Abstract
We report the isolation and physiological characterization of novel, psychrophilic, iron-oxidizing bacteria (FeOB) from low-temperature weathering habitats in the vicinity of the Juan de Fuca deep-sea hydrothermal area. The FeOB were cultured from the surfaces of weathered rock and metalliferous sediments. They are capable of growth on a variety of natural and synthetic solid rock and mineral substrates, such as pyrite (FeS2), basalt glass (∼10 wt% FeO), and siderite (FeCO3), as their sole energy source, as well as numerous aqueous Fe substrates. Growth temperature characteristics correspond to the in situ environmental conditions of sample origin; the FeOB grow optimally at 3 to 10°C and at generation times ranging from 57 to 74 h. They are obligate chemolithoautotrophs and grow optimally under microaerobic conditions in the presence of an oxygen gradient or anaerobically in the presence of nitrate. None of the strains are capable of using any organic or alternate inorganic substrates tested. The bacteria are phylogenetically diverse and have no close Fe-oxidizing or autotrophic relatives represented in pure culture. One group of isolates are γ-Proteobacteria most closely related to the heterotrophic bacterium Marinobacter aquaeolei (87 to 94% sequence similarity). A second group of isolates are α-Proteobacteria most closely related to the deep-sea heterotrophic bacterium Hyphomonas jannaschiana (81 to 89% sequence similarity). This study provides further evidence for the evolutionarily widespread capacity for Fe oxidation among bacteria and suggests that FeOB may play an unrecognized geomicrobiological role in rock weathering in the deep sea.
In the deep-sea environment, hydrothermal habitats are recognized as sites that harbor unique biological ecosystems that are underpinned by chemosynthetic microbial primary production (22, 26). These prokaryotes derive energy from oxidation of reduced chemicals emanating from these sites (e.g., hydrogen sulfide [H2S]) and fix ambient CO2 for cellular organic carbon needs. However, not all reduced chemicals released at these sites are available for immediate conversion to biomass. Rather, the interaction between geothermally heated fluids and cold seawater results in the precipitation of massive mineral deposits, primarily metal sulfides (40). Sulfides occur as chimney structures, brecciated rubble, particulate material (black smoke), and metalliferous sediment formed by settling particles. These deposits are composed of minerals such as pyrite (FeS2), chalcopyrite (CuFeS2), sphalerite (ZnS), marcasite (FeS2), and pyrrhotite (FeS). At circumneutral pH in the presence of O2 (and other suitable oxidants, e.g., Fe3+), these minerals will oxidize abiogenically (33-35). Similar to the oxidation of reduced aqueous chemical species, oxidation of these minerals releases energy, which if mediated or catalyzed by microorganisms can be harnessed for cellular processes.
On a quantitative basis, sulfide and sulfur oxidation may account for a large portion of the geochemical energy available for microbial growth in these ecosystems. In one study, it was calculated that in a typical hydrothermal system, the amount of energy available to support microbial growth based on oxidation of minerals (principally pyrite) exceeded what would be available based on oxidation of aqueous chemicals (principally H2S) by nearly an order of magnitude per kilogram of vent fluid (32).
For most sulfide minerals, pyrite being the most abundant, energy is available from oxidation of ferrous iron (Fe2+), disulfide (2[S−]), or both. For Fe2+, only one electron is transferred per mole of Fe oxidized, and 1 mol is available for oxidation per mol of pyrite. The energetics of this reaction are not very favorable, with an E0′ for the Fe2+-Fe3+ couple of only about +0.2 V at neutral pH (31). By comparison, oxidation of S− is more advantageous than Fe2+ oxidation, with a seven-electron transfer completing the oxidation to sulfate per mole of S− and 2 mol of S− available for oxidation per mol of pyrite. In a natural community, a variety of S oxidation pathways are used, as is dependent on and reflected in the intermediate S products known to form and be used by different S-oxidizing microorganisms (e.g., elemental sulfur [S0] or thiosulfate).
Microbial oxidation of hydrothermal sulfide minerals has been studied in some detail by a few investigators (see, e.g., references 5, 45, and 46). Indeed, S oxidizers of the genus Thiomicrospira, which grow from the oxidation of sulfide minerals or other solid S species (S0) have been cultured and demonstrated to be prominent members of these ecosystems (5), yet these autotrophic organisms are not able to utilize ferrous (Fe2+) iron as an energy source (45). Until recently, the role of Fe-oxidizing species in seafloor hydrothermal mineral oxidation has been largely overlooked. This is perhaps not surprising given the poor energetics of Fe oxidation; however, despite this hurdle, acidophilic Fe-oxidizing organisms have long been recognized in terrestrial sulfide mineral weathering habitats known as acid mine drainage (4). For deep-sea weathering environments, however, several arguments have been made as to why microbial Fe oxidation should not be an important process (4). Specifically, the poor energetics and the well oxygenated, approximately neutral pH conditions that typify the deep sea have been thought to preclude the activity of Fe oxidizers (5).
It is known that in terrestrial ecosystems, Fe-oxidizing bacteria must oxidize enormous quantities of Fe to harness this process for growth (31). In microbial Fe-oxidizing habitats associated with the weathering of terrestrial sulfide mineral deposits, this is generally not a problem. Oxidation of sulfide minerals produces acid, and the resulting low pH retards the abiotic kinetics of Fe oxidation; therefore, the Fe2+ is fairly stable and can be readily obtained by Fe oxidizers to support growth (7). Neutrophilic Fe-oxidizing bacteria are also recognized in terrestrial habits, not as prevalently associated with sulfide weathering but rather associated with subsurface groundwaters and streams, where reducing, Fe2+-containing water flows from anoxic to oxic conditions (11). Low O2 concentrations similarly retard abiotic Fe oxidation, allowing certain specialized species of Fe oxidizers (e.g., Gallionella spp.) to grow. One prominent difference between the neutrophilic and acidophilic Fe oxidizers that have been studied (other than pH and O2 optima) is that most of the Fe oxidizers isolated from acidic sulfide-weathering habitats are autotrophic, whereas with few exceptions (9, 10, 16), chemolithoautotrophic neutrophilic Fe oxidizers have not been cultured.
Neither of the typical terrestrial habitats described above for Fe oxidizers is very similar to conditions expected in deep-sea hydrothermal weathering environments. Most of the Fe that is available in seafloor habitats is in solid form, such as in the terrestrial sulfide mineral-weathering regimens; however, in the deep sea, sulfide deposits are flushed with large amounts of well-buffered seawater during low-temperature weathering, and therefore these habitats are both well oxygenated and at approximately neutral pH.
