Abstract
The Arthrobacter sp. strain SU 4-chlorobenzoate (4-CBA) dehalogenation pathway converts 4-CBA to 4-hydroxybenzoate (4-HBA). The pathway operon contains the genes fcbA, fcbB, and fcbC (A. Schmitz, K. H. Gartemann, J. Fiedler, E. Grund, and R. Eichenlaub, Appl. Environ. Microbiol. 58:4068-4071, 1992). Genes fcbA and fcbB encode 4-CBA-coenzyme A (CoA) ligase and 4-CBA-CoA dehalogenase, respectively, whereas the function of fcbC is not known. We subcloned fcbC and expressed it in Escherichia coli, and we purified and characterized the FcbC protein. A substrate activity screen identified benzoyl-CoA thioesters as the most active substrates. Catalysis of 4-HBA-CoA hydrolysis to 4-HBA and CoA occurred with a kcat of 6.7 s−1 and a Km of 1.2 μM. The kcat pH rate profile for 4-HBA-CoA hydrolysis indicated optimal activity over a pH range of 6 to 10. The amino acid sequence of the FcbC protein was compared to other sequences contained in the protein sequence data banks. A large number of sequence homologues of unknown function were identified. On the other hand, the 4-HBA-CoA thioesterases isolated from 4-CBA-degrading Pseudomonas strains did not share significant sequence identity with the FcbC protein, indicating early divergence of the thioesterase-encoding genes.
During the last century, large quantities of industrially produced 4-chlorobenzoate (4-CBA) or 4-CBA progenitors (herbicides and polychlorinated biphenyl pesticides) (8, 14, 17, 19) have been released into the environment. A wide variety of 4-CBA-degrading bacterial strains, which are capable of growth on 4-CBA as the principal source of carbon, have been isolated from soil (11, 35). In these bacteria, 4-CBA is first converted to 4-hydroxybenzoate (4-HBA), which is then further metabolized via the ortho- or meta-cleavage pathway (11).
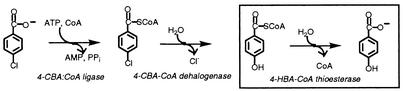
The 4-CBA dehalogenation pathway of the 4-CBA-degrading bacterium Pseudomonas sp. strain CBS3 (21) was the first to be characterized. The pathway consists of three reaction steps (28) (see Fig. 1) which are catalyzed by the enzymes 4-CBA-coenzyme A (CoA) ligase, 4-CBA-CoA dehalogenase, and 4-HBA-CoA thioesterase. Each of these enzymes has been isolated, and their kinetic properties have been defined (7). The three 4-CBA dehalogenation pathway genes are organized within an operon that is under the regulatory control of 4-CBA. In some 4-CBA-degrading bacteria, the gene cluster is located within the chromosomal DNA (6, 23, 26), whereas in others it is carried on a plasmid (22, 25).
FIG. 1.
Illustration of the reaction steps of the bacterial 4-CBA dehalogenation pathway (28).
The Arthrobacter sp. strain SU 4-CBA dehalogenation pathway operon was isolated on a 3.6-kb DNA fragment derived from a 120-kb plasmid (27). The DNA fragment was shown to contain six open reading frames, three of which (fcbA, fcbB, and fcbC) are transcribed in the same direction. Truncation mutants lacking fcbA or fcbB were unable to dehalogenate 4-CBA, whereas the truncation mutant devoid of fcbC was 10-fold less active. The fcbA gene encoded a 57-kDa protein that shares 38% sequence identity with the 57-kDa 4-CBA-CoA ligase of the Pseudomonas sp. strain CBS3 4-CBA dehalogenation pathway. The fcbB gene encoded a 30-kDa protein that shares 50% sequence identity with the 30-kDa 4-CBA-CoA dehalogenase of the Pseudomonas sp. strain CBS3 4-CBA dehalogenation pathway. The fcbA and fcbB genes were thus assumed to encode 4-CBA-CoA ligase and 4-CBA-CoA dehalogenase (27). The neighboring gene, fcbC, encoded a 16-kDa protein that did not share a significant level of sequence homology with the known 16-kDa Pseudomonas sp. strain CBS3 4-HBA-CoA thioesterase. The lack of sequence homology between the Pseudomonas thioesterase and the putative Arthrobacter thioesterase, as well as the different gene order observed in the Pseudomonas (dehalogenase-ligase-thioesterase) and Arthrobacter (ligase-dehalogenase-putative thioesterase) operons, made the assignment of the fcbC as the 4-HBA-CoA thioesterase gene uncertain. In the present study, the fcbC gene was subcloned, and its protein product was characterized.
MATERIALS AND METHODS
4-HBA-CoA thioesterase preparation.
