Abstract
The impact of land use intensity on the diversity of arbuscular mycorrhizal fungi (AMF) was investigated at eight sites in the “three-country corner” of France, Germany, and Switzerland. Three sites were low-input, species-rich grasslands. Two sites represented low- to moderate-input farming with a 7-year crop rotation, and three sites represented high-input continuous maize monocropping. Representative soil samples were taken, and the AMF spores present were morphologically identified and counted. The same soil samples also served as inocula for “AMF trap cultures” with Plantago lanceolata, Trifolium pratense, and Lolium perenne. These trap cultures were established in pots in a greenhouse, and AMF root colonization and spore formation were monitored over 8 months. For the field samples, the numbers of AMF spores and species were highest in the grasslands, lower in the low- and moderate-input arable lands, and lowest in the lands with intensive continuous maize monocropping. Some AMF species occurred at all sites (“generalists”); most of them were prevalent in the intensively managed arable lands. Many other species, particularly those forming sporocarps, appeared to be specialists for grasslands. Only a few species were specialized on the arable lands with crop rotation, and only one species was restricted to the high-input maize sites. In the trap culture experiment, the rate of root colonization by AMF was highest with inocula from the permanent grasslands and lowest with those from the high-input monocropping sites. In contrast, AMF spore formation was slowest with the former inocula and fastest with the latter inocula. In conclusion, the increased land use intensity was correlated with a decrease in AMF species richness and with a preferential selection of species that colonized roots slowly but formed spores rapidly.
A main component of the soil microbiota in most agroecosystems are the arbuscular mycorrhizal fungi (AMF). These obligate mutualistic symbionts colonize the roots of the vast majority of plants, including most crop plants (50). By forming an extended, intricate hyphal network, AMF can efficiently absorb mineral nutrients from the soil and deliver them to their host plants in exchange for carbohydrates. Facilitated nutrient uptake, particularly with respect to immobile nutrients, such as phosphorus, is believed to be the main benefit of the mycorrhizal symbiosis for plants (20, 39). AMF can also enhance tolerance of or resistance to root pathogens (7) or abiotic stresses, such as drought and metal toxicity (37). Furthermore, AMF may play a role in the formation of stable soil aggregates, building up a macroporous structure of soil that allows penetration of water and air and prevents erosion (39).
From all of these beneficial effects on plant performance and soil health, it is evident that AMF are crucial for the functioning of terrestrial ecosystems. Not only their presence but also their genetic and functional diversities are of importance: AMF diversity can be decisive for both plant community structure and ecosystem productivity (3, 4, 9, 28, 41, 54, 55). Therefore, the application of AMF is of interest for the reclamation and revegetation of degraded lands (39, 45, 57), an aspect of particular interest in the tropics (11). Moreover, it is a challenge to develop AMF management strategies applicable for sustainable low-input but reasonably productive and ecologically sound agriculture (2, 36, 40, 43, 49, 51, 52).
Modern intensive farming practices are evidently a threat for AMF, as indicated by studies of AMF performance in agroecosystems (reviewed in references 14 and 22; see also references 6, 34, 35, and 38). In general, these studies have indicated that AMF abundance and effectiveness with respect to root colonization and plant growth promotion are declining upon agricultural intensification. However, little is known about the effect of management practices on the species diversity and community structure of AMF. A recent study on the effects of conventional versus low-input agriculture reported that different management practices did not affect AMF communities in an important way (18); at the site of this trial, however, the nutrient level was extremely high, in particular with regard to phosphorus.
The AMF diversity occurring over a broad range of agricultural land use intensity has, to our knowledge, not yet been investigated. Moreover, only a few data describing AMF diversity in the temperate zone of Central Europe in general are available (5, 12, 23, 24, 31, 32). Therefore, we undertook a study of the impact of increasing land use intensity on agroecosystems in Central Europe. The soils at all sites studied, except for one, developed on Loess in the upper Rhine River valley near Basel (Switzerland). The following land use systems were selected: three low-input, seminatural, and very species-rich grasslands cut once or twice per year; two arable lands with low-input organic and moderate-input integrated conventional farming systems, both with a 7-year crop rotation; and three arable lands with continuous maize monocropping and a high input of fertilizers and pesticides. AMF spores were counted and identified both for samples taken directly from field sites and for samples obtained from “AMF trap cultures” that had been inoculated with field samples.
MATERIALS AND METHODS
Study sites.
Eight field sites were selected for this study. They are all located in the plain of the upper Rhine River valley bounded by the mountainous ranges of the Black Forest, the Vosges Mountains, and the Jura Mountains (an area of less than 1,000 km2 located in France, Germany, and Switzerland). The climate of the region is temperate, with 650 to 850 mm of yearly precipitation and an annual average temperature of about 9.5°C. Seven of the sites have the same geological parent material, namely, Loess, a wind-blown periglacial sediment deposited during the last glacial time 10,000 to 15,000 years ago. The soils that have subsequently developed are Calcaric Regosols (sites R and V), Haplic Luvisols (sites S, L, O, and G), and a Haplic Alisol (site F). An additional site (W) located in the bordering Swiss Jura Mountains is a Rendzic Leptosol that developed on calcareous Jurassic rocks. Table 1 shows some chemical characteristics of the soils.
TABLE 1.
Chemical soil parameters at field sites differing in agricultural land usea
| Land | Site | pH
|
Organic C (g kg−1) | P (mg kg−1)
|
||
|---|---|---|---|---|---|---|
| H2O | KCl | Sodium acetateb | Double lactateb | |||
| Grassland | W | 8.0 | 7.0 | 45.8 | 5.3 | 8.4 |
| V | 7.7 | 7.4 | 38.9 | 4.0 | 5.7 | |
| G | 7.5 | 6.7 | 36.5 | 5.3 | 8.4 | |
| Arable | ||||||
| Crop rotation | O | 6.4 | 5.2 | 15.8 | 5.7 | 7.9 |
| L | 7.1 | 6.0 | 21.8 | 16.3 | 62.0 | |
| Monocropping | F | 5.6 | 3.9 | 10.3 | 11.4 | 47.1 |
| S | 6.8 | 5.8 | 9.8 | 23.3 | 57.6 | |
| R | 8.3 | 7.6 | 10.4 | 18.5 | 35.2 | |
Input and management intensity increase from top to bottom.
Soil extractant; see Materials and Methods.
