Abstract
The antimicrobial susceptibilities of 378 Campylobacter isolates were determined. Resistance to tetracycline was the most common (82%), followed by resistance to doxycycline (77%), erythromycin (54%), nalidixic acid (41%), and ciprofloxacin (35%). Campylobacter coli displayed significantly higher rates of resistance to ciprofloxacin and erythromycin than Campylobacter jejuni, and Campylobacter isolates from turkey meat showed a greater resistance than those from chicken meat.
Campylobacter is a leading cause of human gastroenteritis worldwide, with an estimated 2.4 million cases each year in the United States (8). Campylobacter jejuni and Campylobacter coli are the species most commonly associated with human infections and cause clinically similar illnesses. When an antibiotic is recommended for treatment of patients with severe campylobacteriosis, the most frequently recommended drug is erythromycin or a fluoroquinolone such as ciprofloxacin (16). Tetracycline, doxycycline, and chloramphenicol are sometimes listed as alternative drugs for treatment. However, an increasing number of Campylobacter species that exhibit decreased susceptibility to several of these drugs have been isolated from food and water sources as well as from clinical samples in Europe (9, 14, 15, 18), Canada (5), and the United States (2, 17).
Campylobacter is considered mainly a food-borne pathogen, with raw or undercooked poultry serving as an important source of sporadic Campylobacter infection (1). The identification of antimicrobial-resistant Campylobacter organisms in food has raised concerns that the treatment of food-borne campylobacteriosis may be compromised, because antimicrobial-resistant Campylobacter strains cause more prolonged or more severe illness than do antimicrobial-susceptible strains (19). In this study, we examined the antimicrobial susceptibility of Campylobacter isolates from retail meats and determined the genetic basis of resistance to ciprofloxacin and erythromycin by studying the mutations in the gyrA and 23S rRNA genes, respectively.
Campylobacter was isolated from 159 (22%) of 719 retail meat samples from a previous study (21). To determine whether multiple Campylobacter strains were present, more than one colony per sample was selected, which resulted in a total of 378 isolates, including 196 C. jejuni isolates, 153 C. coli isolates, and 29 isolates from other Campylobacter species. The 378 Campylobacter isolates were examined for susceptibility to chloramphenicol, ciprofloxacin, doxycycline, erythromycin, gentamicin, nalidixic acid, and tetracycline by using the agar dilution method according to NCCLS recommendations (6, 10).
Approximately 94% of the 159 meat samples (149 samples) were contaminated with Campylobacter isolates that were resistant to at least one of the seven antimicrobials tested. Resistance to tetracycline was the most commonly found among 155 poultry isolates (82%), followed by resistance to doxycycline (77%), erythromycin (54%), nalidixic acid (41%), and ciprofloxacin (35%) (Table 1). Two C. coli isolates from two turkey samples showed resistance to chloramphenicol. None of the isolates was resistant to gentamicin.
TABLE 1.
Number of retail poultry meat samples containing C. jejuni, C. coli, and other Campylobacter species resistant to antimicrobial agents
| Antimicrobial agent | MIC breakpoint (μg/ml) | No. (%) of meat samples that contain resistant isolates of:
|
|||
|---|---|---|---|---|---|
| C. jejuni (n = 88) | C. coli (n = 75) | Other species (n = 12) | Totala (n = 155) | ||
| Chloramphenicol | 32 | 0 | 2 (3) | 0 | 2 (1) |
| Ciprofloxacin | 4 | 22 (25) | 30 (40) | 4 (33) | 55 (35) |
| Doxycycline | 16 | 66 (75) | 57 (76) | 7 (58) | 119 (77) |
| Erythromycin | 8 | 37 (42) | 46 (61) | 3 (25) | 84 (54) |
| Gentamicin | 16 | 0 | 0 | 0 | 0 |
| Nalidixic acid | 32 | 28 (32) | 32 (43) | 5 (42) | 64 (41) |
| Tetracycline | 16 | 71 (81) | 58 (77) | 9 (75) | 127 (82) |
Multiple species of Campylobacter were isolated from 15 poultry meat samples.
