with a low number of natural bacteria (<104 cells/ml) to ensure dominance of the added bacteria (final concentration, ∼107 cells/ml). One milliliter of spiked milk was added to 9 ml of a milk clearance reagent (Foss Electric A/S). The mixture was allowed to react for 10 min at 40°C, and the bacteria were then gradient centrifuged into a 3 M KCl solution (3 M KCl, 0.035 M EDTA [pH 7.0]) by using a gradient centrifuge (BactoScan 8000; Foss Electric A/S). One hundred microliters of the gradient was stained with 20 μl of HI working solution and incubated at a temperature of 40°C for 10 min. Subsequently, 10 μl of WGA working solution was added and allowed to react for 4 min at room temperature. Before flow cytometric analysis, the sample was diluted with 3 M KCl solution to give a final volume of 1.0 ml. Samples were measured immediately after dilution by using the FACScan flow cytometer (BD Immunocytometry) as described in “Gram-staining technique for bacterial cultures.”
Data analysis.
The Windows Multiple-Document Interface flow cytometry application was used for data analysis (WinMDI, version 2.8, Joseph Trotter, The Scripps Research Institute, La Jolla, Calif.).
RESULTS
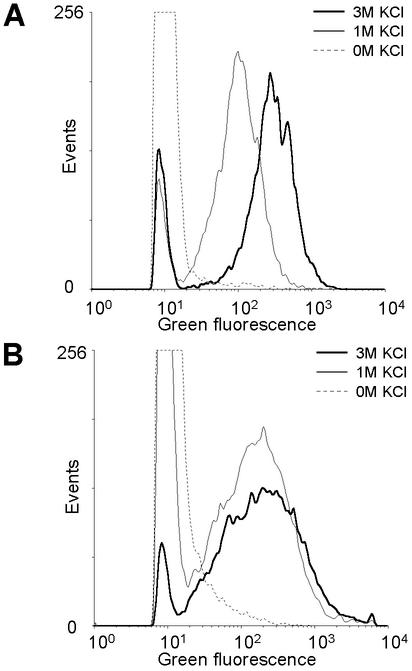
Figure 1 shows the effect of the KCl concentration on the binding of WGA to the gram-positive bacteria M. luteus and S. uberis, when the bacteria are stained by WGA alone, excluding HI. In the histograms the noise is seen to the left, where events with low fluorescence intensities have accumulated (a threshold was used to cut away most of the noise). When bacteria are stained sufficiently to distinguish them from noise, they will appear as a peak entirely separated from noise. Using 0 M KCl, none of the two bacteria were stained enough to separate them from the noise. Using 1 M KCl, an increased fluorescence intensity was observed for both bacteria. M. luteus was then separated from the noise, and S. uberis only slightly overlapped the noise. Increasing the KCl concentration to 3 M further improved the binding (higher fluorescence intensities) of WGA to these bacteria strains, especially for M. luteus.
FIG. 1.
Effect of the potassium chloride concentration on the binding of WGA to the gram-positive bacteria M. luteus (A) and S. uberis (B). The results were obtained by using the procedure described in “Optimizing WGA binding.”
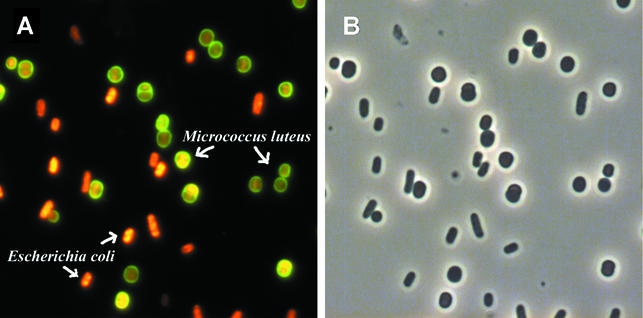
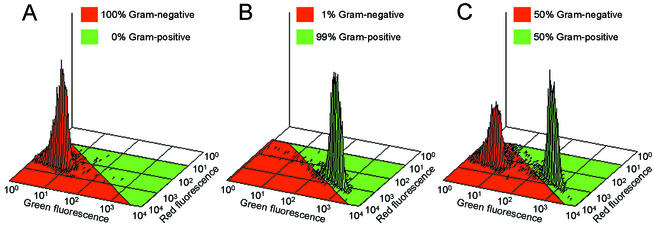
Microscopic observations demonstrated that gram-positive bacteria were stained by WGA, whereas gram-negative bacteria were not stained by WGA. HI stained both the gram-positive and the gram-negative bacteria. Figure 2 shows an example of the staining of a mixture (1:1) of the gram-positive M. luteus and the gram-negative E. coli with both WGA and HI. As a result of the staining, M. luteus appeared with a green surface (WGA) and a red cytoplasm (HI), whereas E. coli only appeared with a red cytoplasm. Isometric plots of flow cytometric analyses of cultures of E. coli, M. luteus, and a mixture (1:1) of these are shown in Fig. 3. Measuring E. coli alone (Fig. 3A), 100% of the events were present in the gram-negative region. For M. luteus alone (Fig. 3B), 99% of the events were in the gram-positive region and 1% of the events entered the gram-negative region. When cultures of E. coli and M. luteus were mixed (Fig. 3C), 50% of the events were observed in each region.
