Abstract
Two tetrachlorethene (PCE)-dechlorinating populations, designated strains BB1 and BRS1, were isolated from pristine river sediment and chloroethene-contaminated aquifer material, respectively. PCE-to-cis-1,2-dichloroethene-dechlorinating activity could be transferred in defined basal salts medium with acetate as the electron donor and PCE as the electron acceptor. Taxonomic analysis based on 16S rRNA gene sequencing placed both isolates within the Desulfuromonas cluster in the δ subdivision of the Proteobacteria. PCE was dechlorinated at rates of at least 139 nmol min−1 mg of protein−1 at pH values between 7.0 and 7.5 and temperatures between 25 and 30°C. Dechlorination also occurred at 10°C. The electron donors that supported dechlorination included acetate, lactate, pyruvate, succinate, malate, and fumarate but not hydrogen, formate, ethanol, propionate, or sulfide. Growth occurred with malate or fumarate alone, whereas oxidation of the other electron donors depended strictly on the presence of fumarate, malate, ferric iron, sulfur, PCE, or TCE as an electron acceptor. Nitrate, sulfate, sulfite, thiosulfate, and other chlorinated compounds were not used as electron acceptors. Sulfite had a strong inhibitory effect on growth and dechlorination. Alternate electron acceptors (e.g., fumarate or ferric iron) did not inhibit PCE dechlorination and were consumed concomitantly. The putative fumarate, PCE, and ferric iron reductases were induced by their respective substrates and were not constitutively present. Sulfide was required for growth. Both strains tolerated high concentrations of PCE, and dechlorination occurred in the presence of free-phase PCE (dense non-aqueous-phase liquids). Repeated growth with acetate and fumarate as substrates yielded a BB1 variant that had lost the ability to dechlorinate PCE. Due to the 16S rRNA gene sequence differences with the closest relatives and the unique phenotypic characteristics, we propose that the new isolates are members of a new species, Desulfuromonas michiganensis, within the Desulfuromonas cluster of the Geobacteraceae.
The first reported anthropogenic production of tetrachloroethene (PCE) dates back to 1821, when Faraday produced PCE by thermal decomposition of hexachloroethane (15). Starting at the beginning of the 20th century, increasing amounts of PCE and trichloroethene (TCE) were manufactured due to the extensive use of these compounds in industry, in the military, and in private households, mainly as nonflammable solvents (summarized in reference 9). This widespread use, along with careless handling and storage, made chlorinated ethenes abundant groundwater pollutants. Often, PCE and TCE contamination coincided with spills of other organic compounds, such as crude oil constituents, other solvents (often seen at industrial and military sites), or starch (at dry cleaning operations). Aerobic microorganisms rapidly degrade the nonchlorinated contaminants, thereby depleting oxygen. Under anaerobic conditions PCE and TCE can be used as alternate growth-supporting electron acceptors by specialized reductively dechlorinating bacteria. This reductive dechlorination process is known as chloridogenesis or chlororespiration (also called dechlororespiration) (23, 25, 32, 39). Several PCE-dechlorinating isolates belonging to different groups of the Proteobacteria, the low-G+C-content gram-positive group (e.g., Desulfitobacterium), and the Dehalococcoides cluster were obtained over the last several years (4, 16, 20, 31, 33, 40, 41, 47, 52). These isolates have different electron donor requirements. Some are versatile and use hydrogen as well as organic electron donors, but not acetate, for reductive dechlorination. Others, such as Dehalobacter and Dehalococcoides populations, are strictly hydrogenotrophic. Reductive dechlorination by Desulfuromonas chloroethenica, a member of the Desulfuromonas cluster in the Geobacteraceae, is supported by acetate rather than hydrogen.
A recent study in which 16S rRNA gene-based primers targeting the PCE-dechlorinating Desulfuromonas group were used suggested that populations of this type are frequently found in anaerobic environments and might contribute to chloroethene dechlorination at contaminated sites (26). Hence, in this study we aimed at isolating and characterizing acetotrophic PCE dechlorinators and comparing the isolates to Desulfuromonas chloroethenica, the only previously described PCE-dechlorinating population that couples chloroethene reduction to the oxidation of acetate (19, 20). Two new isolates were obtained that were distinctive in that they were more versatile with regard to the electron donors utilized, exhibited fermentative metabolism, grew in PCE-saturated medium in the presence of free-phase solvent, exhibited higher growth rates and yields on PCE, and had different 16S rRNA gene sequences. Hence, these isolates are described here as a new species, Desulfuromonas michiganensis.
MATERIALS AND METHODS
Chemicals.
Chlorinated aliphatic and aromatic hydrocarbons, including PCE, TCE, cis-1,2-dichloroethene (cis-DCE), trans-DCE, 1,1-dichloroethene, vinyl chloride, 1,1,2,2-tetrachloroethane, 1,2,3-trichloropropane, 1,3-dichloropropane, 1,2-dichloropropane, 1-chloropropane, 2-chloropropane, trichloroacetate, 2-chlorobenzoate, 3-chlorobenzoate, 4-chlorobenzoate, 2,4-dichlorobenzoate, 3-chloro-4-hydroxybenzoate, 2-chlorophenol, 3-chlorophenol, 4-chlorophenol, 2,3-dichlorophenol, 2,4-dichlorophenol, 3,4-dichlorophenol, 2,6-dichlorophenol, 2,4,6-trichlorophenol, and 2,4-dichlorophenoxyacetate, were purchased from Sigma-Aldrich-Fluka or Supelco (St. Louis, Mo.). Gases were supplied by AGA Gas, Inc. Synonyms for PCE are perchloroethylene, ethylenetetrachloride, carbon dichloride, PER, and Perk.
Medium preparation and growth conditions.
A reduced anaerobic basal salts medium (BS medium) was prepared as described previously (22). HEPES (10 mM) was used as a buffering substance for bicarbonate-free medium. The pH of the bicarbonate-free medium was adjusted to 7.2 with 1 M NaOH prior to autoclaving, and the headspace of the culture bottles contained argon or dinitrogen. Medium with a low chloride content (LC medium) had the following composition (per liter): MgSO4 · 6H2O, 0.5 g; NH4SO4, 0.3 g; K2SO4, 0.3 g; CaSO4 · H2O, 0.015 g; resazurin, 0.25 mg; trace element solution A (25), 1 ml; trace element solution B (25), 1 ml; Na2S · 9H2O, 0.048 g; l-cysteine, 0.035 g; and NaHCO3, 2.52 g. The pH of the medium was adjusted to 7.2 to 7.3 with CO2, and 2 mM sterile, anoxic sodium potassium phosphate (pH 7.2) was added after the medium had been autoclaved. Vitamins (54) and electron donors were added from neutralized, anoxic, sterilized stock solutions. Unless indicated otherwise, electron donors were added in excess to allow for complete reduction of the electron acceptor added. Cultures were incubated in 20-ml vials containing 10 ml of medium or in 160-ml serum bottles containing 100 ml (total volume) (referred to as 100-ml cultures below). Chlorinated aliphatic compounds were added from saturated anoxic aqueous stock solutions or as neat compounds by using Hamilton syringes equipped with reproducibility (Chaney) adapters (Hamilton, Reno, Nev.), or they were dissolved in an organic carrier phase (hexadecane). PCE stock solutions were autoclaved in containers sealed with new, previously autoclaved Teflon-lined rubber stoppers. Routinely, 0.2 ml of hexadecane containing 5 to 25 μl of PCE was added to 100-ml cultures to obtain aqueous PCE concentrations ranging from 0.16 to 0.83 mM (12). Cultures were incubated with the stopper down without shaking at 25°C in the dark; the only exceptions were vessels containing free-phase PCE (dense non-aqueous-phase liquid [DNAPL]), which were incubated with the stopper up to prevent extensive absorption of chloroethenes to the rubber stoppers.
Sources of cultures.