The agricultural use of the soils includes seminatural, extensive grasslands; arable lands with a 7-year crop rotation and moderate-intensity management; and lands with high-input maize monocropping and high-intensity management (Table 2). Sites W (Leymen, France), V (nature reserve near Kaiserstuhl mountain group, Germany), and G (Therwil, Switzerland) are extensive grasslands cut only once or twice per year. The grasslands of sites W and V have an exceptionally high plant diversity (over 80 different plant species per site), with Bromus erectus being the dominant grass (vegetation type classified as Meso-Brometum [15]). These sites were not fertilized in the last 20 years. The grassland of site G, with Arrhenatherum elatius being the dominant grass (vegetation classified as Arrhenatheretum [15]) was fertilized every 2 years with a slow-release P-K fertilizer. Arable site O (Therwil, Switzerland) belongs to a long-term field trial and was managed in a 7-year rotation with 2.5 years of a permanent grass-clover meadow, potatoes, red beets, and several cereals (35). The management of this site followed Swiss guidelines for bio-organic farming (corresponding to European Union regulation no. 2092/91) in the last 21 years; i.e., no synthetic pesticides or mineral nitrogen were used. Fertilization was usually exclusively based on manure produced on the farm, but the application of slow-release phosphate and potassium fertilizers was allowed. Farmyard manure and slurry (1.4 livestock units per ha and year [42]) were applied to the plots (plot size, 100 m2 per field plot replicate). At arable site L (Binningen, Switzerland), about the same quantity of fertilizer as that used at high-input sites S and F (see below) was applied to the maize in the form of mineral fertilizer, farmyard manure, and sewage sludge. However, this site was also subjected to a 7-year crop rotation with wheat, rapeseed, and various legumes. The management complied with the rules of Swiss Integrated Production with respect to thresholds for plant protection and for a nutrient import not exceeding the nutrient export of the harvest. At arable sites F (Burnhaupt-Le Haut, France), S (Biengen, Germany), and R, (Rheinweiler, Germany), maize was grown in 8 of the last 10 years and continuously for at least 5 years before soil sampling. In autumn before the first soil sampling took place, site S was transformed to a temporary grassland (monoculture of Lolium perenne). At all of these sites, about 150 to 170 kg of N ha−1 and 60 to 70 kg of P ha−1 were applied to the maize crop per year (Table 2), allowing an annual corn yield of about 10 to 12 tons ha−1. At sites R and S, only mineral fertilizers were applied, while at site F, farmyard manure also was added.
TABLE 2.
Principal agricultural management practices, crop rotation, and standing crop at sampling date for field sites
| Land use | Site | Farming system, rotation, and/or crop | Fertilization (kg ha−1) | Plant protection | Cultivation intensity | Standing crop in March 2000 | Geographic position (km)a |
|---|---|---|---|---|---|---|---|
| Grassland | W | Extensive; permanent grassland | None | None | Very low, mown once | Meso-Brometum | 1386.2 E, 5,260.5 N |
| V | Nature reserve; permanent grassland | None | None | Very low, mown once | Meso-Brometum | 1402.5 E, 5,326.8 N | |
| Gb | Conventional; permanent grassland | Thomas slag; P (5); every 2 yr | None | Low, mown twice | Arrhenatheretum | 1390.6 E, 5,261.4 N | |
| Arable | |||||||
| Crop rotation | Oc | Bio-organic; 7-yr rotation | Slurry and farmyard manure; N (96), P (27) | According to Swiss bio-organic farming guidelines | Moderate-low | Grass-clover (2nd yr) | 1390.2 E, 5,262.3 N |
| Lb | Conventional, 7-yr rotation | Mineral and organic, sewage sludge; N (150), P (50) | Chemical (with thresholds), mechanical | Moderate | L. multiflorum as intercrop | 1391.4 E, 5, 265.3 N | |
| Monocropping | F | Conventional, continuous maize | Mineral and farmyard manure; N (170), P (70) | Chemical | High | Bare soil | 1359.5 E, 5,287.3 N |
| S | Conventional, continuous maize | Mineral; N (170), P (70) | Chemical, mechanical | High | L. perenne (converted to grassland in autumn 1999) | 1401.2 E, 5,310.2 N | |
| R | Conventional, continuous maize | Mineral; N (170), P (60) | Chemical | High | Bare soil | 390.2 E, 5,284.5 N |
E, east of Greenwich; N, north of the Equator.
Corresponds to conventional farming managed according to Swiss Integrated Production rules (reduced fertilizer and pesticide input).
Plots of site O belong to a long-term field trial at Therwil, Switzerland, in which conventional and organic farming systems have been compared since 1978 (35).
Soil sampling at the field sites and preparation of AMF inocula.
Soil samples (four replicate plots per field site; plot sizes, 5 by 20 m) were taken in March and October 2000. These dates correspond to the beginning and the end of the growing season in the region. At each of the four plots at each field site, six soil core samples were taken up to a depth of 10 cm by using a soil corer with an 8-cm diameter. As AMF inocula for the trap plants (see below), undisturbed soil crumbs were taken from the soil core samples harvested in March 2000 (20 g for each trap plant, representing all six soil core samples taken at a depth of 5 to 7 cm). The rest of each soil core sample was carefully ground by hand, mixed, and air dried. Each composite sample representing one plot was a mixture of six such soil core samples; it was kept at 4°C until further analyses were performed. These analyses included AMF spore counting; determination of species numbers; and determination of several chemical soil parameters, such as pH, organic carbon, and available P (Table 1). The soil parameters were measured in the laboratory of F. M. Balzer, Wetter-Amönau, Germany, according to standard methods. Soils were extracted with sodium acetate and double lactate according to standard methods to estimate available P.
Soil samples taken from three sites (F, G, and V) in October 2000 were also analyzed for AMF species. The species composition and distribution pattern found were similar to those found in March 2000, but the identification of spores was more difficult due to a larger amounts of young, immature spores in autumn than in spring. We take this fact as a justification to present here only the results of the sampling in early spring.
Trap cultures for AMF.
At each of the four replicate plots at each field site, two trap culture pots (300 by 200 by 200 mm) were established. They were equipped with a 20-mm-thick drainage mat (Enkadrain ST; Colbond Geosynthetics, Arnhem, The Netherlands) and filled with 3 kg of an autoclaved substrate consisting of a mixture of Terragreen (American aluminium oxide, oil dry US special, type III R, <0.125 mm; Lobbe Umwelttechnik, Iserlohn, Germany) and Loess from a local site (3:1 [wt/wt]). The mycorrhizal inocula (see above) were placed on the surface of the substrate in the pots at positions at which the trap plantlets subsequently were planted (each of the nine positions per pot received 20 g of inoculum). Each of the inocula was then covered with 1 kg of autoclaved substrate; at the positions of the nine inocula, three 2-week-old AMF-free plantlets each of L. perenne, Trifolium pratense, and Plantago lanceolata were randomly planted in every pot. Each plantlet of T. pratense received 1 ml of a 1:5-diluted overnight culture of Rhizobium trifolii (DSM 30138; Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH [DSMZ], Braunschweig, Germany; grown in DSMZ 98 medium on a rotary shaker at 27°C). There were two reasons to choose these three trap plant species. First, all three species are well-known AMF host plants frequently used for trap cultures; second, they represent important functional groups of grasslands (a grass, a legume, and an additional common forb). The use of such a consortium of trap plants appeared to be superior to the use of a single trap plant for trapping as many AMF species as possible. Eight control pots were also included; they contained autoclaved inocula from field site G and a nonmycorrhizal suspension of soil bacteria from this site. The latter was obtained by fine filtration (LS 141/2; Schleicher & Schuell, Feldbach, Switzerland) of a soil suspension (0.4 kg of air-dried soil suspended in 1 liter of water). Finally, an automated watering system (Tropf-Blumat; Weninger GmbH, Telfs, Austria) was installed. The trap cultures were kept in a greenhouse under ambient natural light and temperature conditions for 8 months until the end of 2000. During this time, the trap plants were cut three times (i.e., every second month) 3 cm above the ground.
Sampling of the trap cultures.
After 2, 4, and 6 months of growth of the trap plants, two soil core samples (15 cm3; sampling depth, 10 cm) were taken from each pot for the extraction of AMF spores and roots. At the end of the growth season (after 8 months), four core samples were taken from each pot. The initial rate of spore formation was determined by extracting and counting the spores formed after 2 and 4 months, and the initial rate of root colonization by AMF was determined after 2 months as described previously (8) by using trypan blue for staining of mycorrhizal structures. At each sampling time, single spore cultures of each of the species newly discovered in the trap cultures were set up.