The Arthrobacter sp. strain SU fcbC was amplified by PCR (13) by using the pAS5 clone (previously prepared from the native plasmid pASU1) (27) as a template and commercial oligonucleotides as primers. The PCR products were digested with the restriction enzymes NdeI and HindIII and then purified by agarose gel chromatography before T4 DNA ligase-catalyzed ligation to the NdeI- and HindIII-digested pET-23b vector (Novagen). The resulting clone was transformed into competent Escherichia coli BL21(DE3) cells for gene expression. The cloned gene was verified by DNA sequencing. A WT-Arthio/pET-23b transformant of E. coli BL21(DE3) was grown aerobically at 37°C in Luria-Bertani medium containing 50 μg of carbenicillin/ml. Protein production was induced with 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at ca. 10 h postinoculation (cell density had reached an optical density at 600 nm of 1.0). After a 5-h induction period, the cells were harvested by centrifugation (5,000 × g for 15 min) and then resuspended in 200 ml of buffer (50 mM K+HEPES [potassium salt of HEPES], 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride; pH 7.5) at 0°C. The cell suspension was passed through a French press at 1,200 lb/in2 twice before centrifugation at 48,000 × g and 4°C for 60 min. The supernatant was applied to a (5 by 40 cm) DEAE-Sepharose (Amersham Pharmacia Biotech) column and eluted at 4°C with a 2-liter gradient of 0 to 0.5 M KCl in 50 mM HEPES (pH 7.5). The chromatography was monitored by measuring the eluant absorbance (280 nm) and thioesterase activity (see below). The thioesterase-containing fractions (eluted at ∼0.2 M KCl) were combined, and then treated with ammonium sulfate. The 60 to 80% ammonium sulfate (wt/vol) protein precipitant was dissolved in 2 ml of 50 mM HEPES-0.2 M KCl (pH 7.5) buffer, applied to a 2-by-100-cm Sephacryl S-200 column (Amersham Pharmacia Biotech), and eluted at 4°C with the same buffer. The thioesterase-containing fractions were pooled and concentrated with a 10-kDa Macrosep Centricon (Pall Filtron). The yield of homogeneous thioesterase (based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] analysis) was 100 mg/g of wet cell.
Thioesterase molecular size determination.
The molecular mass was calculated from the amino acid composition, derived from the gene sequence, by using the EXPASY molecular biology server program Compute pI/Mw (2). The molecular mass was measured by electrospray mass spectrometry (mass spectrometry facility at the University of New Mexico) and by SDS-PAGE (a 4% stacking gel and a 16% separating gel). Commercial protein molecular weight standards were used to generate a plot of log of the molcular mass versus the distance traveled on the gel.
Synthesis of [14C]-labeled 4-HBA-CoA.
[14C]4-HBA-CoA (14C-labeled benzoyl C=O) was prepared by Pseudomonas sp. strain CBS3 4-CBA-CoA dehalogenase (7)-catalyzed dehalogenation of [14C]4-CBA-CoA (34). A typical reaction contained 46 μM [14C]4-CBA-CoA (specific activity = 56 μCi/μmol) and 1.5 μM dehalogenase in 100 μl of 50 mM K+HEPES (pH 7.5). After incubation for 3 h at room temperature, the reaction solution was filtered through a 3-kDa microfilter (Pall Gelman) to remove the enzyme from the [14C]4-HBA-CoA product.
Steady-state kinetic constant determination.
Owing to its small value, the 4-HBA-CoA Km was measured by using a fixed-time, radioisotope-based assay. [14C]4-HBA-CoA (56 μCi/μmol; 1.2 to 4.6 μM) was incubated with 1.8 nM thioesterase in 21 μl of 50 mM K+HEPES (pH 7.5) for a specified time period (typically 0.5 or 1 min) and then mixed with 10 μl of 0.3 M HCl to terminate the reaction within 20% conversion. Unlabeled 4-HBA-CoA and 4-HBA were added to the reaction solution prior to chromatography on a Beckman Ultrasphere analytical reversed-phase C18 column (4.6 by 250 mm; Rainin Dynamax HPLC System). A sequential, linear gradient (10 to 33.5% solvent B for 0 to 12 min and 33.5 to 70% solvent B for 12 to 14 min) was used to elute the column at a flow rate of 1 ml/min. Solvent A was 20 mM ammonium phosphate (adjusted to pH 6.7 with H3PO4), and solvent B was 16:9 (vol/vol) CH3CN-H2O and 20 mM ammonium phosphate (adjusted to pH 6.7 with H3PO4). The eluant was analyzed by measuring the absorbance at 260 nm and by liquid scintillation counting. The retention times of 4-HBA and 4-HBA-CoA were 3.3 and 11.1 min, respectively. The concentration of the product formed in the reaction was calculated from the ratio of the counts per minute (cpm) in the 4-HBA peak to the total cpm in the eluant and the concentration of [14C]4-HBA-CoA in the original reaction solution. The initial velocity was calculated from the concentration of product formed divided by the reaction time. The initial velocity data, measured as a function of substrate concentration, were analyzed by using the following equation and the computer program KinetAsyst (IntelliKinetics): V = Vmax [S]/([S] + Km), where V is the initial velocity, Vmax is the maximum velocity, [S] is the substrate concentration, and Km is the Michaelis constant. The kcat was calculated from Vmax/[E], where [E] is the total enzyme concentration (determined by the Bradford method [5]).