Consistent with the arguments given above, both culture-dependent and culture-independent studies have revealed Fe-oxidizing species or likely Fe-oxidizing phylotypes in only a few marine localities, such as shallow submarine hydrothermal systems that are characterized by fluids that are unusually enriched in Fe2+ (10, 15). These habitats, however, are rather unique compared with typical deep-sea mineral-weathering habitats; the organisms in these shallow hydrothermal systems apparently derive energy from oxidation of aqueous Fe2+ in a way that is rather similar to the terrestrial groundwater-stream example noted above (11).
Despite the infrequency of definitive identifications of Fe-oxidizing bacteria in seafloor environments and the theoretical arguments discussed above, there are abundant identifications of mineral particles that are hypothesized to be indicative of the activity of this physiological group, in both geologically recent and ancient seafloor oxidation deposits (1, 18, 25, 42). In a recent inspection of museum mineral collections and field observation, more than 140 sites which contained evidence of biogenic Fe oxide formation were found (20). Additionally, Fe-containing sheath material, characteristic of Leptothrix ochracea, has been reported from both Loihi and Mid-Atlantic Ridge hydrothermal vent sites (27, 46).
Here we report the isolation and initial physiological assessment of a diverse group of neutrophilic Fe-oxidizing bacteria from low-temperature, deep-sea weathering deposits that occur in the vicinity of the Main Endeavor and Middle Valley segments of the Juan de Fuca Ridge axis off the coast of the Pacific Northwest of the United States. Based on the results presented here and preliminary field studies reported elsewhere (8), we suggest a role for these organisms in mineral weathering and primary production at the seafloor.
MATERIALS AND METHODS
Sample collection.
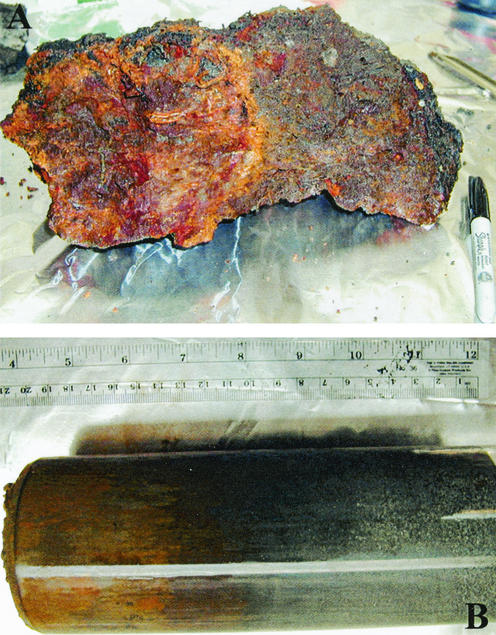
Sulfide mineral samples were collected from the seafloor near active hydrothermal fields along the Main Endeavour and Middle Valley segments of the Juan de Fuca Ridge axis (Fig. 1). Samples were collected during Atlantis cruise 3-54 in July 2000 by using the deep submersible vehicle Alvin. Samples used for culturing were collected from low-temperature (∼4°C) weathering regimes and included rusty reddish-brown oxidized sulfide chimney rubble (Fig. 2A) and metalliferous sediments (Fig. 2B). In contrast to seafloor environments that are influenced by hydrothermal fluids, the ambient bottom seawater from which the samples used here were collected is generally characterized by low temperatures, high chlorinity, high pH, high alkalinity, and low metal and hydrogen sulfide concentrations (see reference 8 and citations therein).
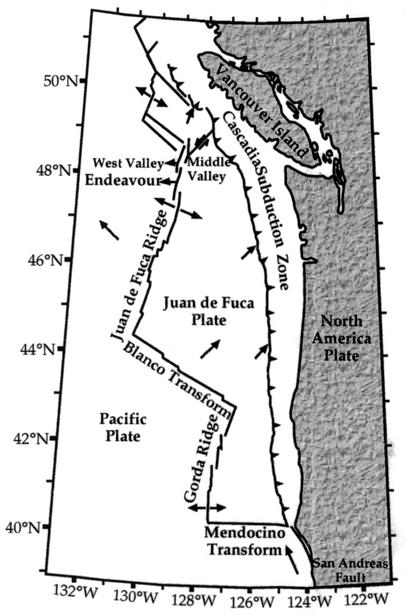
FIG. 1.
Location of the main Endeavour segment of the Juan de Fuca Ridge, northeast Pacific Ocean (modified from reference 13).
FIG. 2.
Examples of oxidized sulfide materials used to enrich for FeOB. (A) Weathered, brecciated sulfide rubble, collected from the vicinity of Hulk Flange (8) along the main Endeavour segment of the Juan de Fuca ridge axis. (B) Push-core sample of fine-grained sulfide sediment collected from the Middle Valley (Fig. 1).
Culturing and isolation.
Enrichment cultures were initiated on board the R/V Atlantis. These were done with an artificial seawater medium (24, 46) that contained no aqueous inorganic energy source. The sulfide minerals pyrite, chalcopyrite, marcasite, and pyrrhotite (Wards Geology) as well as natural hydrothermal chimney sulfide material collected during the cruise were used as energy sources for enrichment cultures. Mineral materials were ground (∼50- to 500-μm diameter), sterilized by autoclaving, and packed in 15-ml sterile Falcon tubes (Fisher Scientific). Aqueous medium was added to tubes containing minerals and then inoculated with small amounts of seafloor sediments or scrapings from the oxidized surface of chimney rubble. Enrichments were maintained for approximately 6 months by infrequent subculturing. Subculturing consisted of removing and replacing a portion of the aqueous medium from culture tubes every 4 to 6 weeks. Sulfide material was not ever replaced or replenished and was observed to become increasingly oxidized over time, as evidenced by increasing accumulations of reddish-brown precipitates within tubes.
Isolations of neutrophilic Fe-oxidizing bacteria (FeOB) were initiated by using the gradient tube method described by Emerson and Moyer (9), as modified as described by Kucera and Wolfe (29). Briefly, the medium is an agarose-stabilized slush medium, which allows a diffusion gradient to form, providing a transition between oxygenated and anoxic environments during descent from the air-medium interface downward in the tubes. At the bottom of the gradient tube is a synthetic ferrous sulfide (FeS) plug, which serves as an Fe2+ source and reductant to the overlying slush. The tubes are inoculated by pulling a Pasteur pipette vertically through the slush medium while inoculum is expelled. These gradient tubes were iteratively inoculated in a dilution-to-extinction method until clonally pure cultures were obtained (see below).