AMF spore isolation and identification.
AMF spores occurring in the original soil samples or produced in the trap cultures were extracted by wet sieving and sucrose density gradient centrifugation (13). The procedure included passage of 25 g of air-dried field soil or 30 cm3 of harvested trap culture substrate through 1,000-, 500-, 125-, and 32-μm sieves. The 1,000-μm sieve was checked for spores adjacent to or inside roots, while the 500-μm sieve was checked for large spores, spore clusters, and sporocarps. The contents of the 125- and 32-μm sieves were layered onto a water-sucrose solution (70% [wt/vol]) gradient and centrifuged at 900 × g for 2 min. The resulting supernatant was passed through the 32-μm sieve, washed with tap water, and transferred to petri dishes. Spores, spore clusters, and sporocarps obtained from all sieves were counted by using a dissecting microscope at a magnification of up to ×90. Thereafter, 50 to 70% of them were mounted on slides with polyvinyl-lactic acid-glycerol (29) or polyvinyl-lactic acid-glycerol mixed 1:1 (vol/vol) with Melzer's reagent (8). Only the healthy-looking spores were mounted. The spores were examined under a stereomicroscope (Zeiss; Axioplan) at a magnification of up to ×400. Only 60 to 85% of the spores mounted on slides could be identified to the species level or attributed to a specific morphospecies; the rest consisted mostly of old and decaying spores with missing clear features. In this study, a species could be a clearly identified morphospecies based on spore morphology, a not-yet-described morphospecies, or an already-described morphospecies that was not yet known to us. A species group comprised more than one morphospecies due to the fact that the features were not clear enough to definitively attribute the majority of the spores to one of the morphospecies in the group. Identifications were based on current species descriptions and identification manuals (46; International Culture Collection of Arbuscular and Vesicular-Arbuscular Endomycorrhizal Fungi [http://invam.caf.wvu.edu/Myc_Info/Taxonomy/species.htm]).
The abundance of spores from all AMF species together was determined for each sample and expressed as the number of AMF spores per gram of soil for the field site samples, whereas for the trap culture samples, it was expressed per milliliter of substrate. Moreover, for each sample from the field sites and the trap cultures, the number of spores belonging to the different AMF species discerned also was determined. For every AMF species and site, the relative abundance and the absolute number of spores identified per site (total amount of soil explored, 100 g) are given. The Shannon-Weaver (H′) index was calculated as an additional measure of AMF diversity, as it combines two components of diversity, i.e., species richness and evenness. It is calculated from the equation H′ = −Σ ρi lnρi, where ρi is the relative spore abundance of the ith species compared to all species identified in a sample. However, diversity measures change with changes in the numbers of individuals in a sample; therefore, the Shannon-Weaver index was corrected by using the formula of Fager (16), yielding corrected H′ values of between 0 and 1. For the same reasons, the number of species, i.e., the species richness, was corrected by using the rarefaction method (33).
Statistics and HCA.
The significance of differences between field sites in spore abundance, species numbers, and AMF diversity (Shannon-Weaver index) was tested by using Fisher's least-significant-difference (LSD) test at a P value of <0.05 after a one-way analysis of variance (ANOVA). For the trap cultures inoculated with soil from the different sites, the same statistical tests were applied for initial mycorrhizal root colonization and spore formation. A hierarchical cluster analysis (HCA) with Ward's minimum-variance method (33) was applied to determine the relationship between field sites and replicate plots of field sites in AMF species communities based on the χ2 distance (33).
RESULTS
AMF species found at field sites.
AMF spores were separately isolated from four replicate original soil samples taken at eight field sites and were separately identified and counted. In total, 37 species could be distinguished on the basis of morphological criteria (Table 3). Twenty-three species could be identified unequivocally according to descriptions in the literature. In four instances, when two species could not be unequivocally distinguished, a species group was defined (e.g., Glomus occultum group) (Table 3). Ten species not described so far, at least not to our knowledge, could not be named at the species level (Acaulospora sp. strain BR1, Glomus sp. strains BR2 to BR9, and Archaeospora sp. strain BR10). The majority of the isolated species were identified as Glomaceae, all of these being Glomus Tulasne and Tulasne species (Table 3). Two of them had formerly been assigned to Sclerocystis Berkeley and Broome (G. sinuosum and G. rubiforme). One species of the genus Archaeospora, resembling Archaeospora leptoticha Morton and Redecker, and one of the genus Paraglomus Morton and Redecker (Paraglomus occultum) were identified. Six species were identified as Acaulosporaceae (five of them were in the genus Acaulospora Gerdemann and Trappe, and one was in the genus Entrophospora Ames and Schneider). Two species were members of the family Gigasporaceae and belonged to the genus Scutellospora Walker and Sanders. Species in the genus Gigaspora Gerdemann and Trappe (emend. Walker and Sanders) were not found.
TABLE 3.
Relative spore abundance of AMF species found at field sites and absolute numbers of spores identified
| Glomales species or strain | % Spore abundance (no. of spores) at site
|
|||||||
|---|---|---|---|---|---|---|---|---|
| W | V | G | O | L | F | S | R | |
| G. aggregatum | 4.1 (12) | 18.8 (34) | 9.8 (8) | |||||
| G. caledonium | 0.1 (1) | 1.6 (8) | 3.6 (10) | 7.9 (23) | 9.9 (18) | |||
| G. mosseae groupa | 1.3 (24) | 3.0 (55) | 1.9 (29) | 5.9 (29) | 17.3 (48) | 10.7 (31) | 31.5 (57) | 54.9 (45) |
| G. geosporum | 1.8 (34) | 0.9 (16) | 3.2 (47) | 4.7 (23) | 6.1 (17) | 2.4 (7) | 5.5 (10) | 3.7 (3) |
| G. occultum groupb | 4.9 (90) | 0.9 (17) | 6.9 (103) | 6.7 (33) | 7.2 (20) | 20.7 (60) | 7.7 (14) | 6.1 (5) |
| G. etunicatum | 1.4 (26) | 1.2 (22) | 3.4 (50) | 10.6 (52) | 10.4 (29) | 21.7 (63) | 5.5 (10) | 3.7 (3) |
| G. constrictum | 1.5 (28) | 3.7 (68) | 2.8 (42) | 6.8 (35) | 6.8 (19) | 1.4 (4) | 2.8 (5) | 1.2 (1) |
| G. diaphanum | 0.3 (5) | 0.1 (2) | 4.5 (67) | 6.3 (31) | 15.2 (44) | 17.6 (51) | 14.4 (26) | 14.6 (12) |
| S. calospora | 0.2 (3) | 5.5 (101) | 1.1 (47) | 6.3 (31) | 4.2 (5) | 5.2 (15) | 6.1 (5) | |
| G. fasciculatum groupc | 6.1 (112) | 1.8 (34) | 6.0 (89) | 2.8 (14) | 4.3 (12) | 8.3 (24) | 3.9 (7) | |
| Glomus sp. strain BR9 | 20.8 (384) | 7.2 (132) | 23.0 (342) | 7.9 (39) | 14.4 (40) | |||
| G. invermaium | 2.8 (51) | 0.3 (6) | 5.8 (86) | 12.8 (63) | 9.7 (27) | |||
| G. dominikii | 0.1 (1) | 0.3 (5) | 0.6 (3) | 1.1 (3) | ||||
| A. laevis groupd | 0.1 (1) | 2.0 (30) | 8.1 (40) | |||||
| A. paulinae | 2.1 (32) | 13.0 (64) | ||||||
| A. longula | 0.8 (4) | |||||||
| Acaulospora sp. strain BR1e | 0.4 (2) | |||||||
| S. pellucida | 3.9 (19) | |||||||
| G. heterosporum | 8.2 (152) | 9.1 (167) | 5.7 (85) | |||||
| G. macrocarpum | 0.8 (14) | 14.6 (270) | 0.2 (3) | |||||
| Glomus sp. strain BR2 | 10.5 (195) | 2.0 (36) | 11.1 (166) | 0.4 (2) | ||||
| Glomus sp. strain BR3f | 0.6 (11) | 7.6 (140) | 1.0 (15) | |||||
| E. infrequens | 0.3 (5) | 0.1 (1) | 0.1 (1) | |||||
| Glomus sp. strain BR4 | 25.2 (466) | 0.2 (5) | 18.0 (268) | |||||
| Glomus sp. strain BR5 | 1.7 (31) | 2.8 (51) | 0.7 (2) | |||||
| G. microcarpum | 0.7 (13) | 1.0 (19) | ||||||
| Glomus sp. strain BR6 | 0.8 (12) | |||||||
| G. mortonii | 18.1 (334) | |||||||
| G. rubiforme | 16.0 (295) | |||||||
| Glomus sp. strain BR7 | 3.4 (63) | |||||||
| Glomus sp. strain BR8 | 0.9 (16) | |||||||
| G. globiferum | 0.1 (2) | |||||||
| G. ambisporum | 5.1 (94) | |||||||
| G. sinuosum | 3.1 (57) | |||||||
| G. versiforme | 2.4 (44) | |||||||
| G. tortuosum | 0.3 (5) | |||||||
| Archaeospora sp. strain BR10g | 0.2 (3) | |||||||
Comprises G. mosseae and G. coronatum.
