Abstract
Accurate laboratory tests for the diagnosis of active human herpesvirus 8 (HHV-8) infection are becoming essential to study the pathogenesis of HHV-8-associated tumors and for the clinical management of HHV-8-infected individuals. We have developed a highly sensitive, calibrated quantitative real-time PCR assay for the measurement of cell-free HHV-8 DNA in body fluids, based on the addition of a synthetic DNA calibrator prior to DNA extraction. The calibrator controls each sample for the presence of PCR inhibitors, determines a cutoff value of sensitivity for negative samples, and normalizes positive samples for the efficiency of DNA recovery. The assay shows a wide dynamic range of detection (between 1 and 106 viral genome equivalents/reaction) and a high degree of accuracy even in the presence of high amounts (up to 1 μg) of human genomic DNA. Moreover, the assay has a very high sensitivity (lower detection limit, 10 genome equivalents/ml) and a high degree of reproducibility and repeatability with a coefficient of variation (CV) of <15 and 23%, respectively. Furthermore, the use of the calibrator improves the accuracy of quantitation and decreases the intersample variability (CV, 9 and 6%, respectively). The sensitivity and specificity of the assay were tested with a series of clinical specimens obtained from patients affected by various HHV-8-related diseases, as well as from a wide number of controls. In conclusion, our calibrated real-time PCR assay provides a reliable high-throughput method for quantitation of HHV-8 DNA in clinical and laboratory specimens.
Human herpesvirus 8 (HHV-8) is a recently characterized gamma herpesvirus associated with the development of Kaposi's sarcoma (KS), body-cavity-based-lymphoma (BCL), and some forms of multicentric Castleman's disease (MCD) (6, 14, 40). This virus is distributed worldwide with five distinct subtypes (A to E); yet, unlike other human herpesviruses, HHV-8 is not ubiquitous. Sero-epidemiological studies demonstrated a low prevalence of anti-HHV-8 antibodies in the general population in the United States and Asia (1 to 2%), an intermediate seroprevalence in Italy and other Mediterranean countries (5 to 35%), and the highest seroprevalence in Southern and Central Africa (30 to 60%) and among Brazilian Amerindians (41 to 65%) (1, 4, 8, 19, 31, 35, 38, 39, 46). HHV-8 seroconversion is associated, in some instances, with KS development (9). In addition, HHV-8 seropositivity can predict progression to KS in human immunodeficiency virus (HIV)-seropositive homosexual men (13, 26, 33).
A variety of serological assays for diagnosis of HHV-8 infection have been described, but most of them fall short in sensitivity, specificity, and interassay concordance. Indeed, a combination of several assays should be utilized in order to obtain the optimal sensitivity and specificity (7, 35, 41). Moreover, the assessment of titers of antibody in serum may not always provide useful clinical information, especially for the therapeutic management of patients, since it does not allow distinguishing between latent and active infection. The measurement of the viral load has been crucial to understanding the pathogenic mechanisms of many viruses (10, 25, 44), and, especially when applied to biological fluids, it represents one of the best systems for diagnosing active infection, an essential marker for the study of herpesviruses (22). However, the first-generation PCR assays for the detection of the HHV-8 DNA load are generally too insensitive to provide a reliable screening test (18, 26, 34). Moreover, they were not designed to be high-throughput systems (21) and are susceptible to PCR cross-contamination (16).
At present, several real-time PCR methods are available for quantification of HHV-8 DNA (17, 42, 43, 47), but none allows controlling both the efficiency of the extraction procedure and the presence of PCR inhibitors, both of which affect quantification. Indeed, the main limitation of this technology is represented by the inability to recognize technical artifacts due to a complete failure of the PCR. We approached this problem by introducing, in our assay, a synthetic DNA molecule termed calibrator, which shows the same kinetics of amplification of the HHV-8 amplicon and is specifically detected by a non-cross-reactive probe. The loss of amplification of the calibrator molecule allows the detection of any false-negative results. Moreover, the addition of a known amount of calibrator molecule before any manipulation of the biological sample permits measuring the yield of DNA recovery for each sample, which varies significantly from sample to sample and according to the source of the clinical specimens.