Growth substrates.
Growth in gradient tubes was tested on a variety of organic and inorganic substrates. All reagents were CP grade unless otherwise indicated. Fe2+ substrates included FeS, FeCO3, ferrous chloride FeCl2 · 4H2O, and pyrite (FeS2) (Wards Geology and natural material from Endeavour [8]) and basalt (∼10% FeO) (2). FeS was prepared as described by Kucera and Wolfe (29), FeCO3 was prepared as described by Hallbeck et al. (17), and the basalt and FeS2 were prepared as described by Edwards et al. (8). Reduced sulfur (S)-containing substrates include FeS, FeS2, sulfide (HS−), and thiosulfate (Na2S2O3). Manganese chloride (MnCl2 · 4H2O) was also tested. Organic substrates tested included yeast extract, peptone, acetate, lactate, dextrose, and sucrose. Agar was also tested as a growth substrate in gradient tubes that were unamended (artificial seawater medium only) or amended with supplemental sulfate to test for anaerobic sulfate reduction.
Growth was also tested in aqueous medium containing FeCl2 · 4H2O and FeCO3. Microaerobic growth in flasks that had been bubbled with nitrogen to remove most of the oxygen and anaerobic growth in the presence of nitrate were tested.
Electron microscopy.
Cells were grown to approximately mid-log phase in aqueous medium anaerobically with FeCO3 and nitrate for transmission electron microscopy (TEM). Cells were harvested by centrifugation, and the cell pellet was fixed for 24 h in 2.5% glutaraldehyde. The cell pellet was embedded in resin and sectioned with a microtome. Thin sections were Pb stained and then viewed with a Zeiss 10CA electron microscope at 60 kV at the Marine Biological Laboratories Microscopy Facility, Woods Hole, Mass.
Growth rates and temperature optima.
Generation times were determined for cells grown in gradient tubes in the presence of FeS. Cells were extracted from growth bands within tubes every 2 to 4 days (depending on growth phase), filtered onto Poretics (Fisher Scientific) 0.22-μm-pore-size black polycarbonate filters, stained with acridine orange, and counted directly with an epifluorescence microscope (Zeiss Axiovert 100) (19). Growth for four strains was tested between 3 and 37°C.
Microelectrode measurements.
Oxygen measurements were made in gradient tubes (FeS based) by using a commercial picoammeter (PA2000) equipped with a Clark-type microelectrode (100-μm tip) with a guard cathode and micomanipulator (MM33) (Unisense, Aarhus, Demark). The depth of the microelectrode tip is controlled and quantified by the micromanipulator. Measurements were made every 200 μm while descending through the agar tube. Zero depth is set at the air-medium interface, and the micromanipulator depth range limits the deepest depth recorded. Some of the measurements were stopped prior to reaching the depth limit of the micromanipulator because oxygen was depleted.
Carbon fixation.
Autotrophic C fixation was determined in batch cultures of cells grown microaerobically in gradient tubes containing FeS (five strains were tested). C fixation was tested for one strain (FO10) grown in gradient tubes containing FeS2 and basalt. C fixation was determined by using the methods for H14CO3− incorporation and calculated as described previously (41, 47). Approximately 0.1 μCi of radiolabeled isotope (NaH14CO3) per ml was added in these experiments. Growth rates were determined by performing counts on cells (using epifluorescence microscopy; see above) grown in parallel cultures at equivalent time points in the absence of radioisotope.
Phylogenetic analyses.
Cultures were grown in FeS-based gradient tubes to stationary phase. Cells were extracted from the growth band and treated with 1 N HCl in order to partially digest agar and Fe oxides. Digestion of Fe oxides aided in concentrating pure cell mass (Fe oxides in culture are more abundant than cells), and upon extraction, the DNA was more readily amplified by PCR (below) after this treatment. Cells were then harvested by centrifugation, and DNA extractions were performed by using a freeze-thaw method described previously (6). Nested PCRs were performed on 16S ribosomal DNA with primers 27F and 1492R, followed by reamplification with primers 515F and 1492R. In order to ascertain clonal purity of the putative isolates, the amplified DNA was cloned by using TOPO-TA (Invitrogen). Restriction fragment length polymorphism analysis (21) was performed on PCR-amplified cloned sequences. Cloned DNAs from cultures determined to be axenic were sequenced at the University of Maine Sequencing Facility. Sequences were aligned with representative sequences obtained from the GenBank database (3) by using ClustalX (38) and were reduced to a comparable length (ca. 639 bp). Sequences were analyzed for chimeric secondary structures by using CHECK_CHIMERA (28). Phylogenetic analyses were also performed with ClustalX, generating neighbor-joining trees.
RESULTS
Culture isolation and growth.
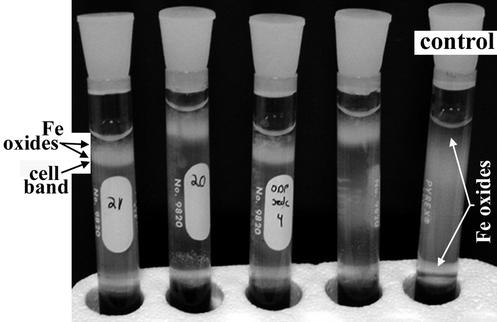
Distinct zones displaying reddish-brown zones of Fe oxide precipitates developed in enrichment cultures after 2 to 3 months. With inocula from these zones, serial dilutions to extinction in FeS-based gradient tubes were used successfully to obtain axenic cultures. In gradient tubes, distinct cell bands developed approximately 1 cm from the air-agar medium interface. The cell bands were overlain by an opaque region that is rich in Fe oxides (Fig. 3). Fe oxides produced in cultures were distinct from those observed in control tubes; however, Fe oxide particle morphologies also varied in different cultures and with different growth conditions. In general, particles in control tubes were often densely aggregated (partially opaque) and amorphous in appearance. In contrast, particles in cultures displayed specific morphologies that included irregularly twisted, dendritic particles (Fig. 4A), straight bundles of fine-grained filaments (particle aggregates of ca. 1 by 5 μm), and elongated, narrow particles that have a braided appearance (ca. 1 by 10 to 20 μm). TEM analysis revealed that the cells were closely associated with the Fe oxide particles produced during growth (Fig. 4B). Fe oxides appeared to be nucleated at the cell surface, and electron-dense particles often occurred in a capsule-like polymeric layer surrounding cells (Fig. 4B). Particles and particle aggregates observed by TEM were all fairly small compared with many of the particles observed by light microscopy; individual particles ranged from ∼2 nm to 2 μm, and particle aggregates were generally smaller than 5 μm.