A sediment sample from the Père Marquette River was taken in April 1995 at Bowman's Bridge near Baldwin, Mich., located in the Manistee National Forest Recreation Area. Aquifer material from the Bachman aquifer was collected in August 1997 as described previously (14). Microcosms were established inside a glove box (Coy Manufacturing, Ann Arbor, Mich.) filled with a mixture of 97% (vol/vol) N2 and 3% (vol/vol) H2. Portions (1 g, wet weight) of sediment or aquifer material were transferred to 20-ml vials, and 9 ml of sterile, anoxic phosphate-buffered saline (5 mM potassium phosphate, 0.85% NaCl; pH 7.2) or 10 mM phosphate buffer (pH 7.2) was added to each vial. All of the vials were amended with 2 mM acetate, 3 ml of H2, and 2 μmol of PCE and sealed with Teflon-lined rubber stoppers. Killed controls were prepared in the same way and autoclaved for 30 min on two consecutive days. A second set of live controls contained all additions except PCE. Microcosms showing the formation of less-chlorinated ethenes were sequentially transferred to 20-ml vials containing bicarbonate-buffered BS medium amended with acetate or acetate plus H2. After sediment-free dechlorinating cultures were obtained, 2 mM 2-bromoethanesulfonate (BES) was added to inhibit the growth of methanogenic archaea (24). Further enrichment was achieved by preparing repeated dilution-to-extinction series in 20-ml vials containing 10 ml of HEPES-buffered BS medium amended with 5 mM acetate and 10 μmol of PCE dissolved in 100 μl of hexadecane. Pure cultures were obtained from dilution-to-extinction soft agar cultures (0.6% low-melting-point agarose [SeaPlaque agarose; BioWhittaker Molecular Applications, Rockland, Maine]) prepared in 10 ml of bicarbonate-buffered BS medium amended with 5 mM acetate and fumarate (20 mM) or PCE (5 μmol) supplied as electron acceptors. Additional PCE (20 μmol in 100 μl of hexadecane) was added by syringe after the agarose solidified. All vials were incubated in the inverted position (the hexadecane phase and the soft agar remained physically separated).
Desulfuromonas acetexigens DSM 1397, Desulfuromonas acetoxidans DSM 684, Desulfuromonas succinoxidans DSM 8964, and Desulfuromonas thiophila DSM 8987 were obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen (Braunschweig, Germany). All cultures were grown as recommended by the Deutsche Sammlung für Mikroorganismen und Zellkulturen before they were transferred (2% [vol/vol] inoculum) to the same medium containing acetate as the only electron donor and 20 μmol of PCE as the electron acceptor. Geobacter metallireducens and Pelobacter acetylenicus were kindly provided by E. Harris and D. Lovley, University of Massachusetts, Amherst, and L. Krumholz, University of Oklahoma, Norman, provided a culture of Desulfuromonas chloroethenica.
PCR amplification of 16S rRNA genes, sequencing, and phylogenetic analysis.
Cells were harvested by centrifugation (20,000 × g for 30 min) from 20 ml of an acetate-PCE-grown culture. Total genomic DNA was extracted with a QIAamp tissue kit (QIAGEN, Santa Clarita, Calif.) used according to the manufacturer's recommendations. DNA yields were estimated spectrophotometrically and by agarose gel electrophoresis. Nearly complete (ca. 1,500-bp) small-subunit rRNA genes were PCR amplified by using bacterium-specific primers and conditions described previously (26). Excess amplification primers were removed prior to sequencing by using commercially available columns (Wizard PCR Preps; Promega, Madison, Wis.) as recommended by the manufacturer. Double-stranded sequencing of PCR products was performed by automated fluorescent Taq cycle sequencing by using a 373A DNA sequencer (Applied Biosystems, Foster City, Calif.) and modified versions of previously described bacterium-specific sequencing primers targeting conserved regions of the 16S rRNA gene (21, 50, 51, 53). Sequences of the nearest relatives were identified and obtained from the Ribosomal Database Project II (RDP) (http://rdp.cme.msu.edu) by using the SIMILARITY_RANK and SUBALIGNMENT programs, respectively (30), and related sequences in other databases were identified by BLAST analysis. Sequences were manually aligned based on both the primary and secondary structures by using the ARB editor (www.biol.chemie.tu-muenchen.de/pub/ARB). Natural relationships were inferred by neighbor-joining analysis (38). A distance matrix was calculated by using the RDP Phylip Interface and applying the Jukes-Cantor method and empirical base frequencies. Sequences of the following organisms were obtained from the RDP or from GenBank (the numbers in parentheses are accession numbers): Desulfuromonas chloroethenica (U49748), Desulfuromonas acetexigens (U23140), Pelobacter venetianus (U41562), Pelobacter acetylenicus (X70955), Pelobacter carbinolicus (U23141), Geobacter metallireducens (L07834), Trichlorobacter thiogenes (AF223382), and Desulfomonile tiedjei (M26635).
Scanning electron microscopy.
Strain BB1 was grown in BS medium with acetate and PCE. The cells were fixed in an equal volume of 4% glutaraldehyde in 100 mM sodium phosphate buffer (pH 7.4) for 30 min. Single drops of cells fixed in suspension were placed on poly-l-lysine-coated glass coverslips. The coverslips were gently washed with several drops of water, and the samples were dehydrated in an ethanol series (25, 50, 75, and 100% ethanol). Then the samples were critical point dried, mounted on aluminum stubs, coated with gold (thickness, 20 nm), and examined with a JEOL 6400V scanning electron microscope (Japan Electron Optics Limited, Peabody, Mass.).
Determination of substrate range.
Serum bottles containing 100 ml of BS medium were amended with a limiting amount of acetate (20 μmol) and excess PCE (100 μmol dissolved in 0.2 ml of hexadecane), and then they were inoculated with an acetate-PCE-grown culture (1%, vol/vol) that had consumed all of the acetate present. The cultures were incubated until dechlorination ceased due to lack of an electron donor. The different electron donors that were tested were added, and dechlorination was monitored for 2 months. PCE was replenished when it was depleted.
The ability of each of the isolates to use alternate electron acceptors was determined in duplicate 20-ml vials amended with 5 mM acetate or 5 mM lactate as an electron donor. Chlorinated alkanes and alkenes were added from sterilized, anoxic, aqueous stock solutions to final concentrations ranging from 50 to 100 μM. Gaseous vinyl chloride was added by syringe to a final aqueous concentration of 0.5 mM. Chlorophenolic compounds and trichloroacetate were added to a final concentration of 0.6 mM, and all other chlorinated aromatic compounds were tested at a concentration of 1 mM in 100-ml cultures. Inorganic electron acceptors and fumarate were tested at an initial concentration of 5 mM. Soluble ferric iron (5 mM) was added from an aqueous neutralized ferric citrate stock solution (concentration, approximately 0.5 M), and poorly crystalline ferric oxide was prepared as described by Lovley (29). Sulfur was added to the cultures as an aqueous powdered S0 suspension that had been pasteurized at 90°C. All cultures received a 1% (vol/vol) inoculum from an acetate-PCE-grown culture that had consumed all of the PCE present. Cultures that did not show any electron donor consumption after 6 weeks received a second inoculum.
Growth of the isolates in anaerobic culture tubes with fumarate (20 mM), fumarate (20 mM) plus acetate (10 mM), fumarate (20 mM) plus pyruvate (20 mM), and reduced anaerobic complex media, such as tryptic soy broth, R2A broth, Luria-Bertani broth, potato dextrose broth, and actinomyces broth (2), was monitored by measuring the increase in absorbance at 600 nm.
Rate measurements.
Dechlorination rates were determined in triplicate 100-ml cultures that had received a 2% (vol/vol) inoculum from an acetate-PCE-grown culture. After complete dechlorination of 10 μmol of PCE, each culture received 40 μmol of PCE, and dechlorination was monitored for 45 h. For biomass estimation, 2-ml samples were withdrawn from the cultures after 23 h, and 1 ml of each cell suspension was appropriately diluted with 50 mM phosphate-buffered saline (pH 7.5) and stained in an aqueous 0.01% acridine orange solution for 30 min. The suspensions were diluted with 8 ml of saline before they were filtered through black 0.2-μm-pore-size polycarbonate filters. The stained cells were then counted by computer-assisted light microscopy (37). The average cell concentration after 23 h was 7.7 × 106 ± 0.9 × 106 cells ml−1, which was equivalent to 1.2 ± 0.14 μg of protein ml−1 assuming that a single cell has a protein content of 156 × 10−15 g (34).
Influence of temperature and pH on growth and PCE dechlorination.
To determine the temperature range for growth, LC medium (100 ml) amended with 5 mM acetate and 20 μmol of PCE was inoculated (2%, vol/vol) from an acetate-PCE-grown culture, and duplicate cultures were incubated at 4, 10, 20, 25, 30, 35, and 45°C. Growth was assessed on the basis of formation of cis-DCE and chloride and an increase in the cell number. The temperature range for dechlorination was determined in 16 100-ml cultures containing 10 mM acetate and 20 μmol of PCE that were inoculated (1%, vol/vol) from an acetate-PCE-grown culture. After all the PCE had been dechlorinated to cis-DCE, duplicate cultures were equilibrated at 4, 10, 17, 20, 25, 30, 37, and 45°C. The cultures were then spiked with 20 μmol of PCE and incubated at the different temperatures, and increases in the cis-DCE concentration were measured after 12, 24, and 48 h.