Here, we describe the development of a calibrated real-time PCR assay for the reliable estimation of both cell-free and cell-associated HHV-8 DNA in body fluids and evaluate its performance with clinical specimens obtained from patients of Italian and African origin with HHV-8-associated diseases.
MATERIALS AND METHODS
Oligonucleotide primers and TaqMan probes.
Primers TAQ8A (5′-GTCCAGACGATATGTGCGC-3′) and TAQ8B (5′-ACTCCAAAATA TCGGCCGG-3′), which amplify a 101-bp fragment of the minor capsid protein (from nucleotides 8221 to 8321; GenBank accession no. U40377) and a probe of 23 bp (5′-TTGGTGGTATATAGATCAAGTTC-3′), complementary to an internal region 15 bp downstream of the forward primer, were selected using the Primer Express software (PE Biosystem, Foster City, Calif.). The primers were synthesized by Primm (Milan, Italy), whereas a 3′-minor groove binder DNA probe synthesized with the reporter dye 6-carboxy-fluorescein covalently linked to the 5′ end of the sequence was prepared by PE Biosystems (Warrington, United Kingdom). An extensive search in the EMBL and GenBank databases indicated that neither the primers nor the probe shared significant homology with other known nucleotide sequences. Moreover, the region is highly conserved among different HHV-8 subtypes.
Primers TAQCA (5′-CAAAGCCAAATTATCCAGAGCG-3′) and TAQCB (5′CGCTGG TTGAGGATGATCGA-3′) were utilized for real-time quantitative PCR of the calibrator molecule. A 25-bp oligonucleotide probe (5′-TAC GCAACGCCAACAGACCTAGCGA-3′) was synthesized (PE Biosystems) with a reporter dye, VIC, and the TAMRA quencher dye (6-carboxytetramethyl rhodamine) covalently linked to the 5′ and 3′ ends, respectively, of the oligonucleotide. The calibrator probe and primers were specifically and carefully selected in order to avoid cross-hybridization with the HHV-8 sequence. The lack of detection of the VIC signal in an amplified specimen was interpreted as an index of either absence of DNA recovery or presence of PCR inhibitors.
Preparation of HHV-8 and calibrator standards.
DNA extracted from a cell line chronically infected with HHV-8 (BCBL-1) was amplified with a PCR mixture containing 100 mM deoxynucleotides, 3 mM magnesium chloride, 1× TaqMan buffer A, primers TaqMan 8A and TaqMan 8B (each at 300 nM), 0.625 U of AmpliTaq Gold, and 10 μl of DNA template. The cycling profile consisted of a first denaturation step of 15 min at 95°C and a second step of 26 cycles of denaturation for 20 s at 95°C, followed by annealing for 30 s at 60°C, extension for 30 s at 72°C, and a final extension step of 10 min at 72°C.
The 101-bp HHV-8 DNA fragment amplified by primers TAQ8A-TAQ8B was cloned into the pCR2.1 plasmid (pVU56) using the TA cloning kit (Invitrogen Corp., San Diego, Calif.) according to the manufacturer's instructions. The 133-bp DNA calibrator fragment was synthesized by PE Biosystems and cloned into the pCRII plasmid (pVU46) to provide a reproducible source of calibrator molecule.
PCR assays.
Optimization of the reactions was achieved by determining the concentrations of magnesium, primers, and probes, as well as the annealing temperature yielding the highest intensity of reporter fluorescent signal without a reduction in specificity or sensitivity. The TaqMan reaction for both assays was performed in a final volume of 25 μl containing 100 mM dATP, dCTP, and dGTP, 200 mM dUTP, 5.5 mM magnesium chloride, 1× TaqMan buffer A, primers at 300 nM, probe at 200 nM, 0.625 U of AmpliTaq Gold, 0.25 U of uracil-N-glycosylase, and 10 μl of DNA template. The TaqMan PCR cycling conditions were the following: 2 min at 50°C, followed by 15 min of denaturation at 95°C and by 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 60 s. The fluorescent signal (Rn) generated by the degradation of the hybridized probe is automatically calculated by a computer algorithm that normalizes the reporter emission signal first, by dividing it by the emission of a control dye (ROX) present in the PCR mix and then by subtracting all the background signals generated in the first 15 cycles of the PCR. Then, the algorithm calculates the cycle of threshold (Ct) at which each PCR amplification reaches a threshold value (usually set at 10 times the standard deviation of the baseline signal) that is inversely proportional to the log number of target copies present in the sample (12). A standard curve was drawn using serial dilutions of known input target copies (x axis) versus the corresponding Ct values (y axis) using the least-squares fit method.