FIG. 3.
Gradient tube (FeS-based) growth of FeOB. The four tubes on the left contain cultures of FeOB, and the single tube on the far right represents an abiotic control. In culture tubes, a distinct band of cells develops ∼1 cm from air-medium interface at the tops of the tubes. The milky region that can be seen clearly at the tops of the tubes is comprised principally of Fe oxide particles, which directly overlie the band of cell growth. In the control tube, Fe oxides develop from ∼1 cm from the top of the tube to ∼3 cm from the FeS-medium interface at the bottom.
FIG. 4.
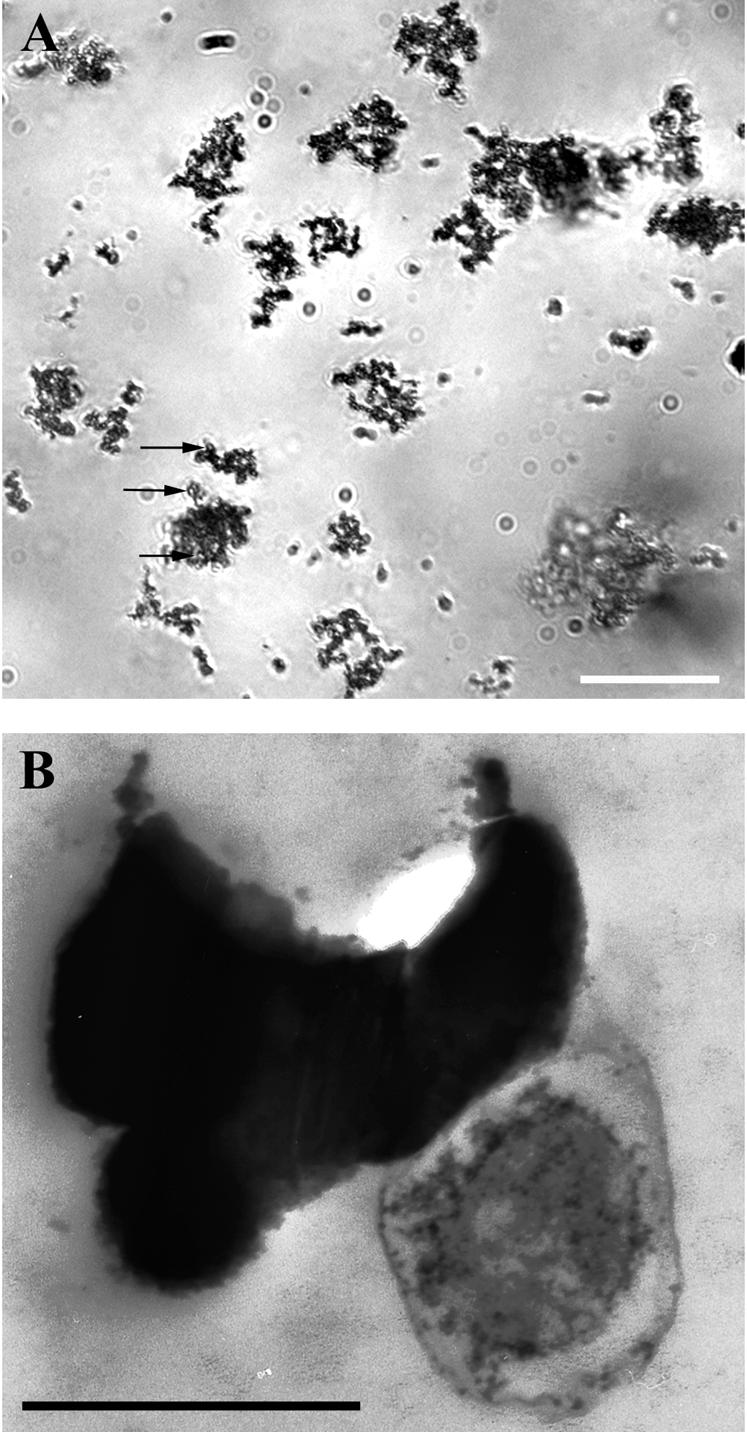
Light (A) and electron (B) microscopic images of FeOB (strain FO10, a member of the γ-Proteobacteria). Cells were grown anaerobically with ferrous carbonate (FeCO3) and nitrate (NO3−). Bars, 20 μm (A) and 0.5 μm (B). Irregularly twisted Fe oxide particles are apparent by light microscopy in this culture. By electron microscopy, cells can be seen closely associated with Fe particles, which appear to nucleate at the cell surface or within a capsule-like structure that surrounds cells. The close association between cells and mineral particles can be seen by light microscopy; cells appear as roughly circular open areas within mineral aggregates (arrows in panel A).
The results of tests concerning growth substrates completed for all strains are summarized in Table 1. All cultures were capable of growth on all Fe-containing growth substrates tested and were unable to grow on any other inorganic or organic substrates provided. Anaerobic growth in the presence of nitrate was supported in the presence of both FeCl2 · 4H2O and FeCO3.
TABLE 1.
Results of growth substrate tests
| Growth substrate (electron donor) | Concn or amt | Electron acceptora | Growth |
|---|---|---|---|
| Solid substrates | |||
| FeS | Plug | O2 | + |
| FeCO3 | Plug | O2 | + |
| NO3− (5 mM) | + | ||
| FeS2 | 1 g of particles | O2 | + |
| Basalt | ∼10 wt% FeO | O2 | + |
| Aqueous substrates | |||
| FeCl2 · 4H2O | 10 mM | O2 | + |
| NO3− (5 mM) | + | ||
| MnCl2 · 4H2O | 10 mM | O2 | − |
| HS− | 100 μM | O2 | − |
| Na2S2O3 | 10 mM | O2 | − |
| Yeast extract | 0.1 g/ml | O2 | − |
| Peptone | 0.1 g/ml | O2 | − |
| Sucrose | 10 mM | O2 | − |
| Dextrose | 10 mM | O2 | − |
| Acetate | 10 mM | O2 | − |
| Lactate | 10 mM | O2 | − |
| Medium only | See text | O2 | − |
| SO42− (30 mM) | − |
The O2 concentrations were not determined.