To test the influence of pH on PCE dechlorination, the following buffer systems were used in BS medium (100-ml cultures; the pH values tested are indicated in parentheses): 2-(N-morpholino)ethanesulfonic acid (MES) (pH 5.5, 6.0, and 6.5), HEPES (pH 7.0, 7.5, and 8.0), 1,3-bis[tris(hydroxymethyl)methylamino]propane (pH 6.5, 7.0, 7.5, 8.0, 8.5, and 9.0), and 2-(N-cyclohexylamino)ethanesulfonic acid (CHES) (pH 9.0, 9.5, and 10.0). The pH of each medium was adjusted with 1 M HCl or 1 M NaOH before autoclaving. An acetate-PCE-grown culture was used as the inoculum (2%, vol/vol). After all the additions to the medium were made, 1-ml samples were withdrawn for pH verification. Cultures that did not show dechlorination activity after 2 weeks received a second inoculum.
Induction studies.
Strain BB1 was grown in 100-ml cultures with acetate plus PCE (25 μl in hexadecane) and acetate plus fumarate (20 mM). When the cultures had consumed all of the PCE or fumarate, the cis-DCE-containing cultures were purged for 10 min with a mixture of 20% CO2 and 80% N2 to remove most of the chloroethene (the hexadecane had previously been removed by syringe). Acetate (5 mM) was added to all cultures, and the pH was adjusted to 7.3 with 1 M KOH. Inside an anaerobic glove box, 25-ml aliquots of the culture fluid were dispensed into 40-ml serum bottles. Chloramphenicol (50 μg per ml of culture fluid) from an ethanol stock solution was added to duplicate cultures that received either 0.4 mM PCE or 1 mM fumarate. Control bottles received only ethanol without the antibiotic. Succinate formation and cis-DCE formation were measured twice a day for 1 week. Additional induction studies were performed with cultures grown with acetate plus PCE (0.33 mM), fumarate (10 mM), or ferric citrate (5 mM). Cultures were challenged with alternate electron acceptors during the exponential growth phase, and electron acceptor consumption was monitored over time.
Inhibition studies.
To test for growth inhibition, citrate (5, 10, or 20 mM), glucose (10 mM), yeast extract (0.1 or 1 g liter−1), or sulfite (1 mM) was added to LC medium containing 5 mM acetate and 20 μmol of PCE prior to inoculation. The effects of these amendments on PCE dechlorination were also tested with actively dechlorinating cultures. Growth was determined by determining the increase in cell number as estimated after acridine orange staining (see above), and dechlorination was monitored by gas chromatography (GC).
Analytical methods.
All volatile compounds were measured in 0.1- or 0.2-ml headspace samples by using a Varian GC (model 3700) or a Hewlett-Packard 6890 GC equipped with flame ionization detectors. The Varian GC was equipped with a Megabore DB-624 column (45 m by 0.543 mm; film thickness, 3 μm; J & W Scientific), and the Hewlett-Packard GC was equipped with an HP-624 column (60 m by 0.32 mm; film thickness, 1.8 μm). Helium was used as the carrier gas. The temperature was kept isocratic at 50°C for 4 min, and this was followed by an increase at a rate of 25°C min−1 to 250°C; then the apparatus was kept at the latter temperature for 4 min. Nonchlorinated alkanes and alkenes were separated and quantified by using a packed stainless steel Porapak Q column (22) or an HP-Plot Q column (30 m by 0.53 mm; film thickness, 40 μm) and flame ionization detection. Elution was isocratic at an oven temperature of 60°C, and N2 or helium was used as the carrier gas. For all headspace measurements, gas-tight 250-μl glass syringes (Hamilton) with gas-tight Teflon valves and Luer Lock adapters were used. Standards for volatile compounds were prepared as described previously (12, 22). Headspace measurements were typically performed at 25°C, and eight-point calibration curves were generated. Additional two-point calibration curves were used to determine dechlorination activity at different incubation temperatures because Henry's Law coefficients are temperature dependent. Aromatic compounds and organic acids were analyzed by high-performance liquid chromatography as reported previously (25, 39). The concentration of chloride ions was determined by using the mercury(II) thiocyanate-iron(III) system with a total assay volume of 1.2 ml (3). Sulfide was quantified spectrophotometrically by the methylene blue method (5). Ferrous iron and total acid-extractable iron were measured spectrophotometrically by the ferrozine assay (18, 45). Except for the rate measurements, protein concentrations were estimated by using a modification of the Lowry assay (46) after alkaline cell lysis of whole cells (10). The standard (ovalbumin) was treated in the same way. For protein estimation with whole cells the variation between two independent measurements of the same sample was less than 20%. Growth in the presence of fumarate was monitored by measuring the A600 in anaerobic culture tubes. Total organic carbon was determined with a Shimadzu TOC-5050A total-organic-carbon analyzer (Shimadzu Scientific Instruments, Kyoto, Japan), and the protein content was calculated based on the assumption that a cell consists of 55% protein and has a composition of C5H7O2N (6, 34). The fraction of electrons consumed in electron acceptor reduction for energy generation (fe) was calculated by assuming that C5H7O2N is the molecular formula of a cell and ammonia is the nitrogen source (6, 27).
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of Desulfuromonas michiganensis strains BB1 and BRS1 have been deposited in the GenBank database under accession numbers AF357915 and AF357914, respectively. Strain BB1 had previously been unofficially named “Desulfuromonas ottawaensis.” The 16S rRNA gene sequence of the heterotrophic sporeformer obtained in pure culture during isolation of strain BB1 has been deposited in the GenBank database under accession number AY221993.
RESULTS
Enrichment and isolation.
Microcosms established with Père Marquette River sediment that were amended with acetate and hydrogen completely dechlorinated PCE to ethene after incubation for 10 weeks. Sequential transfers to medium amended with acetate yielded a culture that rapidly converted PCE to cis-DCE. Dechlorination was not influenced by BES, confirming that PCE-to-cis-DCE dechlorination was not linked to methanogenic metabolism (24). The culture that was obtained after repeated serial dilution-to-extinction series consisted of two populations. The heterotrophic spore-forming population was readily isolated on solidified complex medium under anaerobic conditions. Morphological and physiological characteristics, along with 16S rRNA gene sequence analysis results, suggested that the sporeformer was related to Clostridium sphenoides (49). The isolated sporeformer fermented citrate to acetate as the major product but failed to dechlorinate PCE.
Isolation of the dechlorinating organism was achieved through five additional dilution-to-extinction series in which hexadecane was used as the carrier phase for PCE. Following this procedure, the contaminating sporeformer was eliminated, and consequently, no growth occurred with citrate. Small whitish colonies (diameter, <0.5 mm) developed after 2 months in soft agar cultures when PCE was used as the electron acceptor. With fumarate as the electron acceptor, reddish colonies that were ellipsoidal and 1.5 to 2.5 mm in diameter formed after 2 to 3 weeks. After isolated colonies grown with PCE or fumarate were transferred to fresh medium, PCE-dechlorinating activity was typically recovered within 5 to 10 days. The dechlorinating organism was designated strain BB1, referring to the location where the sediment sample was collected (Bowman's Bridge isolate 1). Additional acetotrophic, PCE-dechlorinating cultures were derived from the same sediment material after 2 years of storage at 4°C and from Père Marquette River sediment collected in the vicinity of Bowman's Bridge in September 1998 (i.e., from the original sampling location and three downstream locations that were approximately 25, 75, and 150 m apart). Observations based on dechlorinating activity, microscopy, and 16S rRNA gene analysis showed that a population similar or identical to isolate BB1 was present in all enrichments (data not shown).
Microcosms established with material from the chloroethene-contaminated Bachman Road site also completely dechlorinated PCE to ethene when acetate and hydrogen were supplied as electron donors. Repeated transfers to medium amended with acetate, PCE, and BES, followed by dilution-to-extinction series, resulted in isolation of strain BRS1 (Bachman Road Site isolate 1). Both strain BB1 and strain BRS1 were investigated further, although the focus was on strain BB1 because it was isolated first and was the first PCE-dechlorinating isolate derived from a pristine environment.
Purity of isolates.
The purity of both isolates was verified as follows: (i) microscopic uniformity, (ii) a dilution-to-extinction series and most-probable-number analysis, (iii) uniform colony morphology in agar shake cultures along with recovery of PCE-dechlorinating activity from single colonies, (iv) a single restriction pattern for PCR-amplified 16S rRNA genes from genomic DNA, (v) a single clone type in 16S rRNA gene clone libraries, and (vi) identical 16S rRNA gene sequences from amplified genomic DNA. Occasionally, an extended lag time prior to dechlorination was observed with inocula from fumarate-grown BB1 cultures. To investigate this phenomenon, a BB1 culture was transferred several times in fumarate medium without PCE, and dilution-to-extinction soft agar cultures were established. Interestingly, colonies developed in fumarate-amended tubes that were diluted 2 orders of magnitude higher than PCE-amended tubes. This procedure yielded an isolate that had lost the ability to dechlorinate PCE but was otherwise indistinguishable from strain BB1. This nondechlorinating isolate was designated strain BB1PCE−
Growth with PCE as the electron acceptor.