Comprises P. occultum and G. albidum.
Comprises G. fasciculatum and G. clarum.
Comprises A. laevis and A. thomii.
Resembles A. scrobiculata.
Resembles G. constrictum.
Resembles A. leptoticha.
Frequencies and patterns of distribution of AMF species at field sites.
Some species of AMF appeared to be “generalists,” since they were found at virtually all field sites: G. mosseae of the G mosseae group, G. geosporum, G. albidum of the G. occultum group, G. etunicatum, G. diaphanum, G. constrictum, species of the G. fasciculatum group, and Scutellospora calospora (Table 3). Of these, G. mosseae, G. geosporum, G. albidum, and G. etunicatum were the dominant species in the arable soils (O, L, F, S, and R), but they were also present abundantly in the grassland soils (W, V, and G). Some species were abundant both in the grasslands and in the low- and intermediate-input arable lands with crop rotation (O and L) but were absent from the intensively managed arable lands with continuous maize monocropping (F, S, and R). More than half of the species were exclusively found in the grasslands, and many of them occurred in all three grasslands investigated (“grassland specialists”), despite the fact that the soil substrate of grassland W was different from that of grasslands V and G. Five species were found exclusively in grassland V, and five others were found exclusively in grassland W (“highly specialized species”). Among these species, some appear to occur in several habitats (e.g., G. sinuosum and Glomus sp. strain BR2 in tropical pastures and forests as well as in South European grasslands with olive trees; unpublished observations), whereas others presumably have been found for the first time (e.g., Glomus sp. strain BR7 and Glomus sp. strain BR8).
In the intensively managed arable lands (F, S, and R), in addition to the generalists mentioned above, only two further species were found, G. aggregatum and G. caledonium. Remarkably, the spores of these species became less abundant (G. caledonium) or were not found (G. aggregatum) in the low- and intermediate-input arable lands with crop rotation (O and L) and were very rare or absent from the grasslands (Table 3).
Dependence of AMF spore abundance, species number, and Shannon-Weaver index on cultivation systems.
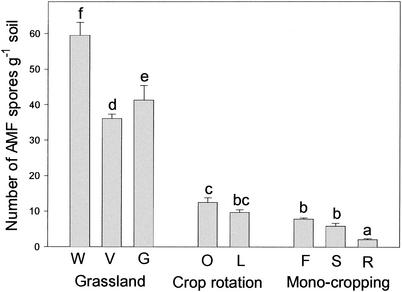
The seminatural grasslands (W, V, and G) contained by far the highest spore abundances (average for the four replicate plots per field site, 35 to 65 spores g−1) (Fig. 1) as well as the highest species numbers (average for the four replicate plots per field site, 17 to 20 species found in 100 g of soil explored) (Table 4). The total species numbers (total number in the four replicate plots per field site) detected at these sites were even higher, namely, 24 in grassland W on Jurassic rock and 25 and 20, respectively, in grasslands V and G on Loess (Table 4). Much lower spore abundances as well as lower species numbers were found in arable lands. The arable lands with crop rotation and low or intermediate input (O and L) showed, respectively, abundances of 12.5 and 9.7 spores g of soil−1 and average species numbers of 15.5 and 11.2 (Fig. 1 and Table 4). Remarkably, the arable land managed organically (O) had an average species number similar to those of the grasslands, while the conventionally managed one (L) had a significantly lower number (Table 4). By far the lowest spore abundances and average species numbers were found in the soils of the intensively managed sites with maize monocropping (F, S, and R), namely, abundances of between 2.5 and 8.0 spores g of soil−1 (Fig. 1) and average species numbers of between 6.0 and 8.0 (Table 4). Processing of additional soil from the same samples as well as from samples taken in other seasons of the year (see Material and Methods) yielded essentially similar spore numbers and species compositions (data not shown).
FIG. 1.
AMF spore abundance at field sites (W, V, G, O, L, F, S, and R) with different cultivation practices. Input and management intensity increase from left to right. Data are reported as averages and standard deviations for four replicate plots per site. Nonsignificant differences between sites are indicated by identical letters above the bars and were determined by using Fisher's LSD test at the 5% level after a one-way ANOVA.
TABLE 4.
Number of AMF species found at field sites and Shannon-Weaver diversity index calculated from the number of species identifieda
| Land use | Site | No. of AMF species
|
Shannon-Weaver diversity index (correctede) | ||
|---|---|---|---|---|---|
| At field sitesb | Cor- rectedb,c | Total at field sitesd | |||
| Grassland | W | 20.5 A | 19.8 A | 24 | 2.29 AB (0.83 AB) |
| V | 19.5 A | 18.9 A | 25 | 2.32 AB (0.86 AB) | |
| G | 17.0 B | 16.8 B | 20 | 2.34 AB (0.87 AB) | |
| Arable | |||||
| Crop rotation | O | 15.5 B | 15.4 B | 18 | 2.45 A (0.94 A) |
| L | 11.2 C | 11.2 C | 13 | 2.14 B (0.74 B) | |
| Monocropping | F | 7.7 D | 7.7 DE | 10 | 1.70 C (0.44 C) |
| S | 8.0 D | 8.0 D | 9 | 1.72 C (0.46 C) | |
| R | 6.0 D | 6.0 E | 8 | 1.32 D (0.16 D) | |
Nonsignificant differences between sites are shown by identical letters and were determined with Fisher's LSD test at the 5% level after a one-way ANOVA. LSD values were 2.07, 1.92, 0.21, and 0.15 for number of AMF species, corrected number of AMF species, Shannon-Weaver diversity index, and corrected diversity index, respectively.