The generation times determined were similar for the four strains. Maximum growth temperatures were approximately 30°C, and temperature optima were between 3 and 10°C. Generation times at the optimal growth temperature (Topt) were 56, 72, 65, and 74 h, respectively, for strains FO10 (Topt = 10°C), FO2 (Topt = 3°C), FO15 (Topt = 10°C), and FO8 (Topt = 10°C).
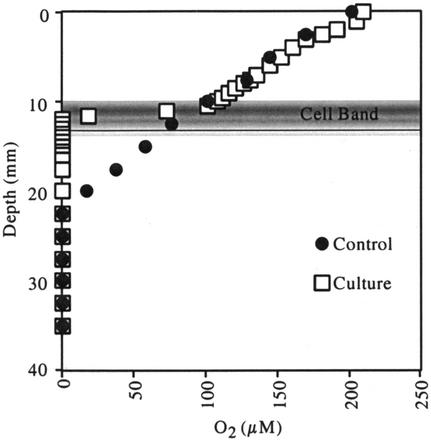
Microelectrode measurements.
Results of microelectrode measurements for one representative culture (strain FO10) are presented in Fig. 5. All gradient tubes containing FeOB cultures displayed similar steep oxygen gradients. In the culture shown in Fig. 4, oxygen became depleted in the culture tube at 10.5 mm relative to the control tube and was undetectable by 12 mm. In contrast, the control tube showed gradual depletion of oxygen from the surface to 22 mm. The shaded area in Fig. 5 displays the growth zone for the FeOB culture, which coincides with the microoxic zone. From these data, a rate of flux of O2 into the cell band can be calculated by using Fick's first law of diffusion, i.e., F = −DδC/δz, where D is the diffusion coefficient (2.2327 × 10−5 cm2 s−1), δC (moles per cubic centimeter) is the change in concentration, and δz (centimeters) is the change in depth. According to this calculation, the flux of O2 in to the cell growth band shown in Fig. 5 was 9 pmol cm−2 s−1.
FIG. 5.
Microelectrode measurements of O2 as a function of depth within gradient tubes containing a culture (data are for strain FO10) and a corresponding abiotic control. The region of cell growth band development is approximated with shading.
Carbon fixation.
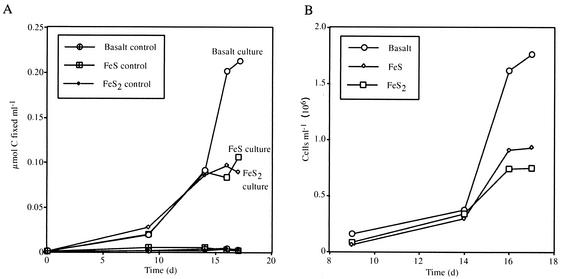
Strain FO10 was tested for 14C fixation microaerobically in gradient tubes by using FeS, FeS2, and basalt as the electron donors. The results for the 22-day experiment are shown in Fig. 6A. Between 100 and 225 nmol of C per ml was fixed by cells grown on these three solid materials. Carbon fixation was higher in the presence of basalt (∼225 nmol/ml) than with the sulfide materials (∼100 nmol/ml each). Total carbon uptake correlated with growth yields determined for parallel (isotope-free) cultures (Fig. 6B). Higher total cell densities were achieved in cultures grown with basalt, followed by those grown with FeS and FeS2. At mid-log growth (14 to 16 days), these growth data corresponded to a total range in carbon fixation of 184 to 194 fmol of C fixed per cell for all three growth substrates.
FIG. 6.
(A) C fixation over time for strain FO10 grown in the presence of basaltic glass, pyrite (FeS2), and FeS (open symbols), with corresponding control data for comparison. C fixation was greatest for the culture grown in the presence of basalt (∼0.22 μmol of C fixed/ml [total]). C fixations in the presence of FeS and FeS2 were approximately equivalent, with a maximum of ∼0.08 μmol of C fixed/ml (total). (B) Cell growth data for strain FO10 grown in parallel cultures in the presence of basaltic glass, FeS2, and FeS. Cell growth was highest in the presence of basalt; this was nearly double the cell yield achieved in the presence of FeS and FeS2.
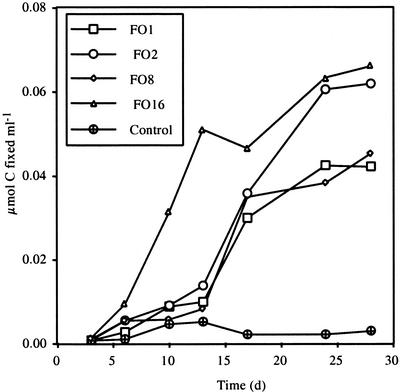
Strains FO1, FO2, FO8, and FO16 were tested for 14C fixation microaerobically in gradient tubes with FeS. C fixation in gradient tubes was similar to that shown for strain FO10 on FeS. C fixation for these strains is shown in Fig. 7.
FIG. 7.
C fixation over time for strains FO1, FO2, FO8, and FO16 and a control. All data are for cells grown in gradient tubes in the presence of FeS. The final C fixation was highest for strains FO2 and FO16, at ∼0.06 μmol of C fixed/ml, and was somewhat less for strains FO1 and FO8, at ∼0.04 μmol of C fixed/ml.
Phylogenetic affiliations.
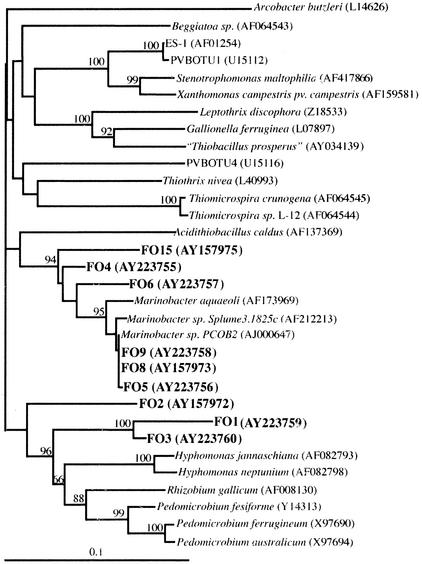
Figure 8 shows the phylogenetic relationships among nine representative strains of FeOB from this study relative to some other FeOB, other autotrophs, and some laboratory cultures and 16S rRNA gene sequences from GenBank that are most closely related to our cultures. One group of isolates fall within the alpha subclass of the Proteobacteria, while the second major grouping clusters with the gamma subclass of the Proteobacteria.