Other kcat and Km determinations were made by using a DTNB [5,5′-dithio-bis(2-nitrobenzoic acid)]-based continuous spectrophotometric assay. Hydrolysis reactions of the acyl-thioester substrates were monitored at 25°C by measuring the absorbance of 5-thio-2-nitrobenzoate at 412 nm (ɛ = 13.6 mM−1 cm−1), formed by reaction of DTNB with the product, CoA. Reaction mixtures (200 μl) contained thioesterase, acyl-CoA substrate (0.5 to 5 Km), DTNB (2 mM), KCl (0.2 M), and 50 mM K+HEPES (pH 7.5). The kinetic parameters of Vmax and Km were determined from initial velocity data, measured as a function of substrate concentration, by using the equation presented above and the computer program KinetAsyst. Inactivation of the thioesterase by the DTNB was tested and ruled out by carrying out enzyme-DTNB preincubation experiments.
pH rate profile analysis.
The initial velocities of the Arthrobacter sp. strain SU thioesterase-catalyzed hydrolysis of saturating 4-HBA-CoA (100 μM) were monitored at 25°C by measuring the decrease in solution absorbance at 300 nm resulting from the disappearance of reactant (i.e., Δɛ = 11.8 mM−1 cm−1.). A universal tribuffer system containing 50 mM acetate, 50 mM 2-(N-morpholino)ethanesulfonate, and 100 mM Tris (pH 4.6 to 9.6) (12) was used to measure the pH dependence of the catalyzed reaction. The kcat was calculated from the ratio of the experimental initial velocity (by assuming equivalence to maximum velocity) and the enzyme concentration of reaction mixture. The enzyme concentration was determined by using a Bradford assay (5).
RESULTS
Thioesterase purification and molecular size determination.
The Arthrobacter sp. strain SU thioesterase gene was overexpressed in E. coli BL21(DE3) cells transformed with the WT-Arthio/pET-23b clone. After induction and cell lysis, the thioesterase was purified by anion exchange and gel filtration column chromatography in a yield of 100 mg/g of wet cells. The purified protein was judged to be homogeneous based on SDS-PAGE analysis (see Fig. 2, estimated size of 17 kDa). The theoretical mass of 16,394.41 Da agrees closely with the actual mass measured by electrospray mass spectrometry of 16394.40 Da, thus showing that the N-terminal Met1 was not removed by posttranslational modification.
FIG. 2.
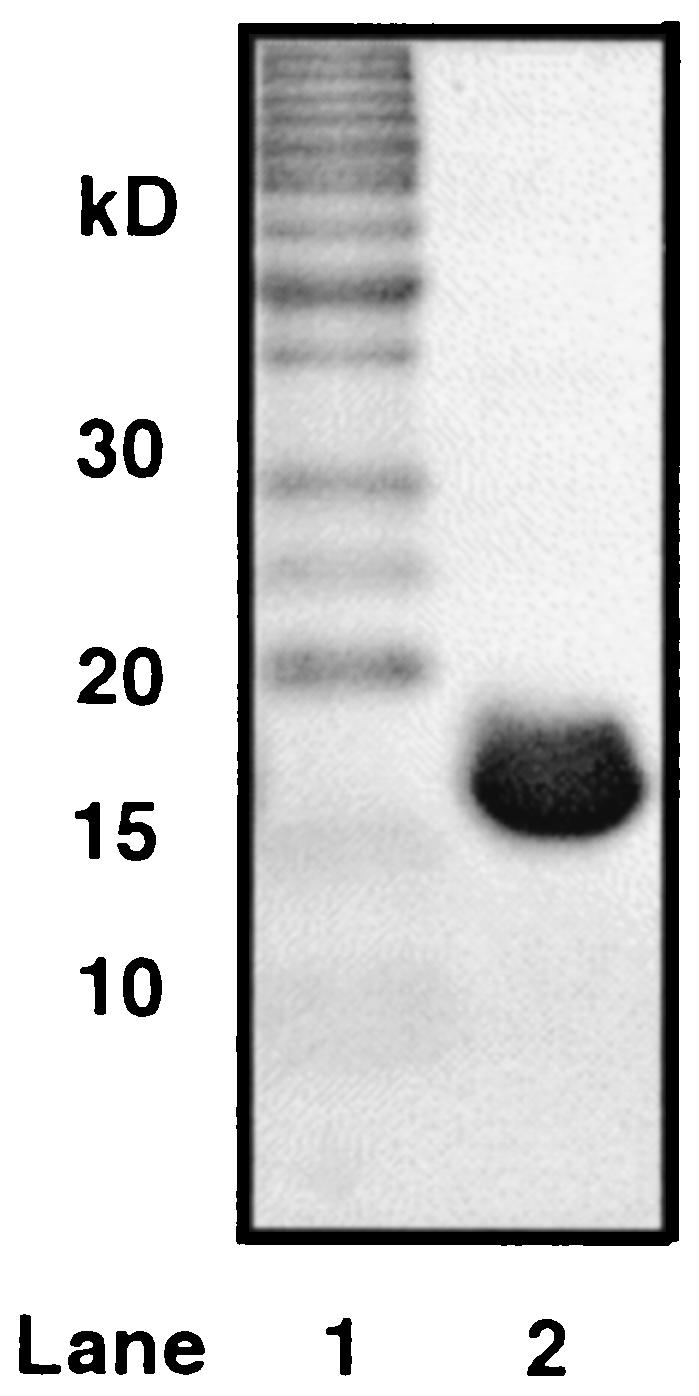
Coomassie blue-stained SDS-PAGE gel of purified Arthrobacter sp. strain SU 4-HBA-CoA thioesterase. Lane 1 shows the Invitrogen benchmark protein ladder. Lane 2 shows the chromatography of the purified Arthrobacter sp. strain SU 4-HBA-CoA thioesterase.