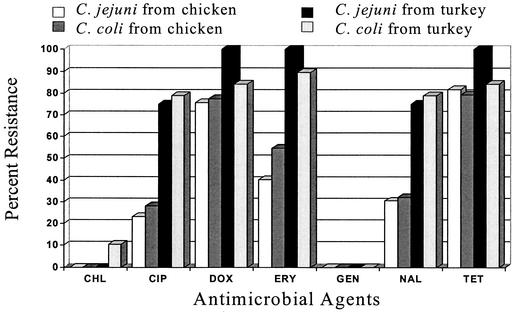
The antimicrobial resistance of Campylobacter isolates differed according to the species of the organism and the source of isolation. C. coli isolates displayed significantly higher rates of resistance (P < 0.05) to ciprofloxacin and erythromycin than did C. jejuni. Therefore, it is important to differentiate Campylobacter isolates at the species level in order to better monitor trends in antimicrobial resistance. Turkey isolates of both species showed significantly higher rates of resistance (P < 0.05) to ciprofloxacin and erythromycin than did chicken isolates (Fig. 1). This may be due to the longer raising period for turkeys (up to 18 weeks) than for chickens (7 weeks). In addition, because turkeys are economically more valuable than chickens, farmers are prone to giving turkeys antibiotics for disease treatment and prevention and for growth promotion (11). Multidrug-resistant Campylobacter isolates were common. Coresistance to ciprofloxacin and erythromycin was found in Campylobacter isolates from 41 (26%) meat samples. The ciprofloxacin MIC for all ciprofloxacin-resistant Campylobacter isolates was ≥16 μg/ml, and that for two of the isolates was 128 μg/ml.
FIG. 1.
Comparison of antimicrobial resistance profiles of C. jejuni and C. coli isolates from chicken (n = 125) and turkey (n = 23) meats. CHL, chloramphenicol; CIP, ciprofloxacin; DOX, doxycycline; ERY, erythromycin; GEN, gentamicin; NAL, nalidixic acid; TET, tetracycline.
Pulsed-field gel electrophoresis (PFGE) was used for subtyping Campylobacter isolates (12). Among the C. jejuni and C. coli isolates, 81 and 67 PFGE patterns were identified, respectively. Multiple Campylobacter species were present in 16 (10%) meat samples, whereas multiple strains with distinct PFGE patterns were recovered from 41 (26%) of the 159 meat samples. The PFGE and antimicrobial susceptibility profiles correlated well. Campylobacter isolates with identical PFGE patterns displayed the same or similar antimicrobial susceptibility profiles. Several of the Campylobacter isolates were found in chicken and turkey products from different chains or stores throughout the 12-month sampling period. Nineteen (68%) of the 28 meat samples that contained isolates of the same species with different PFGE patterns also showed different antibiograms.
For Campylobacter, the resistance to fluoroquinolones and macrolides appears to be due mostly to mutations in genes encoding DNA gyrase and 23S rRNA, respectively (4). DNA sequence analyses of the gyrA gene from 98 ciprofloxacin-resistant Campylobacter isolates and of the 23S rRNA gene from 44 erythromycin-resistant and 15 erythromycin-susceptible Campylobacter isolates were performed to detect mutations associated with resistance. The genes were amplified by using PCR as described in published reports (22, 23). Sequence analyses of the gyrA genes of the ciprofloxacin-resistant C. jejuni isolates identified 14 point mutations, 8 of which were silent mutations, compared to the sequence for ciprofloxacin-susceptible C. jejuni strain UA580 (GenBank accession no. L04566). In addition to the previously reported Thr-86-Ile mutation in 41 of the 42 isolates (13, 20), mutations at several other positions were identified (Table 2). All isolates showed a mutation from Asn at position 203, and 32 had an additional mutation from Ser at position 22. Interestingly, the one isolate lacking the Thr-86-Ile mutation contained novel double mutations, from Asn at position 203 and from Ala at position 206. Up to four mutations have been identified in some C. jejuni isolates. In C. coli, although six point mutations were observed in the gyrA gene of the 58 ciprofloxacin-resistant isolates relative to the sequence for the wild-type C. coli strain ATCC 33559 (accession no. AF092101), the mutation at position 86 was the only one that resulted in an amino acid sequence change (from Thr). However, one ciprofloxacin-resistant C. coli isolate lacked this mutation but possessed other silent substitutions. Among 44 erythromycin-resistant Campylobacter isolates analyzed, 13 of the 15 isolates for which the erythromycin MIC was ≥64 μg/ml had an A-2230-G mutation encoded in the 23S rRNA. The remaining isolates showed a spontaneous mutation at position 2268 (from T to C), which was also found in 15 Campylobacter isolates that were susceptible to erythromycin (MIC, ≤4 μg/ml) (Table 3). Other studies have demonstrated an efflux of fluoroquinolones, which also contributes to antimicrobial resistance in Campylobacter isolates (3, 7).