FIG. 8.
Phylogenetic relationships between cultured strains of FeOB in this study (FO numbers) and other FeOB, other autotrophs, and closely related organisms within the α- and γ-Proteobacteria. Accession numbers of the sequences from databases used for tree construction are given in parentheses. Numbers given at branch notes are bootstrap values where these were greater than 50 (see text).
DISCUSSION
Iron is the fourth most abundant element and the most abundant redox-active element, after oxygen, that comprises the Earth's crust. Thus, it should perhaps be expected that respiratory pathways based on redox transformations of Fe are prevalent among environmental microorganisms. In fact, it has been proposed that the earliest forms of life may have been linked to the formation of pyrite as the first energy source (43). However, our knowledge concerning the physiology, phylogenetic diversity, and environmental abundance and distribution of extant neutrophilic Fe-oxidizing microorganisms has remained poorly elucidated to date, in large part due to our inability to grow and study them in pure culture in the laboratory.
In recent years, application of modified culturing techniques that were originally devised nearly 50 years ago for the enrichment of neutrophilic FeOB have proven remarkably successful for microbiologists studying this historically elusive group, and lithotrophic FeOB cultures have now been obtained from environments that include root plaques (12), groundwaters (9), wetlands (37), hydrothermal systems (10, 15), and now bare-rock systems (this study).
The present study demonstrates that all of these strains are obligate lithotrophic FeOB. No alternate inorganic electron source has been identified for any of our strains (Table 1). No evidence for heterotrophic growth (culture turbidity or cell flocks, such as is observed in lithotrophically grown cultures) has been found for any cultures in the presence of any carbon source tested (Table 1). Furthermore, this study demonstrates autotrophy via 14C fixation in all strains tested thus far (Fig. 6 and 7). Autotrophy among the neutrophilic FeOB is most often inferred by positive growth in the absence of any organic carbon substrate, rather than explicitly demonstrated by 14C fixation, with the one exception of the stalk-forming bacterium Gallionella ferruginea. For this organism, both autotrophic and mixotrophic (with glucose, fructose, and sucrose) growth capabilities have been clearly shown (16). Our studies also provide first evidence for lithoautotrophy among relatives of the long-recognized heterotrophic Fe- and Mn-depositing Hypomicrobia group (Fig. 8).
In many respects the cultures obtained here are similar physiologically to FeOB that have recently been cultured and described (9, 10): the strains presented in this study are similarly obligate lithotrophs, Fe is the only electron donor that has been identified to support growth, and a very similar suite of Fe-bearing substrates (FeS and FeCO3, etc.) (Table 1) support their growth. The notable exception is the identification of several naturally occurring Fe-bearing mineral substrates (pyrite and basaltic glass) that also support the growth of the strains we report here; previously reported FeOB have not been tested on these substrates (9, 10). Also similar to the case for other recently cultured FeOB (9, 10), when cells are grown in gradient tubes a distinct modulation of the O2 gradient developed in comparison with abiotic controls (Fig. 5); O2 is apparently depleted due to growth of the bacteria. Comparable O2 profiles have also been observed in inoculated and control sulfide-oxygen gradient cultures for autotrophic growth studies of Beggiatoa (36).
There are also many significant physiological differences between the strains discussed in this study and other recently cultured FeOB (9, 10). All of the strains presented here grow approximately five times slower than other cultured strains of FeOB, with doubling times ranging between 57 and 74 h, compared, for example, with the 12-h doubling time of FeOB strains ES-1 and ES-2 (9). Also, all of the strains presented here are psychrophilic, with temperature optima ranging from 3° to 10°C, compared with ES-1 and ES-2, which have temperature optima at 25 to 30°C and are incapable of growth at 6°C (9). Slow growth and psychrophily are characteristics that are likely related to the in situ environmental condition of the culture origin. Although in situ temperatures were not directly recorded at all of the sampling locales used for this study, sites that represented ambient seafloor conditions (∼2 to 4°C) were explicitly selected for sample collection in order to obtain representative microorganisms that may be present during low-temperature seafloor rock weathering. Also, the natural materials that were used for enrichment of these organisms likely selected for organisms that were adapted to slow growth on materials (basalt and pyrite) that are less soluble than many of the synthetic solid or aqueous growth substrates used in the laboratory.
The strains reported here share the physiological characteristic of being capable of anaerobic growth with NO3− as the terminal electron acceptor. Although generation times were not calculated, growth yields and rates were notably retarded during anaerobic growth. Preparation of cells for electron microscopy, however, was greatly facilitated by the presence of the aqueous medium used for anaerobic growth (Table 1), allowing us to observe the relationship between cells and the Fe oxides they produce (Fig. 4B). By TEM observation, it was apparent that most cells are closely associated with Fe oxide particles, which often appeared to grow at the cell surface within or around a capsule-like envelope that surrounds them (Fig. 4B).
The close spatial relationship between cells and particles can also be observed by light microscopy (Fig. 4A), and a number of different Fe oxide particle morphologies can be identified. These include short bundles of filaments that are similar to the classical stalks produced by the FeOB G. ferruginea (except for their straight form which is generally shorter) (not shown), long (∼25-μm) Fe oxide particles that have a braided appearance (K. J. Edwards et al., submitted for publication), amorphous-appearing particles, and the irregularly twisted, branching particles shown here for cells grown anaerobically (Fig. 4). Many of the particle types observed are very similar to particle types that have been observed in studies of geologically recent Fe deposits where FeOB are known (10) or hypothesized to be present in deposits that range from recent to ancient (1, 18, 25, 39, 42). The variable morphologies that we have observed cannot be accounted for systematically at this time; variation appears to arise in different strains grown under similar conditions (i.e., strain-specific morphological variation), yet variation has also been observed in a single strain when it is grown under different conditions (solid versus aqueous substrate and aerobic versus anaerobic growth). In general, however, most of the Fe oxide particles that can be observed in cultures are distinct from those particles that occur in corresponding controls. Further detailed studies are required in order to clearly associate specific strains and conditions with specific particle morphologies.