Average for four replicate plots of field site.
Corrected for unequal numbers of spores identified at different field sites (Table 3) by using the rarefaction method (33).
Sum of four field repetitions.
AMF diversities, expressed by the Shannon-Weaver diversity index, in both its original and its corrected versions, were similar for the grasslands (W, V, and G) and the arable lands with crop rotation (O and L). The index was significantly lower, however, at all of the intensively managed sites (R, S, and F) with maize monocropping (Table 4). The index decreased from the highest value of 2.45 (corrected version, 0.94) to the lowest value of 1.32 (corrected version, 0.16) in the order of succession O ≥ G ≥ V ≥ W ≥ L > S ≥ F > R.
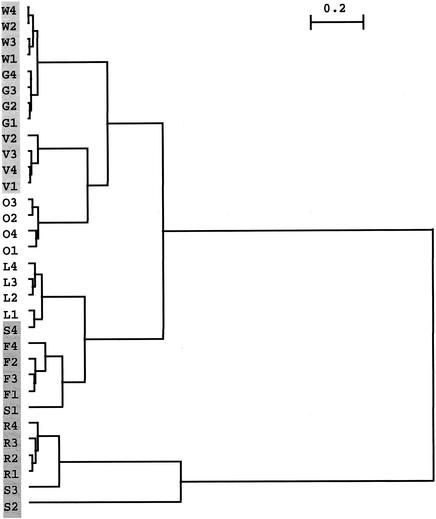
HCA showed the highest similarity in AMF species composition between the four replicate plots of each field site, with the exception of the four replicate plots of intensively managed arable site S, which grouped not with each other but with the other three conventionally managed sites, R, F, and L (Fig. 2). Interestingly, according to this analysis, organically managed site O showed the highest similarity to the grasslands, not to the arable lands.
FIG. 2.
HCA with Ward's minimum-variance method (33) of different field sites (W, G, V, O, L, S, F, and R) and of the four replicate plots per field site for AMF species composition based on the χ2 distance. Samples represented grasslands (W, G, and V), arable lands with crop rotation (O and L), and arable lands with maize monocropping (S, F, and R). Note the similarity of grasslands W and G and the clustering of grasslands W, G, and V with organically managed arable site O with crop rotation. Scale bar, 0.2 U.
Initial AMF root colonization and spore formation in the trap cultures.
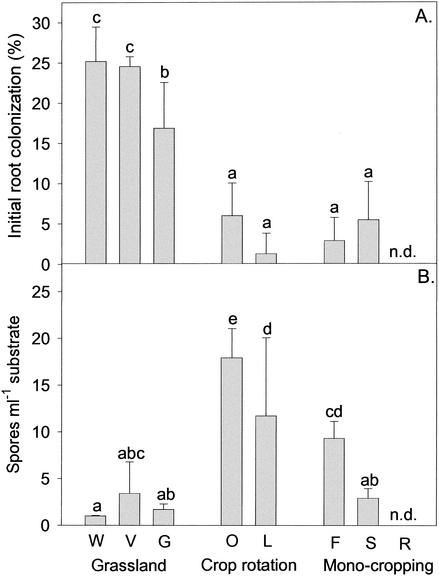
The initial AMF root colonization in the trap cultures established from the different field sites (Fig. 3A) exhibited patterns similar to the spore abundance (Fig. 1) and the total species number (Table 4) detected at the sites. After 2 months of culturing, the roots in the pots inoculated with the grassland soils were colonized to a much higher degree than the roots in the pots inoculated with the soils from the arable sites. No significant difference, however, was observed between the different arable sites.
FIG. 3.
Mycorrhizal root colonization after 2 months (A) and AMF spore formation after 4 months (B) of trapping of AMF in pot cultures. Pots were inoculated with soil derived from field sites (W, V, G, O, L, F, S, and R). Input and management intensity increase from left to right. Data are reported as averages and standard deviations for four replicate plots per field site. Nonsignificant differences between sites are indicated by identical letters above the bars and were determined by using Fisher's LSD test at the 5% level after a one-way ANOVA. n.d., not determined.
Intriguingly, however, spore formation in the trap cultures was initially not correlated with root colonization. The trap cultures of the grasslands contained no spores after 2 months and only a few after 4 months (Fig. 3B). The main AMF spore production started only after 6 to 8 months in these cultures. In contrast, all trap cultures of the arable lands had already produced a few spores after 2 months, and numerous spores were present in these cultures after 4 months (up to 18 spores per ml of substrate) (Fig. 3B). The high standard deviation for site V (Fig. 3B) was due to G. lamellosum, which abundantly sporulated in only one of the four replicate plots.
It was interesting to monitor the appearance of spores of different species in the trap cultures. After only 2 months of culturing, a few species had produced spores in the trap cultures of the arable lands, namely, G. caledonium, G. mosseae, G. geosporum, G. etunicatum, and G. diaphanum; after 4 months, as many as five or six AMF species had sporulated in these cultures (Tables 5 and 6). In contrast, the trap cultures of the grasslands contained the first few spores of one or two species only after 4 months. After 8 months, at the end of the growth season, about the same species numbers were found in the trap cultures of the grasslands (7 to 12 species) and the arable lands (6 to 12 species, excluding the organic site). The highest species number (20 species) was found at this time in the trap cultures of organically managed site O. Interestingly, after 8 months of trap culturing, as many as 75 to 111% of the species found in the original field samples from the arable lands could be recovered, whereas the recovery from the grasslands was only 29 to 60% at that time (Table 4).
TABLE 5.
Number of AMF species found in AMF trap cultures set up from field sitesa
| Land use | Site | No. of AMF species
|
Ratio of no. of AMF species found in samples from trap cultures/field sites | ||||
|---|---|---|---|---|---|---|---|
| Found in trap cultures after the following no. of mo:
|
Total found in samples from field sites and trap cultures | ||||||
| 2 | 4 | 6 | 8 | ||||
| Grassland | W | 0 | 1 | 4 | 7 | 26 | 0.29 |
| V | 0 | 2 | 5 | 12 | 27 | 0.48 | |
| G | 0 | 1 | 7 | 12 | 26 | 0.60 | |
| Arable | |||||||
| Crop rotation | O | 3 | 6 | 13 | 20 | 26 | 1.11 |
| L | 3 | 5 | 8 | 12 | 18 | 0.92 | |
| Monocropping | F | 2 | 5 | 6 | 10 | 13 | 1.00 |
| S | 2 | 6 | 6 | 7 | 10 | 0.78 | |
| R | 1 | 3 | 5 | 6 | 8 | 0.75 | |
AMF spores were isolated from trap cultures and identified repeatedly over a period of 8 months.
TABLE 6.