TABLE 2.
Point mutations observed for sequences from ciprofloxacin-resistant C. jejuni (n = 42) and C. coli (n = 56) isolates
| Species | Mutation(s) | Ciprofloxacin MIC (μg/ml) | No. of isolates |
|---|---|---|---|
| C. jejuni | Ser-22-Gly, Thr-86-Ile, Val-149-Ile, Asn-203-Ser | 16-64 | 22 |
| Ser-22-Gly, Thr-86-Ile, Asn-203-Ser, Ala-206-Val | 32-64 | 3 | |
| Ser-22-Gly, Thr-86-Ile, Asn-203-Ser | 16-64 | 7 | |
| Thr-86-Ile, Asn-203-Ser, Ala-206-Thr | 16 | 1 | |
| Thr-86-Ile, Asn-203-Ser | 64-128 | 8 | |
| Asn-203-Ser, Ala-206-Thr | 64 | 1 | |
| C. coli | Thr-86-Ile | ≥16 | 56 |
TABLE 3.
Point mutations observed for 23S rRNA gene sequences from 44 erythromycin-resistant and 15 erythromycin-susceptible Campylobacter isolates
| Mutation | Erythromycin MIC (μg/ml) | Species | No. of isolates tested | No. of mutated isolates |
|---|---|---|---|---|
| A-2230-G | >64 | C. coli | 14 | 12 |
| A-2230-G | 64 | C. coli | 1 | 1 |
| T-2268-C | 32 | C. coli | 3 | 2 |
| 16 | C. jejuni, C. coli | 11 | 4 | |
| 8 | C. jejuni, C. coli | 15 | 12 | |
| 1-4 | C. jejuni | 15 | 9 |
Since campylobacteriosis is transmitted primarily through food, particularly food of animal origin, the presence of antimicrobial-resistant Campylobacter in raw meat products has important public health implications. Our findings indicated that most Campylobacter isolates from retail poultry were resistant to at least one of the seven antimicrobials tested and that multidrug resistance was common. The coresistance to erythromycin and ciprofloxacin must be considered to be highly undesirable, as the two antimicrobials are generally advocated as first-line drugs for the treatment of campylobacteriosis. These findings suggest that cases of campylobacteriosis acquired from undercooked or mishandled retail meats may not respond to empirical therapy. Therefore, the use of in vitro susceptibility testing of Campylobacter may take on greater importance in ensuring rapid and appropriate management of patients with food-borne campylobacteriosis.
Acknowledgments
We are indebted to Robert Walker, from the Division of Animal and, Food Microbiology, Center for Veterinary Medicine, Food and Drug Administration, for his assistance and comments in the preparation of the manuscript.
This study was supported in part by grants from the Maryland Agricultural Experimental Station, the Joint Institute for Food Safety and Applied Nutrition of the University of Maryland and the Food and Drug Administration.
REFERENCES
- 1.Blaser, M. J. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect. Dis. 176(Suppl. 2):S103-S105. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2000. National Antimicrobial Resistance Monitoring System (NARMS)—1999 annual report. [Online.] http://www.cdc.gov/narms.