The diverse phylogenetic relationships among the strains presented here, and between these strains and other known FeOB, are further evidence for the evolutionarily widespread capacity for Fe oxidation among bacteria (30). The data presented here further extend our knowledge of known neutrophilic lithoautotrophic FeOB to include members of the gamma and alpha subdivisions of the Proteobacteria, although putative evidence for lithoautotrophy among other γ-Proteobacteria has been suggested recently for other novel FeOB (9, 10). It is notable that none of these strains shares a very close phylogenetic relationship with either known FeOB or autotrophic bacteria. The α-Proteobacteria discussed here, strains FO1, FO2, and FO3, which are among the slowest growing and most psychrophilic of these strains, are most closely related to the cultured deep-sea heterotrophic bacterium Hyphomonas jannaschiana (81 to 89% sequence similarity) (44) and are more distally related to other members of the Hypomicrobia that are known Fe- and Mn-depositing bacteria, i.e., Pedomicrobium spp. (Fig. 8). Hyphomonas spp. are prolific and ubiquitous members of deep-sea hydrothermal vent communities (23).
The cultured representative that shares greatest sequence similarity with the γ-Proteobacteria strains presented here (FO4, FO5, FO6, FO8, FO9, and FO15) is the heterotrophic bacterium Marinobacter aquaeolei (87 to 94% sequence similarity). However, among this diverse collection of Fe-oxidizing γ-Proteobacteria, likely representing at least two genera, few cultured representatives exist.
The present study offers support for a growing body of evidence that suggests that FeOB are a globally significant force in Fe cycling near Earth's surface (10). This work is the first to demonstrate growth of neutrophilic FeOB from the dissolution products of natural rock and mineral components of Earth's crust. It is important to recognize that nearly 70% of Earth's surface is covered by basalt, principally oceanic (14). In our study basalt glass was the most ideal growth substrate for our strains, resulting in both the highest growth rate and highest cell yields (Fig. 5B). It is also important to note that these studies establish linkages between (i) Fe oxidation, (ii) mineral dissolution, and (iii) C fixation. This suggests ramifications for the importance of FeOB in geomicrobiological Earth processes that extend well beyond their established important role in near-surface redox cycles in aqueous environments and also suggests coupling between crustal alteration (mineral weathering) and the carbon cycle. Further studies are needed to better define predictive, quantitative relationships between these factors.
Acknowledgments
Funding for this work was provided by NSF grant OCE-0096992 to K.J.E.
We thank Louis Kerr for assistance with electron microscopy and David Emerson for providing protocols and advice on culturing techniques. We thank J. Seewald for inviting our participation in the cruise that allowed this work to be initiated (sponsored by NSF; OCE-9906752).
Footnotes
Contribution 10875 of the Woods Hole Oceanographic Institution.
REFERENCES
- 1.Alt, J. C. 1988. Hydrothermal oxide and nontronite deposits on seamounts in the eastern Pacific. Mar. Geol. 81:227-239. [Google Scholar]
- 2.Bach, W. 1996. Magmatic evolution of SE-Pacific mid-ocean ridges and SW-Pacific back-arc basins: mantle sources, magmagenesis, differentiation, and degassing process. Ph.D. thesis. University of Giessen and Geoforschungs Zentrum, Posdam, Germany.
- 3.Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, B. A. Rapp, and D. L. Wheeler. 2002. GenBank. Nucleic Acids Res. 30:17-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colmer, A. R., and M. E. Hinkle. 1947. The role of microorganisms in acid mine drainage. Science 106:253-256. [DOI] [PubMed] [Google Scholar]
- 5.Eberhard, C., C. O. Wirsen, and H. W. Jannasch. 1995. Oxidation of polymetal sulfides by chemolithoautotrophic bacteria from deep-sea hydrothermal vents. Geomicrobiol. J. 13:145-164. [Google Scholar]
- 6.Edwards, K. J., P. L. Bond, T. M. Gihring, and J. F. Banfield. 2000. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 65:1796-1799. [DOI] [PubMed] [Google Scholar]
- 7.Edwards, K. J., T. M. Gihring, and J. F. Banfield. 1999. Seasonal variations in microbial populations and environmental conditions at an extreme acid mine drainage environment. Appl. Environ. Microbiol. 65:3627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards, K. J., T. M. McCollom, H. Konishi, and P. R. Buseck. Seafloor bio-alteration of sulfide minerals: results from in-situ incubation studies. Geochim. Cosmochim. Acta, in press.
- 9.Emerson, D., and C. L. Moyer. 1997. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl. Environ. Microbiol. 63:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emerson, D., and C. L. Moyer. 2002. Neutrophilic Fe-oxidizing bacteria are abundant at the Loihi seamount hydrothermal vents and play a major role in Fe oxide deposition. Appl. Environ. Microbiol. 68:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerson, D., and N. P. Revsbech. 1994. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: field studies. Appl. Environ. Microbiol. 60:4022-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emerson, D., J. V. Weiss, and J. P. Megonigal. 1999. Iron-oxidizing bacteria are associated with ferric hydroxide precipitates (Fe-plaque) on the roots of wetland plants. Appl. Environ. Microbiol. 65:2758-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortin, D., F. G. Ferris, and S. D. Scott. 1998. Formation of Fe-silicates and Fe-oxides on bacterial surfaces in samples collected near hydrothermal vents on the Southern Explorer Ridge in the northeast Pacific Ocean. Am. Mineral 83:1399-1408. [Google Scholar]
- 14.Francis, P. 1998. Volcanoes: a planetary perspective. Oxford University Press, Oxford, United Kingdom.
- 15.Hafenbrandl, D., M. Keller, R. Dirmeier, R. Rachel, P. Ronagel, S. Burggraf, H. Huber, and K. O. Stetter. 1996. Ferroglobus placidus gen. new., sp. nov., a novel hyperthemophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 166:308-314. [DOI] [PubMed] [Google Scholar]
- 16.Hallbeck, L., and K. Pedersen. 1991. Autotrophic and mixotrophic growth of Gallionella ferruginea. J. Gen. Microbiol. 137:2657-2661. [Google Scholar]
- 17.Hallbeck, L., F. Stahl, and K. Pedersen. 1993. Phylogeny and phenotypic characterization of the stalk-forming and iron-oxidizing bacterium Gallionella ferruginea. J. Gen. Microbiol. 139:1531-1535. [DOI] [PubMed] [Google Scholar]
- 18.Hannington, M. D., and I. R. Jonasson. 1992. Fe and Mn oxides at seafloor hydrothermal vents, p. 351-370. In H. C. Skinner (ed.), Biomineralization processes of iron and manganese; modern and ancient environments, vol. 21. Catena-Verlag, Cremlingen-Destedt, Germany.