AMF species found in trap cultures set up from field sites
| Glomales species or straina | Months when AMF species were found in trap cultures corresponding to the following field samplesb:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Grassland
|
Arable land
|
|||||||
| Crop rotation
|
Monocropping
|
|||||||
| W | V | G | O | L | F | S | R | |
| G. aggregatum | 6 | 4 | 6 | 4 | 6 | |||
| G. caledonium | 4 | 2 | 2 | 2 | ||||
| G. mosseae | 4 | 4 | 4 | 2 | 2 | 2 | 2 | 2 |
| G. geosporum | 8 | 8 | 2 | 2 | 4 | |||
| G. albidum | 6 | 8 | 4 | 6 | 8 | 8 | 6 | |
| G. etunicatum | 8 | 8 | 6 | 2 | 2 | 4 | 4 | 4 |
| G. diaphanum | 8 | 6 | 2 | 4 | 8 | |||
| S. calospora | 8 | 8 | ||||||
| G. fasciculatum | 6 | 6 | 6 | 8 | 8 | 8 | ||
| Glomus sp. strain BR9 | 8 | 6 | ||||||
| A. thomii | 8 | |||||||
| A. paulinae | 6 | 8 | ||||||
| A. longula | 8 | |||||||
| Glomus sp. strain BR2 | 8 | 8 | 8 | |||||
| Glomus sp. strain BR3c | 6 | 6 | 8 | 6 | ||||
| Glomus sp. strain BR4 | 8 | |||||||
| Glomus sp. strain BR5 | 8 | 6 | ||||||
| G. microcarpum | 8 | |||||||
| G. versiforme | 6 | 6 | 6 | |||||
| E. infrequens | 8 | |||||||
| Archaeospora trappeid | 8 | 6 | 4 | 8 | ||||
| G. lamellosumd | 6 | 4 | 6 | 8 | 4 | 4 | ||
| Glomus sp. strain BR11e | 8 | 8 | 6 | 8 | ||||
| G. intraradicesd | 8 | |||||||
AMF species found in trap cultures and in field samples, unless otherwise indicated.
For the first time after the establishment of trap cultures.
Resembles G. constrictum.
AMF morphospecies found in trap cultures but not in field samples.
Resembles G. arborense.
For the arable lands, G. constrictum, Scutellospora pellucida, G. invermaium, G. dominikii, and Acaulospora sp. strain BR1, which were found in the original field samples (Table 3), did not form spores in the trap cultures even after 8 months. All of the other species detected in the field samples of the arable lands could be recovered in the trap cultures at that time (Table 6). Remarkably, G. mosseae and G. etunicatum formed spores much later in the trap cultures of the grasslands than in the trap cultures of the arable lands (4 to 8 versus 2 months) (Table 6). Only after 8 months had a few of the grassland specialists sporulated in the corresponding trap cultures (Glomus sp. strain BR2, Glomus sp. strain BR3, and Glomus sp. strain BR4).
In a number of field sites, some species were not found in the spore analysis but sporulated subsequently in the corresponding trap cultures (Table 6). Examples are G. aggregatum from organically managed arable land O and from grassland G, G. fasciculatum and Acaulospora paulinae from integrated conventionally managed land L, Glomus sp. strain BR3 and G. microcarpum from arable land O, and G. versiforme from low-intensity arable lands L and O and from grassland G. Spores of Archaeospora trappei, G. lamellosum, and G. intraradices and small hyaline spores of a Glomus species (Glomus sp. strain BR11, resembling G. arborense) were not detected in any of the field samples and were discovered only in the trap cultures after 4 to 8 months of culturing. Thereafter, when the trap cultures were analyzed, it became evident that in the field samples, the spores of G. lamellosum had been placed erroneously in the G. fasciculatum group and the spores of Archaeospora trappei and Glomus sp. strain BR11 had been placed in the G. occultum group (Table 3). Control trap cultures, which received autoclaved inoculum, showed neither root colonization by AMF nor spore formation.
DISCUSSION
Overall, we detected a total of 45 AMF species (morphospecies) at the eight field sites investigated; about 20% of them were not previously described, to our knowledge. This is a surprisingly large number, given that so far only about 150 AMF species have been described worldwide for the phylum Glomeromycota (47) and that we took samples in a quite uniform region of less than 1,000 km2 and with similar climatic and edaphic conditions throughout. Altogether, 41 species were detected in the field samples directly, and 4 additional ones were found upon trap culturing for 8 months with a consortium of three AMF trap plant species. A similarly large number of AMF species (namely, 37, one-third of which were not described previously) has been found in a seminatural grassland of 1 ha on the Duke University campus (Durham, N.C.) upon repeated intensive sampling and trap culturing withdifferent substrates and trap plants over a period of 5 years (3). In general, a substantial proportion of the AMF morphospecies detected in such surveys has not been described before, indicating that there must exist many more AMF species than the 150 described so far. We suppose that the actual AMF species number in the region on which we focused our studies must be much larger than we have found because all of our sampling sites, except for one (calcareous grassland W), were located in plains with almost identical climatic and edaphic conditions. Moreover, we used only one type of trap culturing system with a single substrate and a single trap plant consortium.
Natural ecosystems in Europe have been estimated to contain up to 25 AMF species (17), and our findings for the seminatural grasslands (26 or 27 species) corroborate this estimate. In the arable lands, the AMF community contained significantly fewer species, namely, 18 species in the lands with conventional agriculture and crop rotation and 8 to 13 species in the lands with monocropping. A remarkable exception was the organically managed land harboring as many as 26 species, similar to the number in the grasslands. Previously reported data on species richness in temperate European and North American arable lands are in the same range (12, 14, 18, 30, 31, 56).
Our data on the arable lands indicate a marked decrease in AMF species numbers upon intensification of agricultural management. By far the highest AMF species number and Shannon-Weaver diversity index were found in the low-input, organically managed arable land with crop rotation. Not only in species number but also in HCA, this arable land resembled the grasslands. Among the other arable lands, the site with intermediate input and crop rotation had a species number and a diversity index significantly higher than those of the three sites with high input and continuous maize monocropping. These findings are consistent with previous reports in which the AMF community was found to be impoverished in species composition upon agricultural intensification (1, 27, 49), particularly upon a change from crop rotation to monocropping (1). There are also contrasting reports in which no marked differences in AMF community composition and structure were found upon long-term application of conventional or low-input farming practices (18); however, that farming trial took place at a site where the quantities of nutrients—particularly of available phosphorus—were extremely high and nitrogen inputs were similar in all plots.
In the trap cultures, the rates of AMF root colonization and of spore formation differed conspicuously between the grasslands and the arable lands. In the arable lands, rapidly sporulating species, such as G. mosseae, G. geosporum, and G. etunicatum, were among the dominating species with respect to spore abundance. These species are frequently reported to occur in intensively cultivated soils (14, 19, 30-32). Other species, such as G. invermaium, G. dominikii, A. laevis, A. paulinae, A. longula, and S. pellucida, were found in arable lands only when crop rotation was practiced (at sites L and/or O), and most of these species did not sporulate within the 8-month period of trap culturing (although A. paulinae and A. longula sporulated after 6 to 8 months) (Table 6). Root colonization strategies and timing of sporulation may be crucial for the survival of AMF species in arable lands exposed to intensive farming practices, such as herbicide-cleaned monocropping systems with short vegetation and prolonged fallow periods (21). This idea may explain the absence of the slowly sporulating species at the intensively managed monocropping sites (R, S and F). However, these species were present in lands where crop rotation was practiced (sites O and L), particularly in the organically managed one (O), where a grass-clover meadow was part of the 7-year crop rotation for 2.5 years, and also at a lower frequency in the conventionally managed one (L), where overwintering cover crops were generally cultivated.