Thioesterase substrate specificity.
Common soil-dwelling bacteria such as members of the genus Arthrobacter degrade a variety of aromatic compounds, including phenylacetate, benzoate, and mono- and di-hydroxybenzoates (10, 15, 18, 33). The steady-state kinetic constants measured for the thioesterase-catalyzed hydrolysis of several aromatic acyl-CoA thioesters are listed in Table 1. The value of kcat/Km (or specificity constant) is a measure of substrate activity. The large value of the 4-HBA-CoA kcat/Km = 5.4 × 106 M−1 s−1 (which compares to the kcat/Km = 3.1 × 106 M−1 s−1 measured for the 4-HBA-CoA thioesterase of Pseudomonas sp. strain CBS3 [37]) indicates that 4-HBA-CoA is a natural substrate for the thioesterase. Although a kcat/Km value of 7.2 × 105 M−1 s−1 of 3-HBA-CoA was found to be ca. 10-fold lower, this substrate is still considered to be quite active. Interestingly, the kcat of 3-HBA-CoA was ∼3-fold larger than that of the 4-HBA-CoA. The greater reactivity might be explained by the resonance effect (i.e., reduced electron donation of the hydroxyl substituent from the meta position). The 2,5-dihydroxybenzoyl-CoA was, by comparison, a poor substrate. The presence of a hydroxyl group in the ortho position may cause steric hindrance to the nucleophilic attack occurring at the carbonyl carbon.
TABLE 1.
Steady-state kinetic constants for Arthrobacter sp. strain SU thioesterase-catalyzed hydrolysis of acyl-CoA thioesters at pH 7.5 and 25°C determined by using the DTNB spectrophotometric assaya
| Reactant | Mean kcat (s−1) ± SD | Mean Km (μM) ± SD | kcat/Km (M−1 s−1) |
|---|---|---|---|
| BA-CoA | 1.86 ± 0.06 | (2.43 ± 0.16) × 102 | 7.6 × 103 |
| 4-HBA-CoAb | 6.7 ± 0.1 | 1.24 ± 0.06 | 5.4 × 106 |
| 3-HBA-CoA | 15.8 ± 0.2 | (2.2 ± 0.1) × 101 | 7.2 × 105 |
| 2,5-diHBA-CoAd | (8.0 ± 0.1) × 10−2 | (2.9 ± 0.3) × 101 | 2.7 × 103 |
| 4-CBA-CoA | (2.5 ± 0.1) × 10−1 | (1.13 ± 0.08) × 102 | 2.2 × 103 |
| Phenylacetyl-CoA | (9.0 ± 0.2) × 10−4 | (1.4 ± 0.1) × 102 | 6.5 |
| Acetyl-CoA | (1.08 ± 0.02) × 10−2 | (2.2 ± 0.1) × 103 | 5.0 |
| Propionyl-CoA | (2.6 ± 0.1) × 10−2 | (1.1 ± 0.09) × 103 | 2.4 × 101 |
| Butyryl-CoA | (3.19 ± 0.05) × 10−2 | (9.3 ± 0.5) × 102 | 3.4 × 101 |
| Crotonyl-CoA | (7.5 ± 0.1) × 10−2 | (8.1 ± 0.3) × 102 | 9.2 × 101 |
| Octanoyl-CoA | 7.3 × 10−4 | NDc |
See Materials and Methods for details.
The kcat and Km values were obtained by using the radioisotope-based assay described in Materials and Methods. Similar values (within the error limit) were obtained by using the DTNB spectrophotometric assay.
ND, not determined. The micelle-forming properties of octanoyl-CoA prevented the determination of the Km.
diHBA, dihydroxybenzoate.