- 19.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann, B. A., and J. D. Farmer. 2000. Filamentous fabrics in low-temperature mineral assemblages: are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planet. Space Sci 48:1007-1086. [Google Scholar]
- 21.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jannasch, H. W. 1985. The chemosynthetic support of life and microbial diversity at deep-sea hydrothermal vents. Proc. R. Soc. London B 225:277-297. [Google Scholar]
- 23.Jannasch, H. W., and C. O. Wirsen. 1981. Morphological survey of microbial mats near deep-sea thermal vents. Appl. Environ. Microbiol. 41:528-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jannasch, H. W., C. O. Wirsen, D. C. Nelson, and L. A. Roberson. 1985. Thiomicrospira crunogena sp. nov., a colorless, sulfur-oxidizing bacterium from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 35:422-424.
- 25.Juniper, S. K., and Y. Fouquet. 1988. Filamentous iron-silica deposits from modern and ancient hydrothermal sites. Can. Mineral. 26:859-869. [Google Scholar]
- 26.Karl, D. M. 1995. Ecology of free-living hydrothermal vent communities, p. 35-124. In D. M. Karl (ed.), The microbiology of deep-sea hydrothermal vents. CRC Press, Boca Raton, Fla.
- 27.Karl, D. M., A. M. Brittain, and B. D. Tilbrook. 1989. Hydrothermal and microbial processes at Loihi Seamount, a mid-plate hot-spot volcano. Deep-Sea Res. 36:1655-1673. [Google Scholar]
- 28.Kopczynski, E. D., M. M. Bateson, and D. M. Ward. 1994. Recognition of chimeric small-subunit ribosomal DNAs composed of genes from uncultivated microorganisms. Appl. Environ. Microbiol. 60:746-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucera, S., and R. S. Wolfe. 1957. A selective enrichment method for Gallionella ferruginea. J. Bacteriol. 74:344-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane, D. J., A. P. J. Harrison, D. Stahl, B. Pace, S. J. Giovannoni, G. J. Olsen, and N. R. Pace. 1992. Evolutionary relationships among sulfur- and iron-oxidizing eubacteria. J. Bacteriol. 174:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madigan, M. T., J. M. Martinko, and J. Parker. 2000. Brock biology of microorganisms, 9th ed. Prentice-Hall, Inc., Upper Saddle River, N.J.
- 32.McCollom, T. M. 2000. Geochemical constraints on primary productivity in submarine hydrothermal vent plumes. Deep-Sea Res. 47:85-101. [Google Scholar]
- 33.Moses, C. O., and J. S. Herman. 1991. Pyrite oxidation at circumneutral pH. Geochim. Cosmochim. Acta 55:471-482. [Google Scholar]
- 34.Moses, C. O., D. K. Nordstrom, J. S. Herman, and A. L. Mills. 1987. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Cosmochim. Acta 51:1561-1572. [Google Scholar]
- 35.Moses, C. O., D. K. Nordstrom, J. S. Herman, and A. L. Mills. 1985. Initiation of aqueous pyrite oxidation by dissolved oxygen and by ferric iron, p. 669-670. The Geological Society of America, 98th Annual Meeting, vol. 17. Geological Society of America, Boulder, Colo.
- 36.Nelson, D. C., C. O. Wirsen, and H. W. Jannasch. 1989. Characterization of large autotrophic Beggiatoa spp. abundant at hydrothermal vents of the Guaymas Basin. Appl. Environ. Microbiol. 55:2909-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobolev, D., and E. Roden. 2001. Suboxic deposition of ferric iron by bacteria in opposing gradients of Fe(II) and oxygen at circumneutral pH. Appl. Environ. Microbiol. 67:1328-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorseth, I., T. Torsvik, V. Torsvik, F. Daae, R. Pedersen, and K.-S. Party. 2001. Diversity of life in ocean floor basalt. Earth Planet. Sci. Lett. 194:31-37. [Google Scholar]
- 40.Tivey, M. K., S. E. Humphris, G. Thompson, and M. Hannington. 1995. Deducing patterns of fluid flow and mixing within the TAG active hydrothermal mound using mineralogical and geochemical data. J. Geophys. Res. 100:12527-12555. [Google Scholar]
- 41.Tuttle, J. H., and H. W. Jannasch. 1977. Thiosulfate stimulation of microbial dark assimilation of carbon dioxide in shallow marine environments. Microb. Ecol. 4:9-25. [DOI] [PubMed] [Google Scholar]
- 42.Verati, C., P. de Donato, D. Prieur, and J. Lancelot. 1999. Evidence of bacterial activity from micrometer-scale layer analyses of black-smoker sulfide structures (Pito Seamount Site, Easter microplate). Chem. Geol. 158:257-269. [Google Scholar]
- 43.Wächtershäuser, G. 1988. Pyrite formation, the first energy source for life: a hypothesis. Syst. Appl. Microbiol. 10:207-210. [Google Scholar]
- 44.Weiner, R. M., R. A. Devine, D. M. Powell, L. Dagasan, and R. L. Moore. 1985. Hyphomonas oceanitis sp. nov., Hyphomonas hirschiana sp. nov., and Hyphomonas jannaschiana sp. nov. Int. J. Syst. Bacteriol. 35:237-243. [Google Scholar]
- 45.Wirsen, C. O., T. Brinkhoff, J. Kuever, G. Muyzer, S. Molynequx, and H. W. Jannasch. 1998. Comparison of a new Thiomicrospira strain from the Mid-Atlantic Ridge with known hydrothermal vent isolates. Appl. Environ. Microbiol. 64:4057-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirsen, C. O., H. W. Jannasch, and S. J. Molyneaux. 1993. Chemosynthetic microbial activity at Mid-Atlantic Ridge hydrothermal vent sites. J. Geophys. Res. 98:9693-9703. [Google Scholar]
- 47.Wirsen, C. O., J. H. Tuttle, and H. W. Jannasch. 1986. Activities of sulfur-oxidizing bacteria at the 21N East Pacific Rise vent site. Mar. Biol. 92:449-456. [Google Scholar]