It is noteworthy that the extension of the trap culturing time from 4 to 8 months increased the overall AMF species recovery from 9 to 23. If the AMF species numbers would have been judged from the recoveries obtained after 4 months, they would have been smaller in the grasslands than in the arable lands. For the grasslands, even after 8 months of trap culturing, only 30 to 60% of the AMF species found directly in the field samples had produced spores. Most likely, the delayed spore formation in the trap cultures from the grasslands is an intrinsic property of the AMF species present in the corresponding inocula, although it may also be due, in part, to a reduced number of living propagules present in these inocula. Remarkably, species that did not form spores at all in the trap cultures were mostly sporocarpic; these included G. sinuosum, G. tortuosum, G. mortonii, G. rubiforme, G. ambisporum, and Glomus sp. strain BR6. They seemed to be specific for grasslands in our study area. The sporocarps of these species observed in the field samples appeared to be alive and healthy. It will therefore be interesting to determine whether these species eventually, upon prolonged trap culturing, also form spores or whether they were not compatible with the trap plants used. These notions can be tested with newly developed molecular tools for identifying AMF species within roots (23, 44).
Interestingly, not only was the delayed spore formation in the trap cultures of the grasslands compared to the arable lands due to the different AMF species compositions in these two agroecosystems, but also the same delay in spore formation was observed for individual species, e.g., G. mosseae and G. etunicatum. Thus, the different land use practices apparently have selected ecotypes differing in physiological traits. In this context, it is interesting that AMF derived from high-input agroecosystems have repeatedly been shown to be less efficient in plant growth promotion than those derived from low-input systems (10, 25, 26, 48).
Since AMF spore formation is known to be highly variable, depending on AMF species (some species may not form spores at all), host plants, season of the year, and other environmental factors (3), the actual contributions and importance of different AMF species for ecosystem functioning cannot be derived from AMF surveys based solely on an assessment of spore morphotypes. In this context, it is relevant that molecular tools are currently being developed to allow the identification of AMF species directly in host plant roots (12, 23, 44, 53). Currently, we are using such a molecular approach, involving PCR for targeting of specific AMF sequences, to investigate AMF diversity in roots sampled from the field sites and from the trap cultures. In view of the eventual application of selected efficient AMF isolates, these molecular tools for the identification of AMF in roots will be invaluable for testing the compatibility of AMF inocula with different crops and checking their persistence in the field.
Our study clearly indicates that agricultural intensification, as practiced in temperate Central Europe, severely affects AMF abundance and community structure. Remarkably, the AMF communities differed not only in diversity but also in functional aspects (rates of root colonization and spore formation in the trap cultures). These findings were true even for individual AMF species, which appeared to occur as rapidly or slowly sporulating ecotypes depending on the agricultural management practices. It will be interesting to further investigate whether these AMF differ in other functional traits that are of agronomic importance.
Acknowledgments
We thank Endre Laczko for help and advice regarding statistical treatment of the data and Dirk Redecker for critically reading the manuscript. The technical help of Nadja Feddermann, Larissa Vines, and Giacomo Busco is gratefully acknowledged.
This study was supported by the Swiss Agency for Development and Cooperation (SDC) in the frame of the Indo-Swiss Collaboration in Biotechnology (ISCB) program.
REFERENCES
- 1.An, Z. Q., J. W. Hendrix, D. E. Hershman, R. S. Ferriss, and G. T. Henson. 1993. The influence of crop-rotation and soil fumigation on a mycorrhizal fungal community associated with soybean. Mycorrhiza 3:171-182.
- 2.Bethlenfalvay, G. J. 1993. Mycorrhizae in the agricultural plant-soil system. Symbiosis 14:413-425. [Google Scholar]
- 3.Bever, J. D., P. A. Schultz, A. Pringle, and J. B. Morton. 2001. Arbuscular mycorrhizal fungi: more diverse than meets the eye, and the ecological tale of why. BioScience 51:923-931. [Google Scholar]
- 4.Bidartondo, M. I., D. Redecker, I. Hijri, A. Wiemken, T. D. Bruns, L. Domínguez, A. Sérsic, J. R. Leake, and D. J. Read. 2002. Epiparasitic plants specialized on arbuscular mycorrhizal fungi. Nature 419:389-392. [DOI] [PubMed] [Google Scholar]
- 5.Blaszkowski, J. 1993. Comparative studies on the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28:93-140. [Google Scholar]
- 6.Boddington, C. L., and J. C. Dodd. 2000. The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi. II. Studies in experimental microcosms. Plant Soil 218:145-157. [Google Scholar]
- 7.Borowicz, V. A. 2001. Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82:3057-3068. [Google Scholar]
- 8.Brundrett, M., L. Melville, and L. Peterson. 1994. Practical methods in mycorrhiza research. Mycologue Publications, University of Guelph, Guelph, Ontario, Canada.
- 9.Burrows, R. L., and F. L. Pfleger. 2002. Host responses to AMF from plots differing in plant diversity. Plant Soil 240:169-179. [Google Scholar]
- 10.Corkidi, L., D. L. Rowland, N. C. Johnson, and E. B. Allen. 2002. Nitrogen fertilization alters the functioning of arbuscular mycorrhizas at two semiarid grasslands. Plant Soil 240:299-310. [Google Scholar]
- 11.Cuenca, G., Z. De Andrade, and G. Escalante. 1998. Diversity of glomalean spores from natural, disturbed and revegetated communities growing on nutrient-poor tropical soils. Soil Biol. Biochem. 30:711-719. [Google Scholar]
- 12.Daniell, T. J., R. Husband, A. H. Fitter, and J. P. W. Young. 2001. Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Microbiol. Ecol. 36: 203-209. [DOI] [PubMed] [Google Scholar]
- 13.Daniels, B. A., and H. D. Skipper. 1982. Methods for the recovery and quantitative estimation of propagules from soil, p. 29-35. In N. C. Schenck (ed.), Methods and principles of mycological research. The American Phytopathological Society, St. Paul., Minn.
- 14.Douds, D. D., and P. Millner. 1999. Biodiversity of arbuscular mycorrhizal fungi in agroecosystems. Agric. Ecosyst. Environ. 74:77-93. [Google Scholar]
- 15.Ellenberg, H. 1996. Vegetationskunde Mitteleuropas und der Alpen. Ulmer, Stuttgart, Germany.
- 16.Fager, E. W. 1972. Diversity: a sampling study. Am. Nat. 106:293-310. [Google Scholar]
- 17.Fitter, A. H. 2001. Specificity, links and networks in the control of diversity in plant and microbial communities, p. 95-114. In M. C. Press, N. J. Hontly, and S. Levin (ed.), Mycorrhizal functioning. Ecology: achievement and challenge. Blackwell Scientific Publications Ltd., Oxford, England.
- 18.Franke-Snyder, M., D. D. Douds, L. Galvez, J. G. Phillips, P. Wagoner, L. Drinkwater, and J. B. Morton. 2001. Diversity of communities of arbuscular mycorrhizal (AM) fungi present in conventional versus low-input agricultural sites in eastern Pennsylvania, USA. Appl. Soil Ecol. 16:35-48. [Google Scholar]
- 19.Galvez, L., D. D. Douds, L. E. Drinkwater, and P. Wagoner. 2001. Effect of tillage and farming system upon VAM fungus populations and mycorrhizas and nutrient uptake of maize. Plant Soil 228:299-308. [Google Scholar]
- 20.George, E., H. Marschner, and I. Jakobsen. 1995. Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit. Rev. Biotechnol. 15:257-270. [Google Scholar]
- 21.Hart, M. H., and R. J. Reader. 2002. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 153:335-344. [Google Scholar]
- 22.Helgason, T., T. J. Daniell, R. Husband, A. H. Fitter, and J. P. W. Young. 1998. Ploughing up the wood-wide web? Nature 394:431.. [DOI] [PubMed] [Google Scholar]
- 23.Helgason, T., A. H. Fitter, and J. P. W. Young. 1999. Molecular diversity of arbuscular mycorrhizal fungi colonising Hyacinthoides nonscripta (bluebell) in a seminatural woodland. Mol. Ecol. 8:659-666. [Google Scholar]
- 24.Jansa, J., A. Mozafar, T. Anken, R. Ruh, I. R. Sanders, and E. Frossard. 2002. Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza 12:225-234. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, N. C. 1993. Can fertilization of soil select less mutualistic mycorrhizae? Ecol. Appl. 3:749-757. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, N. C., J. H. Graham, and F. A. Smith. 1997. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 135:575-585. [Google Scholar]
- 27.Johnson, N. C., and F. L. Pfleger. 1992. Vesicular-arbuscular mycorrhizae and cultural stresses, p. 71-99. In G. J. Bethlenfalvay and R. G. Linderman (ed.), Mycorrhizae in sustainable agriculture. American Society of Agronomy special publication no. 54. American Society of Agronomy, Madison, Wis.