Serologic assays.
Antibodies against the lytic antigens of HHV8 were detected using an immunofluorescence assay (IFA) based on the BCBL-1 cell line (obtained from M. McGrath and D. Ganem through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health). The cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FCS), antibiotics (100 IU of penicillin/ml and 100 μg of streptomycin/ml), and 5 × 10−5 M 2-mercaptoethanol. For the IFA assay, smears were prepared by pelleting BCBL-1 cells pretreated with 20 ng of tetradecanoyl phorbol ester acetate (Sigma)/ml for 48 h. Ten microliters of a 4 × 106-cell/ml cell suspension were smeared on slides, air dried at room temperature, and fixed with a methanol-acetone solution (1:1 [vol/vol]) at −20°C for 10 min. Fixed smears were preblocked with phosphate-buffered saline supplemented with 3% (vol/vol) FCS for 30 min in a humidified chamber, incubated for 45 min at 37°C with the test serum diluted 1:10 (in phosphate-buffered saline supplemented with 1% glycine-2% FCS) and for 45 min with a fluorescein-isothiocyanate-conjugated goat anti-human antiserum. Titrations were done by twofold serial dilutions. Samples were considered positive when they showed at least two fluorescent cells for each microscopic field at 1:40 dilution.
Patients and sample preparation.
Fifty-eight clinical specimens (plasma, serum, and pleural fluid samples) obtained from 24 patients of different geographic origins (Italy and Uganda) with a broad spectrum of HHV-8-associated diseases were analyzed. In addition, the HHV-8 viral load was measured in plasma samples collected from 36 healthy donors, 64 HIV-seropositive subjects, and 16 patients affected by malignant lymphoma or chronic inflammatory diseases.
To remove cells and debris, the clinical specimens (0.2 to 1 ml) were centrifuged twice (first at 900 × g, 15 min, 4°C; and then at 1,500 × g, 15 min, 4°C). Precleared samples were then subjected to high-speed centrifugation (26,000 × g, 120 min, 4°C) in order to concentrate viral particles. Synthetic calibrator DNA (104 copies of calibrator/sample) was added prior to DNA extraction to control the efficiency of each step of the analytical procedure. DNA extraction from spun material was performed using a phenol-chloroform method, and purified material was resuspended in a final volume of 100 μl of AE buffer, as described previously (20). Ten microliters of the purified material was tested in each PCR in triplicate to measure both the calibrator and the HHV-8 copy number. Data were normalized based on the recovery rate of the calibrator DNA using the following formula:
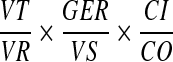 |
where VT indicates the total volume of the extracted material, VR indicates the volume assayed in the PCR, GER indicates the number of HHV-8 genome equivalents measured in each reaction, VS indicates the volume of the analytical sample expressed in milliliters, CI indicates the calibrator input, and CO indicates the calibrator output. On negative samples, the cutoff of sensitivity was calculated as follow:
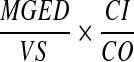 |
where MGED indicates the minimal number of genome equivalent detectable, Vs indicates the volume of the biological sample express in milliliters, CI indicates the calibrator input, and CO indicates the calibrator output.
Statistical analysis.
Accuracy, defined as the level of approximation of a measured value to a reference value taken as a “gold standard,” was estimated by computing the arithmetic differences between DNA copy number evaluated by the real-time PCR assay and the theoretical number inferred from UV spectroscopy. The significance of systematic biases was assessed by a paired-sample Student t test and was adjusted by covariance analysis, when needed. Repeatability and reproducibility were estimated by computing the coefficient of variation (CV) (the ratio between the standard deviation and the mean of repeated measurements) under different conditions. The difference between reference curves was assessed by covariance analysis.
RESULTS
Validation of reference curves, dynamic range, and analytical sensitivity of the TaqMan quantitative PCR assay for HHV-8 DNA.