Benzoyl-CoA, which lacks a ring hydroxyl group, was almost 1,000-fold less reactive than 4-HBA-CoA. However, once bound to the enzyme, the rate of hydrolysis (kcat = 1.9 s−1) was only 3.6-fold lower than that of 4-HBA-CoA. Thus, the ring hydroxyl substituent appears to be an important factor in substrate binding, enhancing binding when located at the para position.
The phenylacetyl-CoA was by far the least reactive substrate among the aromatic acyl-CoA thioesters tested (kcat/Km = 6.5 M−1 s−1). The conjugation of the thioester moiety to the aromatic ring appears to be required for substrate recognition by the thioesterase.
Fatty acyl-CoA and short-chain acyl-CoA thioesters are produced in bacterial cells to perform a variety of roles in metabolism and membrane function. We tested the substrate activities of a variety of short- and medium-chain acyl-CoA thioesters. As indicated in Table 1, none of these thioesters showed substantial substrate activity. Thus, the Arthrobacter sp. strain SU thioesterase appears to be specific for the benzoyl-CoA core structure and most active with the benzoyl-CoA thioester that is hydroxylated at the para position. This substrate specificity profile is consistent with the primary role of the thioesterase in the metabolism of 4-CBA via the dehalogenation pathway of Fig. 1.
Lastly, the substrate activity of 4-CBA-CoA with the thioesterase was tested. In the bacterium, 4-CBA dehalogenation occurs via conversion of the 4-CBA carboxyl group to a thioester, followed by nucleophilic substitution of the ring chloride and regeneration of the ring carboxylate group by thioester hydrolysis (Fig. 1). The conversion of the 4-CBA to the 4-CBA-CoA thioester consumes a molecule of ATP (for 4-CBA-adenylate formation). Therefore, it is essential that thioesterase catalyzed hydrolysis of 4-CBA-CoA does not compete with the dehalogenation of 4-CBA-CoA to 4-HBA-CoA. Otherwise, a futile cycle of 4-CBA-CoA synthesis and hydrolysis would occur at the expense of cellular ATP. The kcat/Km = 2.2 × 103 M−1 s−1 for thioesterase-catalyzed 4-CBA-CoA hydrolysis (Table 1) was 10-fold smaller than the kcat/Km = 2.3 × 104 M−1 s−1 for dehalogenase-catalyzed 4-CBA-CoA dehalogenation measured under the same conditions (pH 7.5, 25°C) (Y. Wei and D. Dunaway-Mariano, unpublished data). This rate difference should prevent loss of the 4-CBA-CoA to thioesterase-catalyzed hydrolysis provided that the cellular dehalogenase concentration is as great or greater than the cellular thioesterase concentration.
Thioesterase pH optimum.
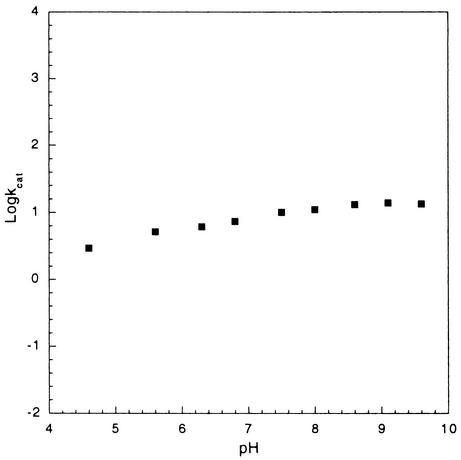
To determine the optimal pH range for thioesterase catalysis, the variation in the value of kcat values for 4-HBA-CoA hydrolysis was measured. As illustrated in Fig. 3, there was no substantial change in the rate of catalysis over a pH range from 4.6 to 9.6. This indicates that acid and base catalysis is not required for the catalyzed reaction or, alternatively, that the pKa values of the participating acid or base groups reside outside of the pH range examined. The C(4)OH of the 4-HBA-CoA in aqueous solution ionizes with a pKa of 8.6 (32). Thus, it might be expected that substrate ionization would impair catalysis at an alkaline pH. Since the kcat is not significantly reduced, it can be inferred that either the environment of the active site increases the pKa of the bound substrate or only the protonated form of the substrate binds to the enzyme. In future studies the pH profiles of the substrate kcat/Km and the Ki for inhibitor binding will be measured to learn more about the pH dependency of substrate binding.
FIG. 3.
pH profile of the kcat for the 4-HBA-CoA thioesterase from Arthrobacter sp. strain SU. See Materials and Methods for details.
4-HBA-CoA thioesterase sequence comparison.
The observation that two bacterial enzymes functioning in the same metabolic pathway and catalyzing the same reaction with equal efficiency share no significant sequence similarity is quite remarkable. A protein-threading study using the program 3D-PSSM (20) with Arthrobacter thioesterase as a query sequence identified the known Pseudomonas 4-HBA-CoA thioesterase fold (3) with 95% certainty. Thus, despite the absence of sequence homology, the two enzymes may share the same fold. The determination of the X-ray crystal structure of the Arthrobacter 4-HBA-CoA thioesterase, which is under way, should provide helpful insight into the structural divergence of these two enzymes.