FIG. 2.
Mixed culture (1:1) of M. luteus (gram-positive cocci) and E. coli (gram-negative rods), stained with HI and Oregon Green-conjugated WGA. (A) Fluorescence image; (B) same image by phase-contrast microscopy.
FIG. 3.
Isometric flow-cytometric plots. (A) E. coli; (B) M. luteus; (C) mixed culture (1:1) of E. coli and M. luteus. Percentages are calculated as the number of events in the red region (gram negative) or green region (gram positive) divided by the total number of events. The results were obtained by using the procedure described in “Gram-staining technique for bacterial cultures.”
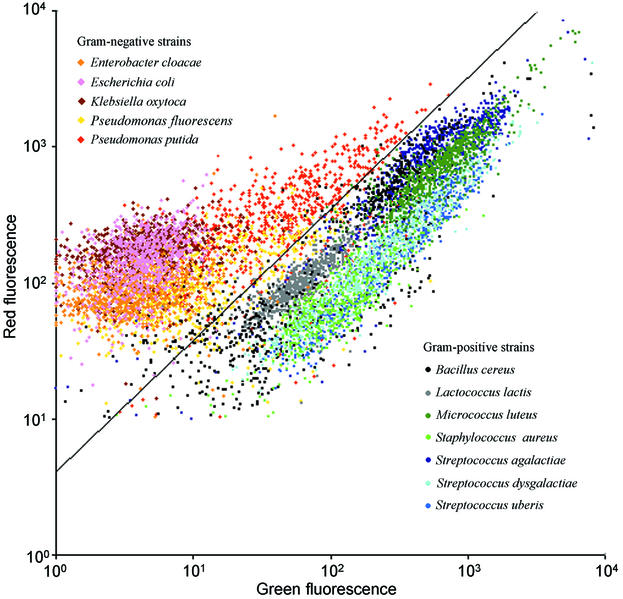
Each of the 12 bacterial strains was measured in duplicate after 6,12, 24, and 48 h of incubation. From each measurement, 100 events were used for calculations. In Fig. 4 an assembled plot is presented which includes all 800 events for each of the 12 bacterial strains tested during 48 h of incubation (a total of 9,600 events). Each bacterial strain is presented with a distinct color. A calculated best line for separating gram-positive and gram-negative bacteria is shown. Overall, the line ensured 97% of the events to be correctly interpreted. The percentages of cells correctly interpreted for the individual strains were calculated and are presented in Table 1. For E. cloacae, E. coli, K. oxytoca, L. lactis, M. luteus, S. aureus, S. agalactiae, S. dysgalactiae, and S. uberis, almost all cells (>96%) were correctly interpreted for any incubation time. For B. cereus, P. fluorescens, and P. putida, >93% of the cells were interpreted correctly after 12 and 24 h of incubation, whereas after 6 and 48 h of incubation only 75 to 89% of the cells were interpreted correctly.
FIG. 4.
An assembled plot is presented which includes 800 events for each of the 12 bacterial strains tested during 48 h of incubation (a total of 9,600 events). Each bacterial strain is presented with a distinct color. Gram-positive strains are presented in green, blue, and gray colors, and gram-negative strains are presented in yellow, orange, red, pink, and brown colors. A calculated best line for separating gram-positive and gram-negative bacteria is shown. The results were obtained by using the procedure described in “Gram-staining technique for bacterial cultures.”