The aim of this study was to design a calibrated quantitative PCR-based assay suitable for quantification of the HHV-8 DNA load in biological samples. In this regard, two independent quantitative PCR methods were optimized and carried out with two separate aliquots of the same DNA specimen. The first real-time PCR was designed to measure the HHV-8 genome equivalent number, and the second one was developed to measure the recovery of the calibrator DNA molecule, which serves to normalize the DNA extraction procedure and to monitor PCR artifacts. To generate the reference curve for HHV-8 quantitation, pVU58 plasmid DNA was measured by UV spectroscopy, and three distinct sets of 10-fold dilutions were prepared, ranging from a DNA concentration of 0.405 μg/μl (equivalent to 1011 copies/μl) to a DNA concentration of 4 × 10−4 fg/μl (equivalent to 10−1 copies per μl). The three series of samples were amplified by PCR in the same run of the 7700 ABI Prism sequence detector. The data generated a log-linear regression plot that showed a strong linear relationship (r2 > 0.99) between the log10 of the starting copy number and the Ct values (Fig. 1). A very wide dynamic range was observed (six orders of magnitude), since the assay could discriminate template concentrations between 100 and 106 genome equivalents in a single reaction. To better mimic the situation in which DNA is extracted from cells, we performed some experiments by adding an excess of human HHV-8-negative genomic DNA (1 μg of peripheral blood mononuclear cells DNA = 150,000 cellular genome equivalents) to a wide range of HHV-8 template DNA (101 to 106 copy equivalent of DNA). As shown in Fig. 1, the sensitivity and the performance of the assay were not affected by the presence of 1 μg of human genomic DNA.
FIG. 1.
Reference curves of the HHV-8 TaqMan assay obtained in the presence and absence of irrelevant human genomic DNA. Serial dilutions (from 101 to 106 HHV-8 genome equivalents) of the HHV-8 template run in the presence (closed circles) or absence (open triangle) of 1 μg of human genomic DNA derived from normal blood donor peripheral blood mononuclear cells. The equations resulting from the regression curves obtained by plotting the Ct values (y axis) against the indicated amount of DNA inputs (x axis) were the following: y = 39.226 − 3.3347 log (x), r2 = 0.9978; and y = 39.667 − 3.4 log (x), r2 = 0.9986 for the curve of the pVU56 construct diluted in water or in 1 μg of human DNA, respectively.
A similar procedure was followed to validate the reference curve using the calibrator construct pVU56. Again, we observed a wide range of detection and a strong linear relationship between the log of the copy number and the PCR cycle of detection of the fluorescent signal (F = 32,043; P < 0.0001) (Fig. 2). In addition, the kinetics profile of calibrator amplification was compared, by covariance analysis, with the one generated with the HHV-8 amplicon (Fig. 2). No significant differences were found between the plots generated for the two constructs (F = 1.32 [P = 0.25] and 1.37 [P = 0.24] for the intercept and the slope of the plots, respectively).
FIG. 2.
Comparison between the reference curves of the HHV-8 and calibrator real-time PCR assays. The number of the pVU56 (HHV-8) and pVU46 (calibrator) molecules is indicated on the x axis, and the cycle of threshold (Ct) is indicated on the y axis. Open triangle, TaqMan assay for HHV-8; closed circles, TaqMan assay for calibrator. The equations for the HHV-8 and the calibrator constructs were y = 39.052 − 3.333 log (x) and y = 39.084 − 3.340 log (x), respectively. The solid lines were obtained by linear regression analysis of the data (r2 > 0.99; P < 0.001), and dotted lines indicate the 95% confidence intervals for the regression.
Accuracy, repeatability, and reproducibility.
Next, we measured the accuracy of the TaqMan assay for HHV-8 DNA. For DNA inputs above 104 copies/reaction the error observed in 10 distinct experiments performed with serial dilutions of the pVU58 reference plasmid was minimal (1.8 to 5.7%), whereas it was greater (12.2 to 14.3%) for inputs below 104 copies/reaction (Table 1).
TABLE 1.