- 28.Klironomos, J. N., J. McCune, M. Hart, and J. Neville. 2000. The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecol. Lett. 3:137-141. [Google Scholar]
- 29.Koske, R. E., and B. Tessier. 1983. A convenient permanent slide mounting medium. Mycol. Soc. Am. Newsl. 34:59. [Google Scholar]
- 30.Kurle, J. E., and F. L. Pfleger. 1996. Management influences on arbuscular mycorrhizal fungal species composition in a corn-soybean rotation. Agron. J. 88:155-161. [Google Scholar]
- 31.Land, S., and F. Schönbeck. 1991. Influence of different soil types on abundance and seasonal dynamics of vesicular arbuscular mycorrhizal fungi in arable soils of North Germany. Mycorrhiza 1:39-44. [Google Scholar]
- 32.Land, S., H. Von Alten, and F. Schönbeck. 1993. The influence of host plant, nitrogen fertilization and fungicide application on the abundance and seasonal dynamics of vesicular-arbuscular mycorrhizal fungi in arable soils of northern Germany. Mycorrhiza 2:157-166. [Google Scholar]
- 33.Legendre, P., and L. Legendre. 1988. Numerical ecology: developments in environmental modelling, 2nd ed. Elsevier Science, Amsterdam, The Netherlands.
- 34.Mäder, P., S. Edenhofer, T. Boller, A. Wiemken, and U. Niggli. 2000. Arbuscular mycorrhizae in a long-term field trial comparing low-input (organic, biological) and high-input (conventional) farming systems in a crop rotation. Biol. Fertil. Soils 31:150-156. [Google Scholar]
- 35.Mäder, P., A. Fliessbach, D. Dubois, L. Gunst, P. Fried, and U. Niggli. 2002. Soil fertility and biodiversity in organic farming. Science 296:1694-1697. [DOI] [PubMed] [Google Scholar]
- 36.Matson, P. A., W. J. Parton, A. G. Power, and M. J. Swift. 1997. Agricultural intensification and ecosystem properties. Science 277:504-509. [DOI] [PubMed] [Google Scholar]
- 37.Meharg, A. A., and J. W. G. Cairney. 2000. Co-evolution of mycorrhizal symbionts and their hosts to metal-contaminated environments. Adv. Ecol. Res. 30:69-112. [Google Scholar]
- 38.Menéndez, A. B., J. M. Scervino, and A. M. Godeas. 2001. Arbuscular mycorrhizal populations associated with natural and cultivated vegetation on a site of Buenos Aires province, Argentina. Biol. Fertil. Soils 33:373-381. [Google Scholar]
- 39.Miller, R. M., and J. D. Jastrow. 1992. The application of VA mycorrhizae to ecosystem restoration and reclamation, p. 438-467. In M. F. Allen (ed.), Mycorrhizal functioning. Chapman & Hall, Ltd., London, England.
- 40.Muthukumar, T., and K. Udaiyan. 2002. Growth and yield of cowpea as influenced by changes in arbuscular mycorrhiza in response to organic manuring. J. Agron. Crop Sci. 188:123-132. [Google Scholar]
- 41.O'Connor, P. J., S. E. Smith, and E. A. Smith. 2002. Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytol. 154:209-218. [Google Scholar]
- 42.Oehl, F., A. Oberson, M. Probst, A. Fliessbach, H. R. Roth, and E. Frossard. 2001. Kinetics of microbial phosphorus uptake in cultivated soils. Biol. Fertil. Soils 34:31-41. [Google Scholar]
- 43.Pimentel, D., C. Harvey, P. Resosudarmo, K. Sinclair, D. Kurz, M. McNair, S. Crist, L. Shpritz, L. Fitton, R. Saffouri, and R. Blair. 1995. Environmental and economic costs of soil erosion and conservation benefits. Science 267:1117-1123. [DOI] [PubMed] [Google Scholar]
- 44.Redecker, D. 2000. Specific PGR primers to identify arbuscular mycorrhizal fungi within colonized roots. Mycorrhiza 10:73-80. [Google Scholar]
- 45.Requena, N., E. Perez-Solis, C. Azcon-Aguilar, P. Jeffries, and J. M. Barea. 2001. Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl. Environ. Microbiol. 67:495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenck, N. C., and Y. Perez. 1990. Manual for the identification of VA mycorrhizal fungi, 3rd ed. Synergistic Publications, Gainesville, Fla.
- 47.Schüssler, A., D. Schwarzott, and C. Walker. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105:1413-1421. [Google Scholar]
- 48.Scullion, J., W. R. Eason, and E. P. Scott. 1998. The effectivity of arbuscular mycorrhizal fungi from high input conventional and organic grassland and grass-arable rotations. Plant Soil 204:243-254. [Google Scholar]
- 49.Sieverding, E. 1989. Ecology of VAM fungi in tropical agrosystems. Agric. Ecosyst. Environ. 29:369-390. [Google Scholar]
- 50.Smith, S. E., and D. J. Read. 1997. Mycorrhizal symbiosis, 2nd ed. Academic Press Ltd., London, England.
- 51.Tilman, D. 1999. Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. Proc. Natl. Acad. Sci. USA 96:5995-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tilman, D., K. G. Cassman, P. A. Matson, R. Naylor, and S. Polasky. 2002. Agricultural sustainability and intensive production practices. Nature 418:671-677. [DOI] [PubMed] [Google Scholar]
- 53.Vandenkoornhuyse, P., R. Husband, T. J. Daniell, I. J. Watson, J. M. Duck, A. H. Fitter, and J. P. W. Young. 2002. Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol. Ecol. 11:1555-1564. [DOI] [PubMed] [Google Scholar]
- 54.van der Heijden, M. G. A., T. Boller, A. Wiemken, and I. R. Sanders. 1998. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082-2091. [Google Scholar]
- 55.van der Heijden, M. G. A., J. N. Klironomos, M. Ursic, P. Moutoglis, R. Streitwolf-Engel, T. Boller, A. Wiemken, and I. R. Sanders. 1998. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69-72. [Google Scholar]
- 56.Vestberg, M. 1995. Occurrence of some Glomales in Finland. Mycorrhiza 5:329-336. [Google Scholar]
- 57.Wu, T. H., W. Y. Hao, X. G. Lin, and Y. Q. Shi. 2002. Screening of arbuscular mycorrhizal fungi for the revegetation of eroded red soils in subtropical China. Plant Soil 239:225-235. [Google Scholar]