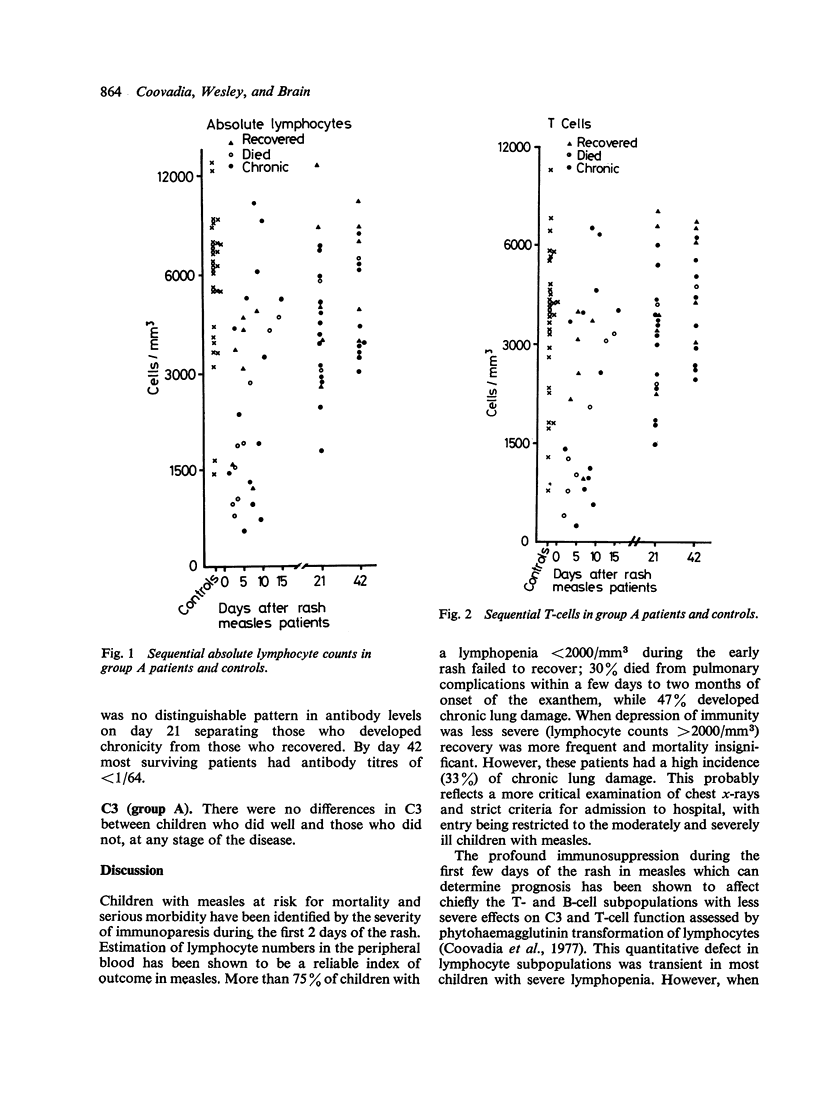
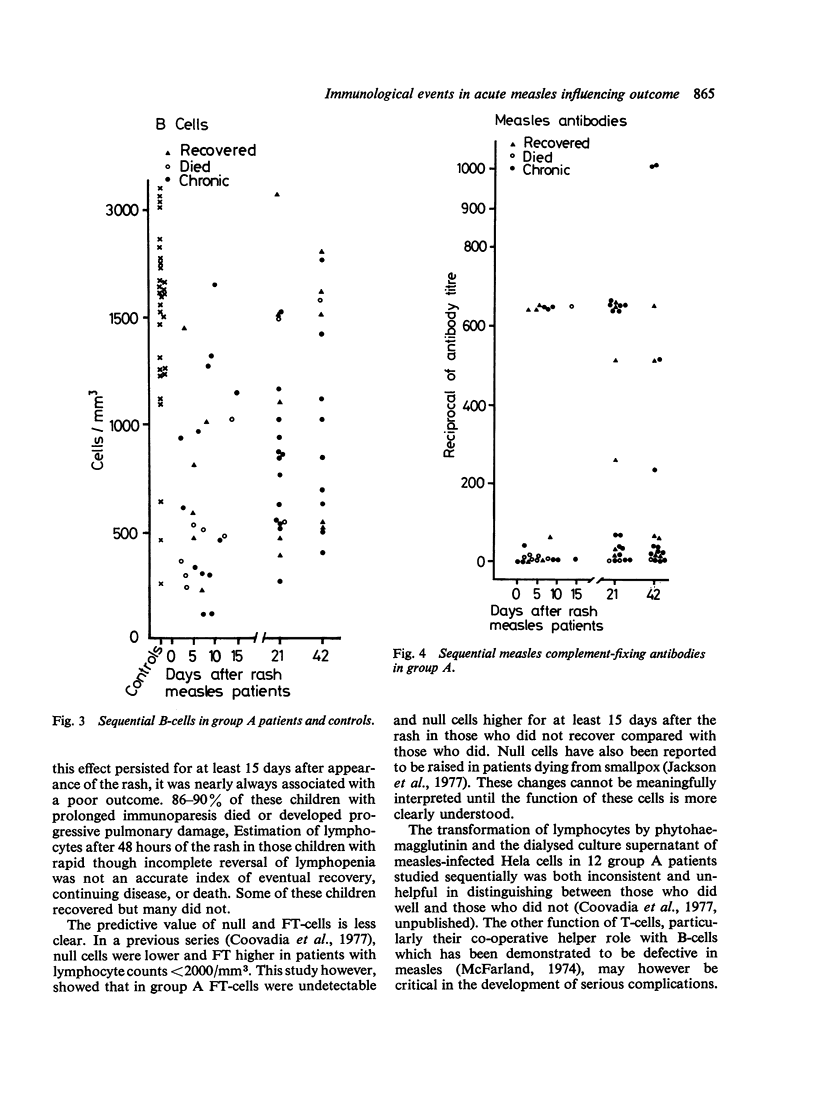
Abstract
77% of 30 children with measles who had severe lymphopenia (less than 2000/mm3; less than 2.0 x 10(9)/1) within 2 days of appearance of rash (group A) subsequently died or progressed to chronic chest disease. This was significantly worse than the outcome in 30 children with measles who had lymphocyte counts more than 2000/mm3 (more than 2.0 x 10(9)/1) (group B) of whom 67% recovered. In group A children the persistence of severe lymphopenia (caused by a reduction in T- and B-cells) for at least 15 days after onset of rash, remained a good predictive index of morbidity and mortality. Reversal of immunoparesis in group A was slower and incomplete 42 days from appearance of the rash in those who subsequently died or developed chronic chest disease compared with those who recovered. All patients who died failed to produce adequate measles antibodies. The therapeutic implications and immunopathological significance of these findings for chronic complications after acute measles are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coovadia H. M., Wesley A., Brain P., Henderson L. G., Hallett A. F., Vos G. H. Immunoparesis and outcome in measles. Lancet. 1977 Mar 19;1(8012):619–621. doi: 10.1016/s0140-6736(77)92056-6. [DOI] [PubMed] [Google Scholar]
- Coovadia H. M., Wesley A., Henderson L. G., Brain P., Vos G. H., Hallett A. F. Alterations in immune responsiveness in acute measles and chronic post-measles chest disease. Int Arch Allergy Appl Immunol. 1978;56(1):14–23. doi: 10.1159/000231998. [DOI] [PubMed] [Google Scholar]
- Dossetor J., Whittle H. C., Greenwood B. M. Persistent measles infection in malnourished children. Br Med J. 1977 Jun 25;1(6077):1633–1635. doi: 10.1136/bmj.1.6077.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans J. L. Lymphocytes. Harvey Lect. 1968 1969;64:87–119. [PubMed] [Google Scholar]
- Jackson T. M., Zaman S. N., Huq F. T and B rosetting lymphocytes in the blood of smallpox patients. Am J Trop Med Hyg. 1977 May;26(3):517–519. doi: 10.4269/ajtmh.1977.26.517. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps A., Kaschula R. O. Virus peneumonia following measles: a virological and histological study of autopsy material. S Afr Med J. 1976 Jul 3;50(28):1083–1088. [PubMed] [Google Scholar]