Accuracy error and CV of standard plasmid pVU58 quantification
| Copy no. | Accuracy error (%), mean ± SE | CV (%) |
|||
|---|---|---|---|---|---|
| Intra-assay |
Inter-assay |
||||
| Ct | Copies | Ct | Copies | ||
| 101 | −14.3 ± 4.7 | 1.21 | 29.4 | 1.05 | 44.4 |
| 102 | −13.1 ± 2.89 | 0.5 | 14.7 | 1.08 | 20.6 |
| 103 | −16.2 ± 2.39 | 0.72 | 7.1 | 0.9 | 17.6 |
| 104 | −12.2 ± 2.71 | 0.68 | 6.1 | 1.09 | 22.8 |
| 105 | 1.8 ± 2.82 | 0.57 | 7.3 | 1.17 | 20 |
| 106 | 5.7 ± 1.46 | 0.31 | 4.8 | 0.83 | 9.2 |
The repeatability and reproducibility of our assay were assessed by calculating the CV for the Ct values experimentally determined as well as for the estimated plasmid copy numbers (Table 1). Intra- and interexperimental variability calculated for the Ct values of 170 measurements of the reference DNA was consistently below 1.2% (101 to 106), thus demonstrating the correctness of the technical setup and its reliability over time (Table 1). Intra- and interexperimental variability calculated on the basis of the copy numbers was always below 25% (15 and 23%, respectively) except for inputs equal to 10 genome equivalents/reaction (29 and 44%, respectively)
To further verify the validity of the calibrated quantification approach, two sets of six independent 1-ml aliquots of human plasma were spiked with different inputs of HHV-8 viral stock dilutions (800 and 300,000 copies/sample) and with 104 copies of calibrator. The DNA from each plasma aliquot was extracted, and 1/10 of the final DNA suspension was tested in each PCR. The mean of recovery, intersample variation, and accuracy error were computed to compare the quantification with and without calibration. Irrespective of the system chosen for the HHV-8 DNA load calculation, the measured values were lower than estimated (Table 2). However, for both DNA concentrations, the accuracy error significantly decreased by virtue of the calibrated quantification (from 50 to 6% and from 49.5 to 15.4% for the high and low DNA amounts, respectively). It was noteworthy that the accuracy errors measured for the viral dilutions were fully comparable to the average accuracy errors computed on the standard HHV-8 template dilutions (Table 1), which were not subjected to any extraction procedure. Plasma samples containing a high viral inoculum showed a decrease of the intersample CV (from 41.3 to 8.7%) when calibrated quantification was used. By contrast, no difference was observed for the low-copy-number input.
TABLE 2.
Quantification of HHV-8 DNA with and without calibrationa
| Genome equivalents/sample | Genome equivalents, mean of recovery ± SD |
Intersample CVa |
Accuracy error (%) |
|||
|---|---|---|---|---|---|---|
| Uncalibrated | Calibrated | Uncalibrated | Calibrated | Uncalibrated | Calibrated | |
| 800 | 404 ± 80 | 677 ± 129 | 19.8 | 19 | 49.5 | 15.4 |
| 300,000 | 149,860 ± 60,670 | 282,040 ± 15,500 | 41.3 | 8.7 | 50 | 6 |
Mean of recovery, inter-sample CV, and accuracy error for different amounts of viral DNA spiked into HHV-8-seronegative human plasma.
Clinical sensitivity and specificity of the cell-free HHV-8 load assay.
To verify the sensitivity and specificity of our assay, the HHV-8 DNA load was measured in plasma of 140 subjects with different clinical statuses: 24 patients with active HHV-8-related disorders (18 with AIDS-related KS, 1 with AIDS-BCL, 1 with HIV-associated MCD, and 4 with classical or posttransplantation KS), 16 patients affected by malignant lymphoma or chronic inflammatory disorders, 64 HIV-seropositive individuals, and 36 normal blood donors (Table 3). HHV-8 DNA load was present in specimens (plasma or serum) derived from 20 of 24 patients affected by active HHV-8-related disorders (sensitivity, 83%). By contrast, HHV-8 DNA was found only in 2 of 116 controls screened (specificity, 98%).
TABLE 3.
Sensitivity and specificity with clinical samples
| Clinical status of subjects (n) | TaqMan assay, no. positive (%) | HHV-8 DNA load (copies/ml, range) | Anti-HHV-8, no. positive (%) |
|---|---|---|---|
| HHV-8-related diseases (24) | 20 (83) | <10-560,000 | 23 (96) |
| HIV seropositivea (64) | 2b (3) | <10-138 | 24 (38) |
| Malignant lymphoma and inflammatory diseaseb (16) | 0 | <32c | 0 |
| Healthy blood donors (36) | 0 | <20c | 2 (5) |
No ongoing or previous HHV-8-related disorders.