TABLE 1.
Flow-cytometric analyses of cultures at different times of incubationa
| Bacterium | % of cells correctly interpretedb after incubation for time (h):
|
|||
|---|---|---|---|---|
| 6 | 12 | 24 | 48 | |
| Gram-positive strains | ||||
| B. cereus | 88 (16) | 93 (4) | 93 (5) | 75 (24) |
| L. lactis | 99 (1) | 96 (1) | 98 (0) | 99 (1) |
| M. luteus | 99 (2) | 99 (0) | 100 (1) | 100 (1) |
| S. aureus | 100 (0) | 100 (0) | 100 (1) | 100 (0) |
| S. agalactiae | 99 (0) | 96 (1) | 100 (1) | 99 (0) |
| S. dysgalactiae | 100 (0) | 100 (0) | 100 (0) | 100 (0) |
| S. uberis | 100 (0) | 100 (0) | 100 (0) | 100 (0) |
| Gram-negative strains | ||||
| E. cloacae | 100 (0) | 100 (1) | 98 (1) | 98 (4) |
| E. coli | 100 (0) | 100 (1) | 100 (0) | 100 (1) |
| K. oxyloca | 100 (0) | 99 (0) | 100 (1) | 100 (0) |
| P. fluorescens | 80 (9) | 98 (1) | 98 (1) | 88 (1) |
| P. putida | 89 (4) | 95 (1) | 99 (1) | 79 (2) |
| Avg | 96 | 98 | 99 | 95 |
The results were obtained by using the procedure described in “Gram-staining technique for bacterial cultures.”
Average of two determinations. Standard deviations are given in parentheses.
In Table 2, the results from flow-cytometric analyses of cultures subjected to different storage conditions are presented. When the cultures were stored at 5°C for 14 days, the staining technique seemed to be influenced by the age of the culture, especially for gram-negative bacteria. The average percentage of correctly interpreted cells was 86%. For E. coli, M. luteus, S. agalactiae, S. dysgalactiae, and S. uberis, most cells (>97%) were correctly interpreted. For B. cereus, E. cloacae, K. oxytoca, L. lactis, and S. aureus, 76 to 89% were correctly interpreted, and for P. fluorescens and P. putida, the figures were 58 and 68%, respectively. Freezing did not seem to influence the staining technique. After storage at −18°C for 24 h, >98% of the cells were interpreted correctly for E. cloacae, E. coli, K. oxytoca, L. lactis, P. putida, S. aureus, S. agalactiae, S. dysgalactiae, and S. uberis. For B. cereus and P. fluorescens, the figures were 83 and 82%, respectively.
TABLE 2.
Flow-cytometric analyses of cultures subjected to different storage conditionsa
| Bacterium | % of cells correctly interpretedb after incubation for:
|
|
|---|---|---|
| 14 days at 5°C | 24 h at −18°C | |
| Gram-positive strains | ||
| B. cereus | 80 (15) | 83 (6) |
| L. lactis | 89 (2) | 100 (2) |
| M. luteus | 100 (1) | 99 (2) |
| S. aureus | 85 (6) | 100 (0) |
| S. agalactiae | 100 (0) | 100 (1) |
| S. dysgalactiae | 100 (1) | 100 (1) |
| S. uberis | 100 (1) | 100 (1) |
| Gram-negative strains | ||
| E. cloacae | 76 (7) | 98 (1) |
| E. coli | 97 (3) | 100 (1) |
| K. oxyloca | 77 (8) | 100 (1) |
| P. fluorescens | 58 (4) | 82 (6) |
| P. putida | 68 (8) | 98 (0) |
| Avg | 86 | 96 |
The results were obtained by using the procedure described in “Gram-staining technique for bacterial cultures.”
Average of two determinations. Standard deviations are given in parentheses.