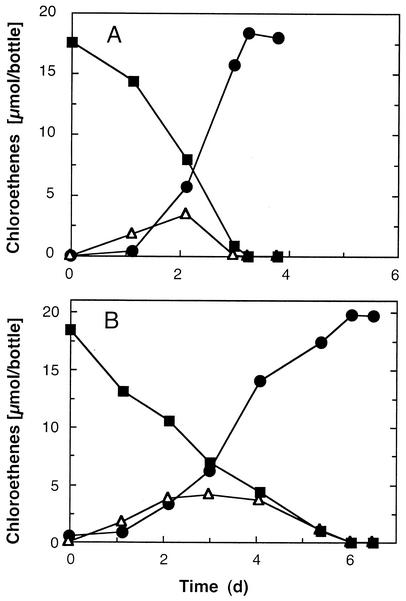
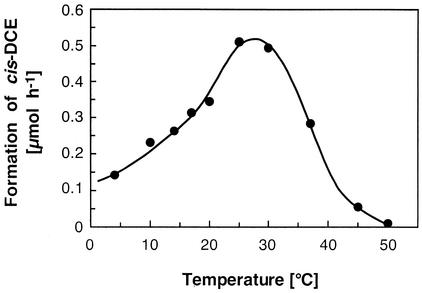
Besides acetate, lactate, pyruvate, succinate, malate, and fumarate supported PCE reduction. Growth with acetate, lactate, pyruvate, or succinate depended strictly on the presence of an electron acceptor (e.g., PCE). Figure 1 shows the dechlorination of PCE to cis-DCE and the intermediate formation of TCE with acetate or lactate as the electron donor. No PCE dechlorination occurred in killed (autoclaved) cultures or in cultures lacking an electron donor. Similar PCE dechlorination rates of at least 139 nmol min−1 mg of protein−1 were obtained with acetate, pyruvate, and succinate as the electron donors. The highest dechlorination rates were measured at temperatures between 25 and 30°C, and 45% of the maximum dechlorination activity was observed at 10°C (Fig. 2). Dechlorination was not supported by the following substrates: hydrogen, formate, propionate, butyrate, methanol, ethanol, 2-propanol, glycerol, citrate, α-ketoglutarate, glucose, fructose, saccharose, lactose, gluconic acid, dl-tryptophan, sulfide, thiosulfate, or ferrous iron.
FIG. 1.
Dechlorination of PCE to cis-DCE with the intermediate formation of TCE. Anaerobic 100-ml cultures in defined BS medium containing 5 mM acetate (A) or 5 mM lactate (B) as the electron donor were inoculated with a 1% (vol/vol) inoculum from an acetate-PCE-grown culture. Dechlorination was monitored by headspace analysis, and data from duplicate cultures were averaged. Symbols: ▪, PCE; ▵, TCE; •, cis-DCE.
FIG. 2.
Temperature dependence of PCE dechlorination. PCE-grown cultures that had consumed 20 μmol of PCE were equilibrated at different temperatures and fed additional PCE. Dechlorination was monitored for 48 h. TCE formation was negligible during the experiment.
Growth at high PCE concentrations.
Both isolates tolerated high concentrations of PCE, although the lag time before PCE dechlorination started increased when the aqueous PCE concentration approached saturation. Following addition of a 2% (vol/vol) inoculum to fresh medium that was saturated with PCE and contained visible PCE DNAPL (total PCE mass added, 200 μmol), strain BB1 consistently produced 197 ± 14 μmol of cis-DCE, 1.05 ± 0.1 μmol of trans-DCE, 0.53 ± 0.03 μmol of 1,1-DCE, and 0.43 ± 0.06 μmol of vinyl chloride. Dechlorination in cultures that received a higher initial dose of PCE ceased when the cis-DCE concentration exceeded 4.3 mM.
Growth without PCE.
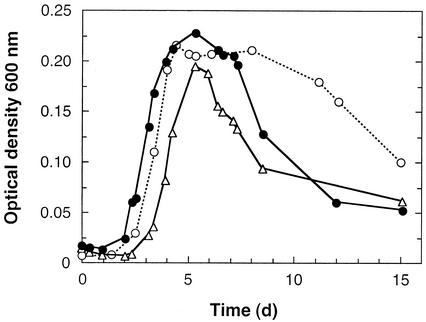
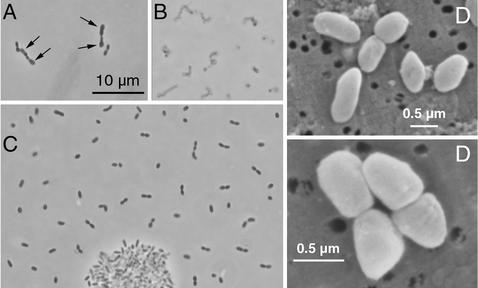
The dechlorinating isolates grew best in the presence of fumarate (Fig. 3). Similar doubling times were obtained when the isolates were grown with fumarate alone, with fumarate plus pyruvate, and with fumarate plus acetate; these doubling times (means ± standard deviations) were 14.4 ± 4.7 h (n = 8), 18.0 ± 2.8 h (n = 12), and 17.1 ± 5.4 h (n = 12), respectively. During fermentative growth with fumarate (or malate) alone, transient accumulation of acetate (<0.1 mM) was observed, and succinate accumulated as the major end product. Rapid decreases in the optical densities of fumarate-grown cultures were observed after the stationary phase was reached. The viscosity of the culture medium increased, and microscopic analysis confirmed that cell lysis occurred (Fig. 4A and B). Fumarate-grown cultures that were incubated for more than 1 month after the stationary phase had been reached contained no viable cells. In contrast, acetate-PCE-grown cultures served as viable inocula for at least 6 months.
FIG. 3.
Growth and changes in optical density at 600 nm in the presence of fumarate at 25°C. Cultures were inoculated with a 1% (vol/vol) inoculum from an acetate-PCE-grown culture. Symbols: •, fumarate; ○, pyruvate plus fumarate; ▵, acetate plus fumarate.
FIG. 4.
Micrographs of Desulfuromonas michiganensis strain BB1. (A) Phase-contrast micrograph of cells grown with fumarate in the early stationary phase. The arrows indicate enlarged and club-shaped cells, a phenomenon observed in fumarate-grown cultures prior to cell lysis. (B) Cells grown with fumarate 3 weeks after the maximum optical density was measured. At this time the culture did not contain viable cells. (C) Phase-contrast micrograph of cells grown with acetate and PCE, showing a cluster of cells typically observed in dechlorinating cultures. Portions of the micrograph were assembled electronically. (D) Scanning electron micrographs of cells grown with acetate and PCE.
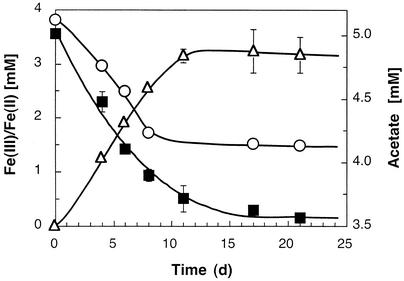
No acetate or lactate consumption and no increase in optical density occurred with sulfate, sulfite, thiosulfate, nitrate, or oxygen as the electron acceptor. Most of the other chlorinated compounds tested were not dechlorinated and did not support growth; the only exception was 1,1,2,2-tetrachloroethane, which was dechlorinated to cis-DCE. Acetate and lactate were oxidized in the presence of elemental sulfur, and there was a concomitant increase in the amount of sulfide. Growth was also supported by ferric iron as an electron acceptor, and ferrous iron accumulated (Fig. 5). Per gram of protein produced, strain BB1 consumed 0.23 mol of acetate and reduced 1.05 mol of ferric iron (as ferric citrate). The production of ferrous iron from poorly crystalline ferric oxide was slower; however, similar ferrous iron formation rates were obtained when anthraquinone-2,6-disulfonate (100 μM) was added to the cultures (data not shown).
FIG. 5.
Reduction of ferric iron (as ferric citrate) coupled to the oxidation of acetate. The production of ferrous iron was linked to growth, as indicated by a visible increase in turbidity, microscopic monitoring, and increased protein concentration (see text). Symbols: ▪, Fe(III); ▵, Fe(II); ○, acetate.
Comparison of growth efficiencies.