Clinical evidence of KS after 8 and 15 months from specimens collection.
For negative samples the highest cutoff value is reported.
Cell-free HHV-8 DNA was detected in the vast majority of the serum or plasma samples from patients with HHV-8-associated diseases, with viral loads that varied between 10 and 560,000 genome equivalents/ml (Table 4), irrespective of the type of disease or the nature of the biological fluid examined. In half of the HHV-8-positive patients (10 of 20), the levels of HHV-8 DNA were below 200 genome equivalents/ml. Similar levels were also measured in 2 of 64 HIV-seropositive patients, 24 of which were HHV-8 seropositive, who later developed KS.
TABLE 4.
Quantitative analysis of cell-free HHV-8 DNA in 26 patients with various HHV-8-associated diseases
| Patient no. | HHV-8-associated disease(s) | Sample (vol in μl) | Amt of HHV-8 (copies/r) | Calibrator recovery (%) | No. of HHV-8 genome equivalents/ml |
HHV-8 antibody titer | |
|---|---|---|---|---|---|---|---|
| Uncalibrated | Calibrated | ||||||
| 1 | AIDS-KSa | Serum (200) | 140 | 36 | 7,000 | 19,440 | 1:1,280 |
| 2 | Serum (200) | 14 | 25 | 700 | 2,800 | 1:320 | |
| 3 | Serum (200) | 0 | 46 | 0 | <109 | >1:1,280 | |
| 4 | Serum (200) | 0 | 100 | 0 | <50 | <1:20 | |
| 5 | Serum (200) | 8 | 100 | 400 | 400 | >1:1,280 | |
| 6 | AIDS-KSb | Plasma (500) | 38 | 71 | 750 | 1,070 | >1:1,280 |
| 7 | Plasma (1,000) | 78 | 32 | 780 | 2,450 | >1:1,280 | |
| 8 | Plasma (1,000) | 1 | 51 | 10 | 20 | >1:1,280 | |
| 9 | Plasma (500) | 2 | 80 | 40 | 50 | >1:1,280 | |
| 10 | Plasma (500) | 28,000 | 100 | 560,000 | 560,000 | >1:1,280 | |
| 11 | Plasma (1,000) | 1 | 100 | 10 | 10 | >1:1,280 | |
| 12 | Plasma (1,000) | 100 | 48 | 1,000 | 2,080 | >1:1,280 | |
| 13 | Plasma (1,000) | 93 | 35 | 930 | 2,660 | >1:1,280 | |
| 14 | Plasma (1,000) | 1 | 100 | 10 | 10 | >1:1,280 | |
| 15 | Plasma (1,000) | 3 | 100 | 30 | 30 | >1:1,280 | |
| 16 | Plasma (1,000) | 3 | 26 | 30 | 115 | >1:1,280 | |
| 17 | Plasma (1,000) | 1 | 94 | 10 | 11 | >1:1,280 | |
| 18 | Plasma (1,000) | 0 | 15 | 0 | <66 | >1:1,280 | |
| 19 | HIV+/HHV-8+ | Plasma (1,000) | 5 | 50 | 50 | 100 | 1:3,560 |
| 20 | Plasma (1,000) | 7 | 50 | 70 | 140 | 1:640 | |
| 21 | Classical KS | Plasma (500) | 30 | 30 | 300 | 2,000 | 1:320 |
| 22 | Plasma (1,000) | 20 | 100 | 200 | 200 | 1:1,280 | |
| 23 | Post-transplantation KS | Plasma (1,000) | 0 | 100 | 0 | <10 | 1:1,280 |
| 24 | Plasma (1,000) | 11 | 65 | 110 | 168 | 1:1,280 | |
| 25 | AIDS-BCL | Plasma (1,000) | 6 | 40 | 60 | 150 | >1:1,280 |
| 26 | AIDS-MCD | Plasma (500) | 111 | 72 | 2,220 | 3,080 | >1:1,280 |
Patients from Uganda.