The results from sequence homology searches carried out with the Pseudomonas and Arthrobacter thioesterase sequences further underscored their evolutionary distance. The NCBI PSI/PHI-BLAST searches (1), carried out through six iterations by using the Pseudomonas and Arthrobacter thioesterase sequences as queries, respectively, identified two unique groups of protein homologs (i.e., none of the homologs were common to both groups of sequences). Additionally, when the two groups of sequences (40 representative sequences from each) were combined and analyzed by using the GCG PILE-UP program, the Pseudomonas thioesterase-derived sequences separated into one group and the Arthrobacter thioesterase homologs separated into a second group. Based on these results, it was concluded that these two groups of sequences represent two distinct subfamilies within the hotdog-fold enzyme superfamily, to which the Pseudomonas sp. strain CBS3 4-HBA-CoA thioesterase has been assigned (3).
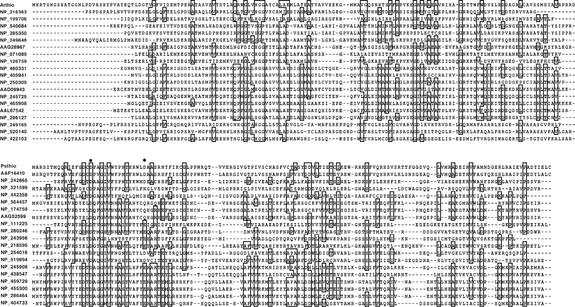
The first 20 sequences taken from each of the two sequence families are shown in Fig. 4. The active-site carboxylate residues Asp17 and Asp32 (30) of the Pseudomonas thioesterase are indicated by an asterisk. Asp17 functions in base or nucleophilic catalysis (30) and is stringently conserved among the subfamily members. Remarkably, there are no polar residues found in the Arthrobacter thioesterase, which are stringently conserved among its subfamily members.
FIG. 4.
Twenty representative sequences derived from the GCG PILE-UP of the sequence homologues to the Arthrobacter sp. strain SU 4-HBA-CoA thioesterase (Arthio) at the top and the Pseudomonas sp. strain CBS3 4-HBA-CoA thioesterase (Psthio) at the bottom. The protein sequences are labeled with their accession numbers (NCBI protein database).
DISCUSSION
The present work has demonstrated that the Arthrobacter sp. strain SU fcbC gene encodes 4-HBA-CoA thioesterase. Therefore, it is evident that the operons encoding 4-CBA dehalogenation in Pseudomonas sp. strain CBS3 and Arthrobacter sp. strain SU display different gene orders. Examination of the literature reveals that the ligase-dehalogenase-thioesterase arrangement observed in Arthrobacter sp. strain SU is also observed in Arthrobacter sp. strain TM1 (GenBank accession no. AF042490). Moreover, the dehalogenase-ligase-thioesterase gene order observed in the Pseudomonas sp. strain CBS3 is observed in the Pseudomonas sp. strain DJ-12 (6). However, in this latter strain, three transport protein genes are inserted between the ligase and thioesterase genes. The differences observed in pathway gene order in different host strains suggests that the 4-CBA pathway operon has been subjected to reorganization.
In addition to differences in the gene order, the proteins themselves have undergone sequence divergence. Whereas the respective 4-CBA dehalogenation pathway proteins of the two Arthrobacter strains are matched in sequence, those of the two Pseudomonas strains are not. Sequence comparisons between paired enzymes from the two Pseudomonas dehalogenation pathways show that the sequence identity between the ligases is 58%, that between the dehalogenases is 86%, and that between the thioesterases is only 65%. Even greater sequence divergence is evident from the Arthrobacter-Pseudomonas 4-CBA pathway protein sequence comparisons (50% identity for dehalogenases, 38% for ligases and ca. 10% for thioesterases).
The divergence in the 4-CBA pathway operon at the level of gene order and at the level of protein sequence indicates that the 4-CBA dehalogenation pathway genes are not the result of recent adaptation. Thus, it is unlikely that the evolution of the dehalogenation pathway was triggered by the release of 4-CBA and 4-polychlorinated biphenyls into the environment by industry as was originally thought (4). Enzymes, such as atrazine chlorohydrolase (29), known to have evolved in response to global pollution by a novel substrate do not deviate in amino acid sequence from one host bacterial strain to another. In addition, a recently evolved enzyme will display a close sequence match (>90% identity) with a protein (its progenitor) that performs a different catalytic function in a classical metabolic pathway (29). This is not the case with any one of the three 4-CBA pathway enzymes.