The mass balances for strain BB1 grown under PCE-, fumarate-, and Fe(III)-reducing conditions are summarized in Table 1. Under the conditions used and with PCE as the electron acceptor, strain BB1 consumed 0.95 mol of acetate and released 2.56 mol of chloride per g of protein produced. Higher growth yields were observed with fumarate as the electron acceptor, and per gram of protein formed, 0.57 mol of fumarate and 0.2 mol of acetate were consumed. From the yield data, the fe values were calculated to be 0.94 and 0.78 for growth with PCE and fumarate, respectively. Cultures in the stationary phase continued to produce cis-DCE and consume acetate; however, essentially all reducing equivalents were directed towards reductive dechlorination. Obviously, dechlorination became uncoupled from growth, and fe approached the theoretical maximum, 1. In contrast, prior to reaching the stationary growth phase, growing cultures exhibited fe values ranging from 0.6 to 0.7, which is characteristic of chloridogenic populations (27).
TABLE 1.
Mass balances in cultures of Desulfuromonas michiganensis strain BB1 under different growth conditionsa
| Initial substrate concn (mM) | Concn of products formed (mM)b
|
Biomass formed (mg 100 ml−1)
|
Electron recovery (%) | Distribution of reducing equivalents (fe) by method:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCEc | Fumarate | Fe(III) | Acetate | cis-DCEd | Succinate | Fe(II) | Acetate | Protein | Cells (dry wt) | 1g | 2h | |
| 19.2 (0.04)e | 8.9 (0.2) | 18.4 (0.15) | 2.2 | 3.2 (0.16) | 3.7 (1.2) | 93 | 0.69 | 0.78 | ||||
| 12.25 | 17.2 (0.5) | 5.3 (0.2) | 13.3 | 0.41 (0.11) | 0.73 (0.4) | 94 | 0.68 | 0.94 | ||||
| 5 (0.1) | 10.2 (0.02) | 3.68 (0.24) | 9.41 (0.17) | 0.34c | 0.62 (0.02)f | 94 | 0.58 | 0.77 | ||||
PCE cultures were analyzed after 35 days; all other cultures were analyzed after 28 days. The dry weight measurements were obtained after 45 days.
In cultures containing pyruvate, two unidentified peaks were detected by high-performance liquid chromatography analysis. Fumarate was completely consumed in fumarate-fed cultures.
Initial concentration of PCE assuming that all PCE was in the aqueous phase. Hexadecane (10 ml) containing 125 μl of PCE was added to each bottle. At the conclusion of the experiment dechlorination had ceased because the pH of the medium had dropped below 6.7. Dechlorination, however, resumed after addition of base and adjustment of the pH to 7.2.
The amount of cis-DCE formed was determined by measuring the increase in chloride concentration in the medium. Only negligible amounts of TCE were present after 35 days.
The values in parentheses are standard deviations.
The amount of total organic carbon was determined with a total-organic-carbon analyzer, and dry weight was calculated by assuming that C5H7O2N is the molecular formula of a cell.
Method 1, calculated from the amount of reducing equivalents released during complete acetate oxidation and consumed during electron acceptor reduction (27).
Method 2, calculated from yield (fs) data by using fe = 1 − fs and the following equations: fe=[electron acceptor reduced (molar)]×(1 e− equivalent accepted/mol)/[acetate consumed (molar)] ×(8e− equivalents/mol)/and fs = [cell concentration (molar)]×(20e− equivalents/mol]/[acetate consumed (molar)] × (8e− equivalents/mol). Molecular weight of cells, 113.
Inhibition and induction studies.
PCE dechlorination was not influenced by addition of 20 mM citrate or 10 mM glucose to the medium. Also, addition of 0.1 or 1 g of yeast extract per liter to dechlorinating cultures did not affect PCE reduction. When yeast extract (1 g liter−1) was added prior to inoculation, dechlorination started following a prolonged lag phase, after which similar dechlorination rates were observed compared with cultures that did not receive yeast extract. Only negligible dechlorination and no growth occurred in full-strength reduced complex media like tryptic soy broth and Luria-Bertani medium after inoculation (1%, vol/vol) from an acetate-PCE-grown culture. Addition of 5 mM acetate did not stimulate dechlorination, indicating that the isolates were inhibited under nutrient-rich conditions. Sulfite had a strong inhibitory effect, and neither growth nor dechlorination occurred in the presence of 1 mM sulfite. Addition of 1 mM sulfite to PCE-dechlorinating cultures resulted in rapid and complete inhibition of dechlorination activity (data not shown).
The culture medium was routinely reduced with sodium sulfide plus l-cysteine (0.2 mM each). Interestingly, growth and dechlorination ceased when the strains were repeatedly transferred to medium that was reduced with 1 mM dl-dithiothreitol. Growth and dechlorination resumed after addition of 0.01 mM sodium sulfide or elemental sulfur to inhibited cultures, whereas addition of 1 mM sulfate had no effect. Sulfide was not an electron donor for dechlorination, and hence, the most plausible explanation is that sulfide was a required sulfur source.
Resting cell experiments were conducted to determine whether fumarate reductase, Fe(III) reductase, and PCE reductase activities were constitutively present or whether these enzyme systems were induced by their respective substrates. In the presence of the protein biosynthesis inhibitor chloramphenicol, fumarate-grown cells did not dechlorinate PCE but continued to reduce fumarate to succinate. Similarly, cells grown with PCE continued to dechlorinate PCE to TCE and cis-DCE in the presence of chloramphenicol, but no fumarate reduction occurred. In the absence of chloramphenicol, fumarate-grown cells started to dechlorinate PCE after a lag phase of 3 to 5 days. A similar lag time was observed with acetate-PCE-grown cells before fumarate reduction occurred. PCE- or fumarate-grown cells reduced Fe(III) with a lag period of no more than 4 days, and a lag time of 3 to 8 days was observed before Fe(III)-grown cells reduced PCE or fumarate (data not shown). These findings suggest that the different reductases were induced by, and are specific for, their respective substrates.
PCE dechlorination in the presence of alternate electron acceptors.
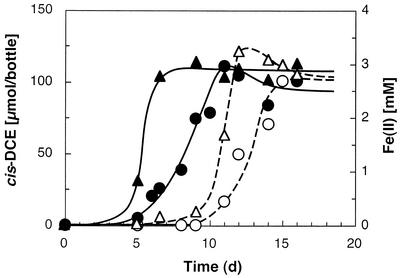
Experiments were performed to determine the influence of fumarate and Fe(III) on PCE dechlorination. As illustrated in Fig. 6, PCE and the competing electron acceptor were simultaneously reduced. Similar observations were made with fumarate as a competing electron acceptor.
FIG. 6.
Concomitant reduction of PCE and Fe(III) in cultures of strain BB1. Symbols: ○ and •, cis-DCE formed; ▵ and ▴, Fe(II) formed. Culture bottles amended with acetate received a 0.5% (vol/vol) inoculum from a culture grown with acetate and PCE (solid symbols) or from a culture grown with acetate and Fe(III) (open symbols). Since cis-DCE was measured in the headspace, it apparently trailed Fe(II) formation, which was measured in the aqueous phase.
Morphology and phylogeny.
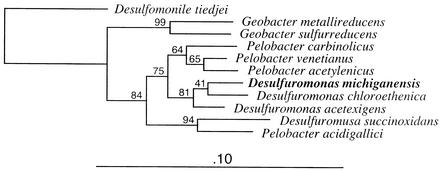
Single cells were short ovoid rods with rounded ends that were 0.8 to 1.4 μm long and 0.4 to 0.5 μm in diameter (Fig. 4C and D). In old cultures cells occurred predominantly in pairs. A small percentage (<5%) of single cells were motile. Strains BB1, BB1PCE−, and BRS1 did not grow on solid surfaces; however, colonies developed in semisolid medium. Comparative 16S rRNA gene sequencing revealed that the isolates had identical sequences and placed them in the δ subgroup of the Proteobacteria; Desulfuromonas chloroethenica was the closest relative (Fig. 7). Several other members of the Geobacteraceae, including Desulfuromonas acetexigens DSM 1397 and DSM 8988, Desulfuromonas acetoxidans DSM 684, Desulfuromonas succinoxidans DSM 8964, Desulfuromonas thiophila DSM 8987, and Geobacter metallireducens, were unable to dechlorinate PCE, confirming that the ability to reductively dechlorinate is not uniformly distributed in this group.
FIG. 7.
Inferred phylogenetic relationships of the new isolates and selected nearest relatives. The tree was generated by using the neighbor-joining method and ca. 1,300 nucleotide positions. The bootstrap values at the nodes are based on 1,000 replicates. Desulfomonile tiedjei was included as the outgroup to root the phylogenetic tree.