- 3.Charvalos, E., Y. Tselentis, M. M. Hamzehpour, T. Köhler, and J. C. Pechere. 1995. Evidence for an efflux pump in multidrug-resistant Campylobacter jejuni. Antimicrob. Agents Chemother. 39:2019-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudreau, C., and H. Gilbert. 1998. Antimicrobial resistance of clinical strains of Campylobacter jejuni subsp. jejuni isolated from 1985 to 1997 in Quebec, Canada. Antimicrob. Agents Chemother. 42:2106-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge, B., S. Bodeis, R. D. Walker, D. G. White, S. Zhao, P. F. McDermott, and J. Meng. 2002. Comparison of the Etest and agar dilution for in vitro antimicrobial susceptibility testing of Campylobacter. J. Antimicrob. Chemother. 50:487-494. [DOI] [PubMed] [Google Scholar]
- 7.Lin, J., L. O. Michel, and Q. Zhang. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 46:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore, J. E., M. Crowe, N. Heaney, and E. Crothers. 2001. Antibiotic resistance in Campylobacter spp. isolated from human faeces (1980-2000) and foods (1997-2000) in Northern Ireland: an update. J. Antimicrob. Chemother. 48:455-457. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard M31-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.National Research Council. 1999. The use of drugs in food animals: benefits and risks. National Academy Press, Washington, D.C. [PubMed]
- 12.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39: 1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz, J., P. Goni, F. Marco, F. Gallardo, B. Mirelis, T. Jimenez De Anta, and J. Vila. 1998. Increased resistance to quinolones in Campylobacter jejuni: a genetic analysis of gyrA gene mutations in quinolone-resistant clinical isolates. Microbiol. Immunol. 42:223-226. [DOI] [PubMed] [Google Scholar]
- 14.Sáenz, Y., M. Zarazaga, M. Lantero, M. J. Gastañares, F. Baquero, and C. Torres. 2000. Antibiotic resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997-1998. Antimicrob. Agents Chemother. 44:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjogren, E., G. B. Lindblom, and B. Kaijser. 1997. Norfloxacin resistance in Campylobacter jejuni and Campylobacter coli isolates from Swedish patients. J. Antimicrob. Chemother. 40:257-261. [DOI] [PubMed] [Google Scholar]
- 16.Skirrow, M. B., and M. J. Blaser. 2000. Clinical aspects of Campylobacter infection, p. 69-88. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 17.Smith, K. E., J. M. Besser, C. W. Hedberg, F. T. Leano, J. B. Bender, J. H. Wicklund, B. P. Johnson, K. A. Moore, M. T. Osterholm, et al. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N. Engl. J. Med. 340:1525-1532. [DOI] [PubMed] [Google Scholar]
- 18.Talsma, E., W. G. Goettsch, H. L. Nieste, P. M. Schrijnemakers, and M. J. Sprenger. 1999. Resistance in Campylobacter species: increased resistance to fluoroquinolones and seasonal variation. Clin. Infect. Dis. 29:845-848. [DOI] [PubMed] [Google Scholar]
- 19.Travers, K., and M. Barza. 2002. Morbidity of infections caused by antimicrobial-resistant bacteria. Clin. Infect. Dis. 34(Suppl. 3):S131-S134. [DOI] [PubMed] [Google Scholar]
- 20.Wang, Y., W. M. Huang, and D. E. Taylor. 1993. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob. Agents Chemother. 37:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao, C., B. Ge, J. De Villena, R. Sudler, E. Yeh, S. Zhao, D. G. White, D. Wagner, and J. Meng. 2001. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, D.C., area. Appl. Environ. Microbiol. 67:5431-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zirnstein, G., L. Helsel, Y. Li, B. Swaminathan, and J. Besser. 2000. Characterization of gyrA mutations associated with fluoroquinolone resistance in Campylobacter coli by DNA sequence analysis and MAMA PCR. FEMS Microbiol. Lett. 190:1-7. [DOI] [PubMed] [Google Scholar]
- 23.Zirnstein, G., Y. Li, B. Swaminathan, and F. Angulo. 1999. Ciprofloxacin resistance in Campylobacter jejuni isolates: detection of gyrA resistance mutations by mismatch amplification mutation assay PCR and DNA sequence analysis. J. Clin. Microbiol. 37:3276-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]