- Koprowski H., Barbanti-Brodano G., Katz M. Interaction between papova-like virus and paramyxovirus in human brain cells: a hypothesis. Nature. 1970 Mar 14;225(5237):1045–1047. doi: 10.1038/2251045a0. [DOI] [PubMed] [Google Scholar]
- MITUS A., ENDERS J. F., CRAIG J. M., HOLLOWAY A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med. 1959 Oct 29;261:882–889. doi: 10.1056/NEJM195910292611802. [DOI] [PubMed] [Google Scholar]
- McFarland H. F. The effect of measles virus infection on T and B lymphocytes in the mouse. I. Suppression of helper cell activity. J Immunol. 1974 Dec;113(6):1978–1983. [PubMed] [Google Scholar]
- Modlin J. F., Jabbour J. T., Witte J. J., Halsey N. A. Epidemiologic studies of measles, measles vaccine, and subacute sclerosing panencephalitis. Pediatrics. 1977 Apr;59(4):505–512. [PubMed] [Google Scholar]
- Papatestas A. E., Kark A. E. Peripheral lymphocyte counts in breast carcinoma. An index of immune competence. Cancer. 1974 Dec;34(6):2014–2017. doi: 10.1002/1097-0142(197412)34:6<2014::aid-cncr2820340620>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- RUCKLE G., ROGERS K. D. Studies with measles virus. II. Isolation of virus and immunologic studies in persons who have had the natural disease. J Immunol. 1957 May;78(5):341–355. [PubMed] [Google Scholar]
- STARR S., BERKOVICH S. EFFECTS OF MEASLES, GAMMA-GLOBULIN-MODIFIED MEASLES AND VACCINE MEASLES ON THE TUBERCULIN TEST. N Engl J Med. 1964 Feb 20;270:386–391. doi: 10.1056/NEJM196402202700802. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H., Agnarsdottir G., Lachmann P. J. Cellular immunity in subacute sclerosing panencephalitis. Proc R Soc Med. 1974 Nov;67(11):1125–1129. [PMC free article] [PubMed] [Google Scholar]
- White R. G., Boyd J. F. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973 Mar;13(3):343–357. [PMC free article] [PubMed] [Google Scholar]
- Zacharski L. R., Linman J. W. Lymphocytopenia: its causes and significance. Mayo Clin Proc. 1971 Mar;46(3):168–173. [PubMed] [Google Scholar]