DISCUSSION
Desulfuromonas michiganensis couples oxidation of acetate to reduction of PCE to cis-DCE. Two dechlorinating isolates, strain BB1 from pristine river sediment with a high organic matter content and strain BRS1 from an oligotrophic chloroethene-contaminated aquifer, were obtained. Although the organisms were obtained from dissimilar environments, they had identical 16S rRNA genes and were indistinguishable on the basis of physiological characteristics. Other PCE-dechlorinating organisms have been isolated from contaminated environments, including digestor sludge, an aquifer, soil, and sediment materials (summarized in references 4, 16, and 47). The presence of PCE-dechlorinating organisms is generally explained by an adaptation to the presence of the chlorinated pollutant. The sampling sites in the Père Marquette River are located in a quality fly fishing zone, and this environment is considered pristine and nonpolluted. Surprisingly, we never failed to enrich for acetate-dependent PCE-to-cis-DCE dechlorinating activity using sediment material from this stretch of the river, indicating that PCE-dechlorinating Desulfuromonas species were broadly distributed. A recent 16S rRNA gene-based study corroborated the presence and distribution of Desulfuromonas in this river sediment (26). The stable spatial and temporal occurrence of the PCE-dechlorinating organism in a pristine environment with no known history of anthropogenic exposure to the pollutant indicates that PCE-dechlorinating populations can be common members of natural anaerobic microbial communities. Why the ability to dechlorinate PCE is maintained in pristine environments is unclear. The increased cognizance of natural chlorine cycles provides an intriguing explanation: dechlorinating populations take advantage of naturally produced chlorinated compounds as favorable electron acceptors and are part of anaerobic food webs that involve chloroorganic compounds (1, 13, 23, 36, 48).
Although a conservative approach was used to estimate the protein contents of growing cultures, the specific dechlorination rates measured for both strains were within the ranges reported for other high-rate PCE-dechlorinating populations. Unfortunately, rate data for the different PCE-dechlorinating populations were obtained under various conditions (e.g., in growing cultures, in resting cell experiments, in chemostats) and with different PCE and electron donor concentrations; hence, the ranges vary by an order of magnitude and are not directly comparable.
A BB1 variant strain that lacked the ability to reduce PCE was obtained. Strain BB1PCE− was isolated from a fumarate-grown culture that had previously been transferred at least 35 consecutive times to PCE-acetate medium. Since growth under these conditions depended on reductive dechlorination, the most likely explanation is that strain BB1PCE− spontaneously lost the ability to dechlorinate PCE when it was grown with fumarate. Interestingly, a nondechlorinating variant of Dehalospirillum multivorans was isolated from the same activated sludge material as the well-studied dechlorinating strain, suggesting that loss of dechlorinating activity might not be an uncommon event (42). Further investigations are necessary to elucidate the nature of this loss of physiological function and to explore its relevance for bioremediation approaches. The existence of the BB1PCE− strain with a 16S rRNA gene that is identical to the 16S rRNA gene of the dechlorinating Desulfuromonas michiganensis strains raises the question of whether 16S rRNA gene-targeted primers are useful for evaluating the dechlorination potential at chloroethene-contaminated sites (26). So far, we have never failed to detect acetotrophic PCE-to-cis-DCE dechlorinating activity in samples that tested positive for the presence of a PCE-dechlorinating Desulfuromonas species. Hence, isolation of strain BB1PCE− is possibly a laboratory artifact, and the 16S rRNA gene-based approach targeting PCE-dechlorinating Desulfuromonas populations in environmental samples is valid. To rule out false-positive results with confidence, microcosm studies verifying dechlorination activity are recommended.
16S rRNA gene analysis demonstrated that the new isolates belong to the Desulfuromonas cluster in the Geobacteraceae (28). The 16S rRNA molecules of strains BB1 and BRS1 have the secondary structural characteristics of the 16S rRNA molecules of members of the Desulfuromonas cluster (28). The family Geobacteraceae is physiologically diverse; however, its members have the ability to reduce Fe(III) and/or S0. Reductive dechlorination is not a common trait in this family, and only two dechlorinating organisms in the Geobacteraceae have been described thus far: Trichlorobacter thiogenes and Desulfuromonas chloroethenica. Trichlorobacter thiogenes grows with trichloroacetate as an electron acceptor, converting it to dichloroacetate in the presence of acetate and sulfide. Interestingly, this organism requires sulfide as the direct electron donor for reductive dechlorination (7). Sulfide did not support reductive dechlorination in strains BB1 and BRS1, and the sulfur-sulfide redox cycle described for Trichlorobacter thiogenes seems not to be commonly distributed among members of the Geobacteraceae, as recently suggested (43). The other known dechlorinating organism in the Geobacteraceae is Desulfuromonas chloroethenica, which reduces PCE to cis-DCE. Table 2 shows a similarity matrix based on the sequences of the new isolates and members of the Geobacteraceae. The 16S rRNA gene sequences of strains BB1 and BRS1 are 97.5% similar to the sequence of Desulfuromonas chloroethenica. Bootstrap analysis showed that strains BB1 and BRS1 formed a coherent phylogenetic group with Desulfuromonas chloroethenica and Desulfuromonas acetexigens. Stackebrandt and Goebel (44) have shown that strains with 16S rRNA gene sequence similarities of less than 97 to 97.5% do not exhibit 70% DNA-DNA homology and should therefore be considered members of different species. For classification of an isolate as a new species, 16S rRNA gene sequence dissimilarities and phenotypic differences between it and closely related organisms must be considered. A detailed comparison of available physiological data for Desulfuromonas chloroethenica and Desulfuromonas michiganensis is presented in Table 3, demonstrating that there are relevant physiological differences. For instance, the new isolates have a fermentative metabolism, and they use a wider range of electron donors, including lactate and succinate, exhibit higher growth yields and shorter doubling times, have a broader temperature range, and tolerate higher levels of PCE than Desulfuromonas chloroethenica. Based on 16S rRNA gene sequence dissimilarities and key phenotypic differences compared with their closest relatives, we propose that the new isolates belong to a new species, Desulfuromonas michiganensis.
TABLE 2.
Pairwise similarity values for Desulfuromonas michiganensis and selected bacterial speciesa
| Species | % Similarity
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Desulfuro- monas michiganensis | Desulfuro- monas chloroethenica | Desulfuro- monas acetexigens | Pelobacter venetianus | Pelobacter acetylenicus | Pelobacter carbinolicus | Geobacter metalli- reducens | Trichloro- bacter thiogenes | Desulfo- monile tiedjei | |
| Desulfuromonas michiganensis | 100 | ||||||||
| Desulfuromonas chloroethenica | 97.5 | 100 | |||||||
| Desulfuromonas acetexigens | 97.0 | 97.0 | 100 | ||||||
| Pelobacter venetianus | 95.5 | 94.1 | 95.0 | 100 | |||||
| Pelobacter acetylenicus | 95.1 | 94.1 | 95.2 | 97.8 | 100 | ||||
| Pelobacter carbinolicus | 94.6 | 94.2 | 94.4 | 96.5 | 96.4 | 100 | |||
| Geobacter metallireducens | 90.9 | 90.3 | 90.8 | 90.8 | 90.1 | 90.3 | 100 | ||
| Trichlorobacter thiogenes | 88.1 | 87.8 | 88.1 | 88.4 | 88.1 | 87.7 | 91.7 | 100 | |
| Desulfomonile tiedjei | 84.9 | 85.1 | 85.0 | 85.5 | 86.1 | 86.3 | 85.2 | 84.2 | 100 |
Similarity values were calculated for 1,234 aligned positions by using the Phylip interface program available from RDP (http://rdp.cme.msu.edu). The Jukes-Cantor method and empirical base frequencies were used.
TABLE 3.
Comparison of phenotypic characteristics of Desulfuromonas chloroethenica and Desulfuromonas michiganensis
| Strain | Electron donors for dechlorination | Electron acceptors | Growth with Fe(III) | Growth yield with PCEa
|
PCE tolerance (mM) | Inhibition of dechlori- nation by 0.1% yeast extract | Temp range for growth (°C) | Temp range for dechlorination (°C) | pH rangea | Optimum pHa | Doubling time (days) with:
|
Motility | Source | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g of protein/ mol of Cl− | g of protein/ mol of acetate | Acetate and fumarate | Acetate and PCE | ||||||||||||
| Desulfuromonas chloroethenica TT4Bb | Acetate, pyruvate | PCE, TCE, fumarate, polysulfide, ferric iron | No visible growth | 0.15 | 0.65 | <0.1 | Yes | 21-31 | NA | 6.5-7.4 | 7.4 | 4 | 2-4 | Nonmotile | TCE-contami- nated stream sediment |
| Desulfuromonas michiganensis BB1 | Acetate, lactate, pyruvate, succi- nate, fumarate, malate | PCE, TCE, fumarate, malate, sulfur (poly- sulfide),c ferric iron | Growthd | 0.39 | 1.05 | ∼1 (saturation in the pres- ence of free- phase PCE) | No | 10-35e | 4-40f | 6.8-8 | 7.0-7.5 | 0.71 | <2 | Motileg | Pristine river sediment |
Tested with acetate and PCE as the substrates.