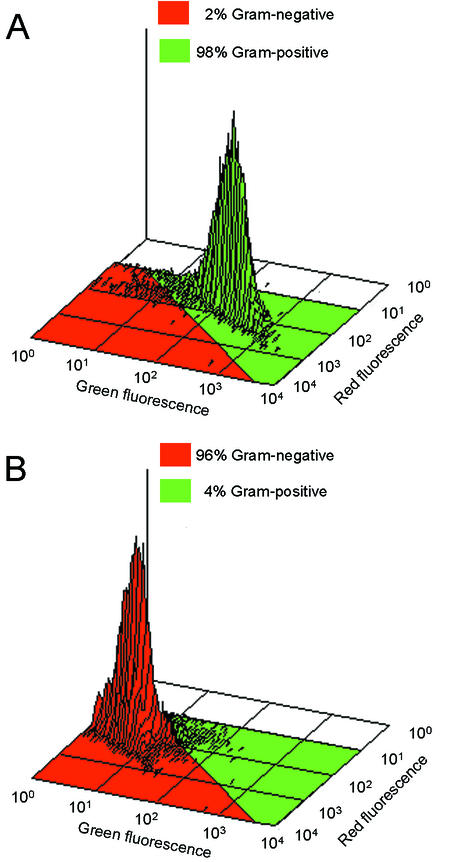
Figure 5 shows the isometric plots of flow-cytometric analyses of S. aureus and E. coli added to bulk tank milk. For S. aureus (Fig. 5A), 98% of the events were in the gram-positive region and 2% of the events entered the gram-negative region. For E. coli (Fig. 5B), 96% of the events were in the gram-negative region and 4% of the events entered the gram-positive region. The contribution from the natural bacteria present in the milk accounted for less than 0.1%.
FIG. 5.
Three-dimensional isometric flow-cytometric plots of bacteria added to milk (∼107 cells/ml). (A) S. aureus; (B) E. coli. Percentages are calculated as the number of events in the red region (gram negative) or green region (gram positive) divided by the total number of events. The results were obtained using the procedure described in “Gram-staining technique for spiked milk samples.”
DISCUSSION
The addition of a high concentration of KCl to the staining solution improved the ability of WGA to stain gram-positive bacteria. A possible explanation is that gram-positive bacteria have teichoic acids attached perpendicularly to their cell walls, which, for some gram-positive bacteria, may block the access of WGA to the cell wall. When the concentration of KCl is low, the structure of the teichoic acids is rigid, but by increasing the concentration of KCl, the structure will be loosened as the potassium ions eliminate the negative charges on the teichoic acids, causing the structure to collapse (8, 9). The composition and structure of teichoic acids vary between gram-positive species (24), which may explain why an increase in KCl concentration from 1 to 3 M had a larger effect on M. luteus than on S. uberis. In the present work, 3 M KCl was used to maximize the binding of WGA to gram-positive bacteria. Techniques for elimination of the organization of the teichoic acids have previously only been reported for microscopic applications. These techniques include the use of osmium tetroxide fixation followed by acetone addition and evaporation (2) and heat fixation of bacteria on a microscopic slide (29). However, these treatments could not be considered in the present study, as a dehydration step is not suitable for flow cytometry.
The Gram-staining technique showed reliable results for all strains after 6, 12, 24, and 48 h of incubation, for which almost all (97%) cells were correctly interpreted. These results conform to the findings of the WGA Gram-staining method reported by Sizemore et al. (29). Their work included 92 bacterial strains and showed consistent results after 24, 48, 72, and 144 h of incubation. A discrepancy between the technique described by Sizemore et al. (29) and the present technique is that the latter combines WGA with HI to stain all cells. Having stained both gram-positive and gram-negative bacteria permits the use of flow cytometry instead of a combination of epifluorescence and phase-contrast microscopy for detection, allowing analysis of higher numbers of cells in a less labor-intensive way.
The different stressful conditions used in this study were intended to simulate some of the injurious treatments that bacteria in milk may be subjected to prior to analysis. During prolonged refrigerated storage, bacteria will slowly start to die, degrade, and eventually lyse (27). The percentage of cells correctly interpreted after 14 days at 5°C (average of 86%) is clearly lower than that of cultures incubated for 24 h (average of 99%). In particular, gram-negative bacteria were affected by the prolonged storage at 5°C, which might be due to degradation of the lipopolysaccharide layer that normally prevents WGA from binding to the cell wall of gram-negative bacteria (29). However, 14 days at 5°C should be considered a worst-case treatment, as milk is normally collected at least every second day in industrialized countries (6). On the contrary, little effect was observed after freezing the cultures at −18°C for 24 h. An average of 96% of the cells was correctly interpreted, even though freezing has been shown to destroy the lipopolysaccharide layer of E. coli, and relatively high freezing temperatures (−18°C) are reported to cause larger injuries to bacteria than low freezing temperatures (10, 25).