Patients from Europe.
A significant degree of variability in the DNA recovery rate (between 15 to 100% of the calibrator input) was observed, irrespective of the nature of the clinical sample, even among samples derived from the same individual (data not shown). Thus, the use of the calibrator allowed an appreciable correction of the HHV-8 DNA load in 12 of 26 individuals tested (Table 4).
Furthermore, the analysis of the kinetics of amplification of the calibrator DNA permitted excluding the presence of PCR inhibitors (data not shown) and determining, for each negative sample, a specific cutoff value for HHV-8 detection, which varied between 10 and 109 genome equivalents/ml (Table 4).
DISCUSSION
We have developed a new, fully controlled, real-time PCR assay for the detection and quantification of the HHV-8 DNA load in body fluids. This assay combines a state-of-the-art PCR technique, the TaqMan real-time PCR, with the use of a calibrator, a synthetic DNA molecule that monitors both the sample manipulation procedures and the presence of PCR artifacts due to the occasional copurification of PCR inhibitors. The system has been specifically designed to allow the simultaneous amplification of both amplicons under the same PCR conditions. Thus, particular attention has been devoted to ensuring that the HHV-8 and the calibrator amplicons possess the same kinetics of amplification and to avoiding primer competition and probe cross-detection phenomena. Similar to the classical competitor molecules developed for the quantitative-competitive PCR techniques, the calibrator represents a high-quality internal PCR standard that can be added prior to any manipulation of the biological sample. Indeed, as demonstrated by others and us (43), the extraction procedure causes a 50% average error in viral load measurements, an error that can be significantly increased by the choice of the extraction procedure (43). Moreover, clinical samples subjected to standard extraction procedures might still contain PCR inhibitors that can contribute to a miscalculation of the viral DNA load (16, 42, 43). The drastic reduction in accuracy error that we measured using the calibrated system attests to the efficacy of our strategy, which permits avoidance of the accuracy error due the introduction of the DNA extraction procedures. Moreover, whereas the detection and the amplification profile of the calibrator construct allow establishment of the presence of PCR inhibitors, the measurement of the sample recovery rate permits establishment of a precise cutoff of sensitivity for each negative sample. The combination of this controlled real-time assay with a preliminary step of sample's concentration allows quantification, with a good level of accuracy and reproducibility (<14 and <30%, respectively), of even minute amounts of HHV-8 DNA (10 genome equivalents/ml). Strikingly, the extreme sensitivity of the assay is not hampered by a reduced degree of specificity, as demonstrated by the results obtained with biological fluid samples derived from 140 individuals with different clinical conditions and geographic origins.
The detection and quantification of viral genetic material, either DNA or RNA, in body fluids provides a reliable marker for tracking an active viral infection (36, 37), especially useful for those viruses, such as herpesviruses, which establish a lifelong persistent infection with an alternating pattern of latency and reactivation. Indeed, the finding of cell-free HHV-8 DNA in plasma, serum, saliva, and secretory fluids from patients with AIDS-KS has been reported (11, 15, 45), but its clinical relevance, given the low sensitivity of the methods used, remains to be established. More recently, Campbell and colleagues, using a real-time PCR assay, have reported high levels of HHV-8 plasma viremia in the vast majority of AIDS-KS patients, as well in individuals from Zimbabwe who were dually infected with HIV and HHV-8 without signs of KS (5). Unfortunately, they were unable to confirm these results in a cohort of patients from the United States who were dually infected with HIV and HHV-8 (T. B. Campbell, K. A. Staskus, R. Evans, et al., Abstr. 7th Conf. Retrovir. Opportunistic Infect., abstr. 4a, 2000). Conversely, Tedeschi and colleagues have documented the presence of persistent HHV-8 viremia in a considerable fraction (71%) of European AIDS-KS patients (43). In agreement with their observations, we found that HHV-8 plasma viremia was detectable in a large fraction (83%) of biological samples derived from individuals suffering of HHV-8-associated diseases. Furthermore, signs of active HHV-8 replication were not featured solely by HIV-coinfected individuals but also were shown by patients affected by both classical and posttransplantation KS. Of note, we documented very low levels of HHV-8 DNA load in half of the patients. These levels were similar to the ones measured in two HIV-coinfected individuals who developed KS lesions a few months later (23). Taken together, these and other findings (2, 3, 5, 24, 27, 30, 32) support the hypothesis of a direct involvement of HHV-8 replication in KS pathogenesis and lesion dissemination, thus emphasizing the usefulness of sensitive, accurate, fast, and high-throughput diagnostic tests to monitor HHV-8 infection.