Data from references 19 and 20.
Sulfur was added from a pasteurized sulfur flower suspension in water to a sulfide-reduced medium.
Growth occurred with soluble ferric citrate and poorly crystalline ferric oxide.
No growth occurred at 4 and 45°C.
About 25% of the maximum dechlorinating activity was obtained at 4 and 40°C in resting cell experiments.
Motility was observed microscopically. A small percentage of cells were motile during the logarithmic growth phase.
Although the new isolates do not promote complete dechlorination, they have unique physiological properties that make them attractive for bioremediation. For instance, (i) dechlorination occurs at temperatures typically encountered in aquifers, (ii) dechlorination is not inhibited by competing terminal electron-accepting processes [e.g., Fe(III) reduction], (iii) both strains tolerate high concentrations of PCE and have possible applications near source zones with free-phase solvent (e.g., PCE DNAPL), and (iv) both strains utilize lactate and acetate as electron donors. Lactate is frequently used to stimulate microbial activity at contaminated sites (e.g., to supply reducing equivalents to reductively dechlorinating populations). In most natural environments, lactate is readily fermented by soil microbes, yielding propionate, acetate, and hydrogen as major fermentation end products. Most chloroethene-dechlorinating populations require hydrogen for reductive dechlorination and compete with other hydrogenotrophic populations (e.g., acetogens and methanogens) for this electron donor. The PCE-dechlorinating Desulfuromonas strains, in contrast, use acetate (or lactate directly) as an electron donor for reductive dechlorination. Hence, the fierce competition with hydrogenotrophic populations is alleviated, and a greater proportion of reducing equivalents is consumed in reductive dechlorination, which increases the efficiency of biostimulation approaches.
The ability of the new isolates to tolerate high PCE concentrations is unusual, as dechlorination occurred at saturating concentrations of PCE. Most other PCE-dechlorinating isolates, including Dehalobacter restrictus, Desulfuromonas chloroethenica, Dehalospirillum multivorans, and Dehalococcoides ethenogens isolates, were completely inhibited at high PCE concentrations (17, 20, 31, 35). Clostridium bifermentans strain DPH-1, Desulfitobacterium sp. strain Y51, and Enterobacter agglomerans strain MS-1 were reported to tolerate PCE concentrations near saturation (4, 41, 47). Strain MS-1, however, failed to dechlorinate more than 1 mM PCE to cis-DCE, probably due to cis-DCE toxicity (41).
Relatively little is known about how the presence of alternate electron acceptors affects reductively dechlorinating populations, although such information is relevant for engineered bioremediation approaches. Typically, electron acceptors are consumed sequentially, with the thermodynamically most favorable reaction occurring first and the least favorable electron acceptor consumed last. As expected, PCE dechlorination in Enterobacter agglomerans strain MS-1 was completely inhibited by oxygen or nitrate, and oxygen and nitrate were the preferred electron acceptors (41). Gerritse et al. (11) observed that PCE dechlorination by Desulfitobacterium frappieri strain TCE1 was not inhibited by nitrate or fumarate in PCE-limited chemostat cultures. In contrast, dechlorination with strain TCE1 ceased under electron donor-limiting conditions when excess nitrate or fumarate was present (11). PCE dechlorination in batch cultures of Dehalospirillum multivorans was inhibited by fumarate, even though the PCE-dechlorinating enzyme system was constitutively expressed (35). Strains BB1 and BRS1 consumed PCE and ferric iron or fumarate concomitantly, suggesting that the influence of alternate electron acceptors on the reductive dechlorination process is species specific and cannot be explained by thermodynamics alone. Many contaminated sites are rich in bioavailable ferric iron, and iron reduction may interfere with the reductive dechlorination process (8). PCE dechlorination by the new Desulfuromonas isolates, however, was not inhibited by ferric iron (or fumarate), suggesting that the reductive dechlorination process can be viable even in the presence of alternate energetically favorable electron acceptors (e.g., bioavailable ferric iron).
Description of Desulfuromonas michiganensis sp. nov.
Desulfuromonas michiganensis (mich.i.gan.en′sis). N.L. adj. michiganensis, from the state of Michigan, which contains the Manistee Forest with the Père Marquette River and the Bachman Road aquifer in Oscoda. The species epithet indicates the geographic location (the state of Michigan) where the sediment and aquifer samples used for isolation of strains BB1 and BRS1 were collected.
Cells are small ovoid rods, about 0.4 to 0.5 μm wide by 0.8 to 1.4 μm long. Cells occur singly or in pairs, and chains develop in fumarate-grown cultures. Rapid cell lysis occurs in the stationary phase when cultures are grown with fumarate. A small proportion of single cells are motile. Growth is obligately anaerobic. Sulfide is required for growth. No colonies are formed on solid surfaces. Reddish colonies are formed in semisolid medium amended with fumarate. PCE, TCE, sulfur, ferric iron, fumarate, and malate are growth-supporting electron acceptors. PCE is reduced to cis-DCE as the major product by an inducible enzyme system. Competing electron acceptors (e.g., PCE, fumarate, and ferric iron) are reduced concomitantly. Electron donors include fumarate, malate, succinate, pyruvate, lactate, and acetate. The optimum pH ranges from 7.0 to 7.5, and the optimum temperature for dechlorination is 25°C. The isolates tolerate high concentrations of PCE and dechlorinate in the presence of free-phase PCE (DNAPL). 16S rRNA gene analysis groups both isolates in the Desulfuromonas cluster in the Geobacteraceae in the δ subgroup of the Proteobacteria. The habitats are anoxic freshwater environments (mud, sediment, aquifer). Phenotypic characteristics and the 16S rRNA gene sequence distinguish the new isolates from previously described members of the genus Desulfuromonas. Strain BB1 is the type strain of the new species Desulfuromonas michiganensis.
Acknowledgments
This research was supported by the Michigan Department of Environmental Quality, by the Department of Defense's Strategic Environmental Research and Development Program (contract DACA72-00-C-0023), and by NSF CAREER award 0090496 to F.E.L.
We thank H. G. Trüper for advice concerning prokaryote nomenclature and Shirley Owens at the Michigan State University Center for Advanced Microscopy for help with scanning electron microscopy.
REFERENCES
- 1.Abrahamsson, K., A. Ekdahl, J. Collén, E. Fahlström, and M. Pedersén. 1995. The natural formation of trichloroethylene and perchloroethylene in seawater, p. 327-331. In A. Grimwall and E. W. B. de Leer (ed.), Naturally-produced organohalogens. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 2.Atlas, R. M. 1997. Handbook of microbiological media. CRC Press, Inc., Boca Raton, Fla.
- 3.Bergmann, J. G., and J. Sanik, Jr. 1957. Determination of trace amounts of chlorine in naphtha. Anal. Chem. 29:241-243. [Google Scholar]
- 4.Chang, Y. C., M. Hatsu, K. Jung, Y. S. Yoo, and K. Takamizawa. 2000. Isolation and characterization of a tetrachloroethylene dechlorinating bacterium, Clostridium bifermentans DPH-1. J. Biosci. Bioeng. 89:489-491. [DOI] [PubMed] [Google Scholar]
- 5.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 6.Criddle, C. S., L. M. Alvarez, and P. L. McCarty. 1991. Microbial processes in porous media, p. 641-691. In J. Bear and M. Y. Corapcioglu (ed.), Transport processes in porous media. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 7.De Wever, H., J. R. Cole, M. R. Fettig, D. A. Hogan, and J. M. Tiedje. 2000. Reductive dehalogenation of trichloroacetic acid by Trichlorobacter thiogenes gen. nov., sp.nov. Appl. Environ. Microbiol. 66:2297-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, P. J., and S. S. Koenigsberg. 2001. A bioavailable ferric iron assay and relevance to reductive dechlorination, p. 209-215. In A. Leeson, B. C. Alleman, P. J. Alvarez, and V. S. Magar (ed.), Bioaugmentation, biobarriers, and biogeochemistry. Battelle Press, Columbus, Ohio.
- 9.Fetzner, S. 1998. Bacterial dehalogenation. Appl. Microbiol. Biotechnol. 50:633-657. [DOI] [PubMed] [Google Scholar]
- 10.Gerhardt, P., R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.). 1981. Manual of methods for general bacteriology. American Society for Microbiology, Washington, D. C.