The Gram-staining technique was applied to bulk tank milk with added bacteria to assess the influence of the milk matrix on the technique. In order to avoid interferences from the milk matrix, a pretreatment of the milk involving enzymatic degradation of proteins and removal of fat was required. Combining the pretreatment with the Gram-staining technique enabled almost all cells (97%) of the added cultures of S. aureus and E. coli to be correctly interpreted. Other flow cytometric techniques for determining the TBC (5, 16, 30) or the use of antibodies for the detection of S. enterica serovar Typhimurium and L. monocytogenes in milk have been described previously (7, 23). However, no other publications to date have dealt with a flow-cytometric Gram-staining technique for milk.
Using cultural methods incorporating different media and culture conditions may give detailed bacterial information for milk samples, which can be used for understanding and solving problems on the farm (3, 4, 13, 20). However, the integrated problem of cultural methods is the 2 to 3 days needed to obtain results, which inhibits the dynamics of identifying and solving problems. The present flow-cytometric technique may not provide as detailed information as an array of cultural methods but can be used as an initial screening of milk samples and has the potential to support cultural methods by suggesting the sources of contamination. The technique described in the present study is practically independent of the state of the bacterial cells and has the potential to analyze samples without precultivation. Combining these properties and the use of flow cytometry allows the development of a rapid automated Gram-staining technique for the analysis of milk.
Acknowledgments
The present work is supported by The Royal Veterinary and Agricultural University, The Danish Dairy Board, Foss Electric A/S, and the Danish Academy of Technical Sciences.
Mogens Jakobsen, Henrik Siegumfeldt, and Peter Nissen of The Royal Veterinary and Agricultural University, Department of Dairy and Food Science, Food Microbiology, are thanked for useful discussions. Christina Olsson of Foss Electric A/S is thanked for competent assistance with the practical work.
REFERENCES
- 1.Allman, R., R. Manchee, and D. Lloyd. 1993. Flow cytometric analysis of heterogeneous bacterial populations, p. 27-47. In D. Lloyd (ed.), Flow cytometry in microbiology. Springer-Verlag, London, England.
- 2.Birdsell, D. C., R. J. Doyle, and M. Morgenstern. 1975. Organization of teichoic acid in the cell wall of Bacillus subtilis. J. Bacteriol. 121:726-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blowey, R., J. Davis, and P. Edmondson. 1997. Bacterial counts in bulk milk-an underused investigation technique. In Pract. 19:122-127. [Google Scholar]
- 4.Blowey, R., P. Edmondson, and J. Davis. 1999. Bacterial counts in bulk milk-an update. In Pract. 21:531-534. [Google Scholar]
- 5.Bolzoni, G., A. Marcolini, and G. Varisco. 2000. Evaluation of the BactoScan FC. 1. Accuracy, comparison with the BactoScan 8000 and somatic cells effect. Milchwissenschaft 55:67-70. [Google Scholar]
- 6.Bramley, A. J., and C. H. McKinnon. 1990. The microbiology of raw milk, p. 163-208. In R. K. Robinson (ed.), Dairy microbiology, vol. 1. Elsevier, London, England.
- 7.Donnelly, C. W., and G. J. Baigent. 1986. Method for flow cytometric detection of Listeria monocytogenes in milk. Appl. Environ. Microbiol. 52:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle, R. J., M. L. McDannel, U. N. Streips, D. C. Birdsell, and F. E. Young. 1974. Polyelectrolyte nature of bacterial teichoic acids. J. Bacteriol. 118:606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle, R. J., and M. Slifkin. 1994. Introduction to lectins and their interactions with microorganisms, p. 1-65. In R. J. Doyle and M. Slithin (ed.), Lectin-microorganism interactions. Marcel Dekker, New York, N.Y.