In view of the variety of halogenated aromatic compounds made by both prokaryotes and eukaryotes (9, 16, 31), it is suspected that natural sources of 4-CBA exist (24). The 4-CBA degradation pathway may have emerged in opportunistic bacteria once these sources became established, and the genes of the 4-CBA dehalogenation pathway may have thus had the opportunity to diverge through the process of random mutation and selection over a very long period of time. As with other operons that offer survival advantages to bacteria in particular niches, the 4-CBA dehalogenation pathway operon appears to have been passed from one strain to another (with some disruption). Indeed, evidence for horizontal operon transfer can be found in the transposon sequence (GenBank accession no. AF537222) that flanks the 4-CBA dehalogenation pathway operon (36) in Alcaligenes sp. strain AL3007 (22).
Acknowledgments
This work was supported in part by NIH grant GM28688 to D.D.-M.
We thank Lawrence Kelley in the Biomolecular Modelling Lab at Imperial College of Science of the United Kingdom for assistance with the 3D-PSSM threading analysis.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel, R. D., A. Bairoch, and D. F. Hochstrasser. 1994. A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server. Trends Biochem. Sci. 19:258-260. [DOI] [PubMed] [Google Scholar]
- 3.Benning, M. M., G. Wesenberg, R. Liu, K. L. Taylor, D. Dunaway-Mariano, and H. M. Holden. 1998. The three-dimensional structure of 4-hydroxybenzoyl-CoA thioesterase from Pseudomonas sp. strain CBS-3. J. Biol. Chem. 273:33572-33579. [DOI] [PubMed] [Google Scholar]
- 4.Benning, M. M., K. L. Taylor, R. Q. Liu, G. Yang, H. Xiang, G. Wesenberg, D. Dunaway-Mariano, and H. M. Holden. 1996. Structure of 4-chlorobenzoyl coenzyme A dehalogenase determined to 1.8 Å resolution: an enzyme catalyst generated via adaptive mutation. Biochemistry 35:8103-8109. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chae, J. C., Y. Kim, Y. C. Kim, G. J. Zylstra, and C. K. Kim. 2000. Genetic structure and functional implication of the fcb gene cluster for hydrolytic dechlorination of 4-chlorobenzoate from Pseudomonas sp. DJ-12. Gene 258:109-116. [DOI] [PubMed] [Google Scholar]
- 7.Chang, K. H., P. H. Liang, W. Beck, J. D. Scholten, and D. Dunaway-Mariano. 1992. Isolation and characterization of the three polypeptide components of 4-chlorobenzoate dehalogenase from Pseudomonas sp. strain CBS-3. Biochemistry 31:5605-5610. [DOI] [PubMed] [Google Scholar]
- 8.Cork, D. J., and J. P. Krueger. 1991. Microbial transformations of herbicides and pesticides. Adv. Appl. Microbiol. 36:1-66. [DOI] [PubMed] [Google Scholar]
- 9.de Jong, E., and J. A. Field. 1997. Sulfur tuft and turkey tail: biosynthesis and biodegradation of organohalogens by Basidiomycetes. Annu. Rev. Microbiol. 51:375-414. [DOI] [PubMed] [Google Scholar]
- 10.Diaz, E., A. Ferrandez, M. A. Prieto, and J. L. Garcia. 2001. Biodegradation of aromatic compounds by Escherichia coli. Microbiol. Mol. Biol. Rev. 65:523-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunaway-Mariano, D., and P. C. Babbitt. 1994. On the origins and functions of the enzymes of the 4-chlorobenzoate to 4-hydroxybenzoate converting pathway. Biodegradation 5:259-276. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, K. J., and J. F. Morrison. 1982. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol. 87:405-426. [DOI] [PubMed] [Google Scholar]
- 13.Erlich, H. A. 1992. PCR technology principles and applications for DNA amplification. W. H. Freeman and Co., New York, N.Y.