- 11.Gerritse, J., O. Drzyzga, G. Kloetstra, M. Keijmel, L. P. Wiersum, R. Hutson, M. D. Collins, and J. C. Gottschal. 1999. Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl. Environ. Microbiol. 65:5212-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gossett, J. M. 1987. Measurement of Henry's Law constants for C1 and C2 chlorinated hydrocarbons. Environ. Sci. Technol. 21:202-208. [Google Scholar]
- 13.Gribble, G. W. 1992. Naturally occurring organohalogen compounds—a survey. J. Nat. Prod. 55:1353-1395. [DOI] [PubMed] [Google Scholar]
- 14.He, J., Y. Sung, M. E. Dollhopf, B. Z. Fathepure, J. M. Tiedje, and F. E. Löffler. 2002. Acetate versus hydrogen as direct electron donors to stimulate the microbial reductive dechlorination process at chloroethene-contaminated sites. Environ. Sci. Technol. 36:3945-3952. [DOI] [PubMed] [Google Scholar]
- 15.Hickman, J. C. 1993. Tetrachloroethylene, p. 50-59. In J. I. Kroschwits and M. Howe-Grant (ed.), Kirk-Othmer encyclopedia of chemical technology, 4th ed., vol. 6. John Wiley & Sons, Inc., New York, N.Y.
- 16.Holliger, C., D. Hahn, H. Harmsen, W. Ludwig, W. Schumacher, B. Tindall, F. Vazquez, N. Weiss, and A. J. B. Zehnder. 1998. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra- and trichloroethene in an anaerobic respiration. Arch. Microbiol. 169:313-321. [DOI] [PubMed] [Google Scholar]
- 17.Holliger, C., G. Schraa, A. J. M. Stams, and A. J. B. Zehnder. 1993. A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Appl. Environ. Microbiol. 59:2991-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostka, J. E., and G. W. Luther III. 1994. Partitioning and speciation of solid phase iron in saltmarsh sediments. Geochim. Cosmochim. Acta 58:1701-1710. [Google Scholar]
- 19.Krumholz, L. R. 1997. Desulfuromonas chloroethenica sp. nov. uses tetrachloroethylene and trichloroethylene as electron acceptors. Int. J. Syst. Bacteriol. 47:1262-1263. [Google Scholar]
- 20.Krumholz, L. R., R. Sharp, and S. Fishbain. 1996. A freshwater anaerobe coupling acetate oxidation to tetrachloroethene dehalogenation. Appl. Environ. Microbiol. 62:4108-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane, D. J., N. Pace, G. J. Olsen, D. A. Stahl, and M. L. Sogin. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 82:6955-6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löffler, F. E., J. E. Champine, K. M. Ritalahti, S. J. Sprague, and J. M. Tiedje. 1997. Complete reductive dechlorination of 1,2-dichloropropane by anaerobic bacteria. Appl. Environ. Microbiol. 63:2870-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löffler, F. E., J. R. Cole, K. M. Ritalahti, and J. M. Tiedje. Diversity of dechlorinating bacteria, p. 53-87. In M. M. Häggblom and I. D. Bossert (ed.), Dehalogenation: microbial processes and environmental applications, in press. Kluwer Academic Press, Dordrecht, The Netherlands.
- 24.Löffler, F. E., K. M. Ritalahti, and J. M. Tiedje. 1997. Dechlorination of chloroethenes is inhibited by 2-bromoethanesulfonate in the absence of methanogens. Appl. Environ. Microbiol. 63:4982-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Löffler, F. E., R. A. Sanford, and J. M. Tiedje. 1996. Initial characterization of a reductive dehalogenase from Desulfitobacterium chlororespirans Co23. Appl. Environ. Microbiol. 62:3809-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Löffler, F. E., Q. Sun, J. Li, and J. M. Tiedje. 2000. 16S rRNA gene-based detection of tetrachloroethene (PCE)-dechlorinating Desulfuromonas and Dehalococcoides species. Appl. Environ. Microbiol. 66:1369-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Löffler, F. E., J. M. Tiedje, and R. A. Sanford. 1999. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl. Environ. Microbiol. 65:4049-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lonergan, D. J., H. L. Jenter, J. D. Coates, E. J. P. Phillips, T. M. Schmidt, and D. R. Lovley. 1996. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J. Bacteriol. 178:2402-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovley, D. R. 1December2000, posting date. Fe(III)- and Mn(IV)-reducing prokaryotes. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes. [Online.] Springer-Verlag, Inc., New York, N.Y. http://www.prokaryotes.com.
- 30.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schimdt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maymó-Gatell, X., Y.-T. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 32.McCarty, P. L. 1997. Breathing with chlorinated solvents. Science 276:1521-1522. [DOI] [PubMed] [Google Scholar]
- 33.Miller, E., G. Wohlfahrt, and G. Diekert. 1997. Comparative studies on tetrachloroethene reductive dechlorination by Desulfitobacterium sp. strain PCE-S. Arch. Microbiol. 168:513-519. [DOI] [PubMed] [Google Scholar]
- 34.Neidhardt, C. F., and E. Umbarger. 1996. Chemical composition of Escherichia coli, p. 13-16. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 35.Neumann, A., H. Scholz-Muramatsu, and G. Diekert. 1994. Tetrachloroethene metabolism of Dehalospirillum multivorans. Arch. Microbiol. 162:295-301. [DOI] [PubMed] [Google Scholar]
- 36.Öberg, G. 2002. The natural chlorine cycle—fitting the scattered pieces. Appl. Microbiol. Biotechnol. 58:565-581. [DOI] [PubMed] [Google Scholar]
- 37.Paul, E. A., D. Harris, M. Klug, and R. Ruess. 1999. The determination of microbial biomass, p. 291-317. In G. P. Robertson, C. S. Bledsoe, D. C. Coleman, and P. Sollins (ed.), Standard soil methods for long term ecological research. Oxford University Press, New York, N.Y.
- 38.Saitou, N., and M. Nei. 1987. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 39.Sanford, R. A., J. R. Cole, F. E. Löffler, and J. M. Tiedje. 1996. Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl. Environ. Microbiol. 62:3800-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholz-Muramatsu, H., A. Neumann, M. Meßmer, E. Moore, and G. Diekert. 1995. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch. Microbiol. 163:48-56. [Google Scholar]
- 41.Sharma, P. K., and P. L. McCarty. 1996. Isolation and characterization of a facultative bacterium that reductively dehalogenates tetrachloroethene to cis-1,2-dichloroethene. Appl. Environ. Microbiol. 62:761-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siebert, A., A. Neumann, T. Schubert, and G. Diekert. 2002. A non-dechlorinating strain of Dehalospirillum multivorans: evidence for a key role of the corrinoid cofactor in the synthesis of an active tetrachloroethene dehalogenase. Arch. Microbiol. 178:443-449. [DOI] [PubMed] [Google Scholar]
- 43.Snoeyenbos-West, O., C. Gaw Van Praagh, and D. R. Lovley. 2001. Trichlorobacter thiogenes should be renamed as a Geobacter species. Appl. Environ. Microbiol. 67:1020-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stackebrandt, E., and B. M. Goebel. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 45.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 46.Stoscheck, C. M. 1990. Quantitation of protein. Methods Enzymol. 182:50-68. [DOI] [PubMed] [Google Scholar]
- 47.Suayama, A., M. Yamashita, S. Yoshino, and K. Furukawa. 2002. Molecular characterization of the PceA reductive dehalogenase of Desulfitobacterium sp. strain Y51. J. Bacteriol. 184:3419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Pée, K.-H. 2001. Microbial biosynthesis of halometabolites. Arch. Microbiol. 175:250-258. [DOI] [PubMed] [Google Scholar]
- 49.Walther, R., H. Hippe, and G. Gottschalk. 1977. Citrate, a specific substrate for the isolation of Clostridium sphenoides. Appl. Environ. Microbiol. 33:955-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic analysis. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weisburg, W. G., Y. Oyaizu, H. Oyaizu, and C. R. Woese. 1985. Natural relationship between bacteroides and flavobacteria. J. Bacteriol. 164:230-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wild, A., R. Hermann, and T. Leisinger. 1996. Isolation of an anaerobic bacterium which reductively dechlorinates tetrachloroethene and trichloroethene. Biodegradation 7:507-511. [DOI] [PubMed] [Google Scholar]
- 53.Woese, C. R., R. Gutell, R. Gupta, and H. F. Noller. 1983. Detailed analysis of the higher-order structure of the 16S-like ribosomal ribonucleic acids. Microbiol. Rev. 47:621-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolin, E. A., M. J. Wolin, and R. S. Wolfe. 1963. Formation of methane by bacterial extracts. J. Biol. Chem. 238:2882-2886. [PubMed] [Google Scholar]