- 10.Elkest, S. E., and E. H. Marth. 1992. Freezing of Listeria monocytogenes and other microorganisms. J. Food Prot. 55:639-648 [DOI] [PubMed] [Google Scholar]
- 11.Fife, D. J., D. F. Bruhn, K. S. Miller, and D. Stoner. 2000. Evaluation of a fluorescent lectin-based staining technique for some acidophilic mining bacteria. Appl. Environ. Microbiol. 66:2208-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedlander, C. 1883. Die Mikrokokken der Pneumonie. Fortschr. Med. 1:715-733
- 13.Godkin, M. A., and K. E. Leslie. 1993. Culture of bulk tank milk as a mastitis screening test: a brief review. Can. Vet. J. 34:601-605. [PMC free article] [PubMed] [Google Scholar]
- 14.Gram, C. 1884. Über die isolirte Farbung der Schizomyceten in Schnitt-und Trockenpräparaten. Fortschr. Med. 2:185-189.
- 15.Gregersen, T. 1978. Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur. J. Appl. Microbiol. Biotechnol. 5:123-127. [Google Scholar]
- 16.Gunasekera, T. S., P. V. Attfield, and D. A. Veal. 1999. A flow cytometry method for rapid detection and enumeration of total bacteria in milk. Appl. Environ. Microbiol. 66:1228-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heeschen, W. H. 1996. Bacteriological quality of raw milk: legal requirements and payment systems, p. 1-18. In Bacteriological quality of raw milk. International Dairy Federation, Brussels, Belgium.
- 18.International Dairy Federation. 1991. IDF Standard 100B. Milk and milk products, enumeration of microorganisms, colony count technique at 30°C. International Dairy Federation, Brussels, Belgium.
- 19.Jayarao, B. M., and L. Wang. 1999. A study of the prevalence of Gram-negative bacteria in bulk tank milk. J. Dairy Sci. 82:2620-2624. [DOI] [PubMed] [Google Scholar]
- 20.Jayarao, B. M., S. R. Pillai, D. R. Wolfgang, D. R. Griswold, and L. J. Hutchinson. 2001. Herd level information and bulk tank milk analysis: tools for improving milk quality and herd udder health. Bov. Pract. 35:23-35. [Google Scholar]
- 21.Jeffrey, D. C., and J. Wilson. 1987. Effect of mastitis-related bacteria on the total bacterial count of bulk milk supplies. J. Soc. Dairy Technol. 40:23-26. [Google Scholar]
- 22.Mason, D. J., S. Shanmuganathan, F. C. Mortimer, and V. A. Gant. 1998. A fluorescent gram stain for flow cytometry and epifluorescence microscopy. Appl. Environ. Microbiol. 64:2681-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClelland, R. G., and A. C. Pinder. 1994. Detection of Salmonella typhimurium in dairy products with flow cytometry and monoclonal antibodies. Appl. Environ. Microbiol. 60:4255-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naumova, I. B., and A. S. Shashkov. 1997. Anionic polymers in cell walls of Gram-positive bacteria. Biochemistry (Moscow) 62:809-840. [PubMed] [Google Scholar]
- 25.Ray, B., M. L. Speck, and W. J. Dobrogosz. 1976. Cell-wall lipopolysaccharide damage in Escherichia coli due to freezing. Cryobiology 13:153-160. [DOI] [PubMed] [Google Scholar]
- 26.Ryu, E. 1938. On the Gram-differentiation of bacteria by the simplest method. J. Jpn. Soc. Vet. Sci. 17:58-63. [Google Scholar]
- 27.Shah, N. P., W. E. V. Lankaputhra, M. L. Britz, and W. S. A. Kyle. 1995. Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in commercial yoghurt during refrigerated storage. Int. Dairy Sci. 5:515-521. [Google Scholar]
- 28.Shapiro, H. M. 1995. Practical flow cytometry, 3rd ed. Alan R. Liss, Inc., New York, N.Y.
- 29.Sizemore, R. K., J. J. Caldwell, and S. Kendrick. 1990. Alternate Gram staining technique using a fluorescent lectin. Appl. Environ. Microbiol. 56:2245-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suhren, G., and H. G. Walte. 1998. First experiences with automatic flow cytometric determination of total bacterial count in raw milk. Kieler Milchwirtschaftliche Forsch. Ber. 50:249-273. [Google Scholar]