- 14.Furukawa, K. 1994. Molecular genetics and evolutionary relationship of PCB-degrading bacteria. Biodegradation 5:289-300. [DOI] [PubMed] [Google Scholar]
- 15.Gerischer, U. 2002. Specific and global regulation of genes associated with the degradation of aromatic compounds in bacteria. J. Mol. Microbiol. Biotechnol. 4:111-121. [PubMed] [Google Scholar]
- 16.Gribble, G. W. 1996. Naturally occurring organohalogen compounds: a comprehensive survey. Prog. Org. Nat. Prod. 68:1-498. [PubMed] [Google Scholar]
- 17.Haggblom, M. M. 1992. Microbial breakdown of halogenated aromatic pesticides and related compounds. FEMS Microbiol. Rev. 9:29-71. [DOI] [PubMed] [Google Scholar]
- 18.Harwood, C. S., G. Burchhardt, H. Herrmann, and G. Fuchs. 1999. Anaerobic metabolism of aromatic coumpounds via the benzoyl-CoA pathway. FEMS Microbiol. Rev. 22:439-458. [Google Scholar]
- 19.Higson, F. K. 1992. Microbial degradation of biphenyl and its derivatives. Adv. Appl. Microbiol. 37:135-164. [DOI] [PubMed] [Google Scholar]
- 20.Kelley, L. A., R. M. MacCallum, and M. J. Sternberg. 2000. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J. Mol. Biol. 299:499-520. [DOI] [PubMed] [Google Scholar]
- 21.Klages, U., and F. Lingens. 1980. Degradation of 4-chlorobenzoic acid by Pseudomonas sp. Zentbl. Bakteriol. Parasitenkd. Infectionskr. Hyg. Abt. 1 Orig. C 1:215-223. [Google Scholar]
- 22.Layton, A. C., J. Sanseverino, W. Wallace, C. Corcoran, and G. S. Sayler. 1992. Evidence for 4-chlorobenzoic acid dehalogenation mediated by plasmids related to pSS50. Appl. Environ. Microbiol. 58:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks, T. S., R. Wait, A. R. Smith, and A. V. Quirk. 1984. The origin of the oxygen incorporated during the dehalogenation/hydroxylation of 4-chlorobenzoate by an Arthrobacter sp. Biochem. Biophys. Res. Commun. 124:669-674. [DOI] [PubMed] [Google Scholar]
- 24.Niedan, V., and H. F. Scholer. 1997. Natural formation of chlorobenzoic acids (CBA) and distinction between PCB-degraded CBA. Chemosphere 35:1233-1241. [Google Scholar]
- 25.Ruisinger, S., U. Klages, and F. Lingens. 1976. Degradation of 4-chlorobenzoic acid by an Arthrobacter species. Arch. Microbiol. 110:253-256. (In German.) [DOI] [PubMed]
- 26.Savard, P., L. Peloquin, and M. Sylvestre. 1986. Cloning of Pseudomonas sp. strain CBS3 genes specifying dehalogenation of 4-chlorobenzoate. J. Bacteriol. 168:81-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz, A., K. H. Gartemann, J. Fiedler, E. Grund, and R. Eichenlaub. 1992. Cloning and sequence analysis of genes for dehalogenation of 4-chlorobenzoate from Arthrobacter sp. strain SU. Appl. Environ. Microbiol. 58:4068-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholten, J. D., K. H. Chang, P. C. Babbitt, H. Charest, M. Sylvestre, and D. Dunaway-Mariano. 1991. Novel enzymic hydrolytic dehalogenation of a chlorinated aromatic. Science 253:182-185. [DOI] [PubMed] [Google Scholar]
- 29.Seffernick, J. L., and L. P. Wackett. 2001. Rapid evolution of bacterial catabolic enzymes: a case study with atrazine chlorohydrolase. Biochemistry 40:12747-12753. [DOI] [PubMed] [Google Scholar]
- 30.Thoden, J. B., H. M. Holden, Z. Zhuang, and D. Dunaway-Mariano. 2002. X-ray crystallographic analyses of inhibitor and substrate complexes of wild-type and mutant 4-hydroxybenzoyl-CoA thioesterase. J. Biol. Chem. 277:27468-27476. [DOI] [PubMed] [Google Scholar]
- 31.van Pee, K. H. 1996. Biosynthesis of halogenated metabolites by bacteria. Annu. Rev. Microbiol. 50:375-399. [DOI] [PubMed] [Google Scholar]
- 32.Webster, L. T., Jr., J. J. Mieyal, and U. A. Siddiqui. 1974. Benzoyl and hydroxybenzoyl esters of coenzyme A: ultraviolet characterization and reaction mechanisms. J. Biol. Chem. 249:2641-2645. [PubMed] [Google Scholar]
- 33.Williams, P. A., and J. R. Sayers. 1994. The evolution of pathways for aromatic hydrocarbon oxidation in Pseudomonas. Biodegradation 5:195-217. [DOI] [PubMed] [Google Scholar]
- 34.Yang, G., P. H. Liang, and D. Dunaway-Mariano. 1994. Evidence for nucleophilic catalysis in the aromatic substitution reaction catalyzed by (4-chlorobenzoyl)coenzyme A dehalogenase. Biochemistry 33:8527-8531. [DOI] [PubMed] [Google Scholar]
- 35.Yi, H., K. Min, C. Kim, and J. Ka. 2000. Phylogenetic and phenotypic diversity of 4-chlorobenzoate-degrading bacteria isolated from soils. FEMS Microbiol. Ecol. 31:53-60. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, W. 2000. Ph.D. thesis. University of Maryland, College Park.
- 37.Zhuang, Z., F. Song, W. Zhang, K. Taylor, A. Archambault, D. Dunaway-Mariano, J. Dong, and P. R. Carey. 2002. Kinetic, Raman, NMR, and site-directed mutagenesis studies of the Pseudomonas sp. strain CBS3 4-hydroxybenzoyl-CoA thioesterase active site. Biochemistry 41:11152-11160. [DOI] [PubMed] [Google Scholar]