In conclusion, the calibrated real-time PCR technique described herein will offer clinicians a fast and reliable readout for establishing an etiologic link between HHV-8 and human diseases, as well as to monitor therapeutic approaches against HHV-8 infection.
Acknowledgments
We thank L. Dagna and M. G. Viganò for providing patient samples and Stefania Laus for editorial assistance.
F.B. and L.G. contributed equally to this work.
This work was partially supported by the European Union Biomed 2 (to P.L., grant P 1951301) and from grants (to P.L., grant 50 C.17; to M.S.M., grants 30C.46 and 40C.56) of the III Italian National AIDS project, Ministry of Health—Rome.
REFERENCES
- 1.Biggar, R. J., D. Whitby, V. Marshall, A. C. Linhares, and F. Black. 2000. Human herpesvirus 8 in Brazilian Amerindians: a hyperendemic population with a new subtype. J. Infect. Dis. 181:1562-1568. [DOI] [PubMed] [Google Scholar]
- 2.Boivin, G., A. Gaudreau, and J. P. Routy. 2000. Evaluation of the human herpesvirus 8 DNA load in blood and Kaposi's sarcoma skin lesions from AIDS patients on highly active antiretroviral therapy. AIDS 14:1907-1910. [DOI] [PubMed] [Google Scholar]
- 3.Boivin, G., A. Gaudreau, E. Toma, R. Lalonde, J. P. Routy, G. Murray, J. Handfield, and M. G. Bergeron. 1999. Human herpesvirus 8 DNA load in leukocytes of human immunodeficiency virus-infected subjects: correlation with the presence of Kaposi's sarcoma and response to anticytomegalovirus therapy. Antimicrob. Agents Chemother. 43:377-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabro, M. L., J. Sheldon, A. Favero, G. R. Simpson, J. R. Fiore, E. Gomes, G. Angarano, L. Chieco-Bianchi, and T. F. Schulz. 1998. Seroprevalence of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 in several regions of Italy. J. Hum. Virol. 1:207-213. [PubMed] [Google Scholar]
- 5.Campbell, T. B., M. Borok, L. Gwanzura, S. MaWhinney, I. E. White, B. Ndemera, I. Gudza, L. Fitzpatrick, and R. T. Schooley. 2000. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi's sarcoma clinical stage. AIDS 14:2109-2116. [DOI] [PubMed] [Google Scholar]
- 6.Cesarman, E., Y. Chang, P. S. Moore, J. S. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 7.Chandran, B., M. S. Smith, D. M. Koelle, L. Corey, R. Horvat, and E. Goldstein. 1998. Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology 243:208-217. [DOI] [PubMed] [Google Scholar]
- 8.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 9.Gao, S. J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 25:233-241. [DOI] [PubMed] [Google Scholar]
- 10.Hagiwara, H., N. Hayashi, E. Mita, M. Naito, A. Kasahara, H. Fusamoto, and T. Kamada. 1993. Quantitation of hepatitis C virus RNA in serum of asymptomatic blood donors and patients with type C chronic liver disease. Hepatology 17:545-550. [DOI] [PubMed] [Google Scholar]
- 11.Harrington, W. J., Jr., O. Bagasra, C. E. Sosa, L. E. Bobroski, M. Baum, X. L. Wen, L. Cabral, G. E. Byrne, R. J. Pomerantz, and C. Wood. 1996. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi's sarcoma patients. J. Infect. Dis. 174:1101-1105. [DOI] [PubMed] [Google Scholar]
- 12.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson, L. P., F. J. Jenkins, G. Springer, A. Munoz, K. V. Shah, J. Phair, Z. Zhang, and H. Armenian. 2000. Interaction of human immunodeficiency virus type 1 and human herpesvirus type 8 infections on the incidence of Kaposi's sarcoma. J. Infect. Dis. 181:1940-1949. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy, M. M., S. B. Lucas, R. R. Jones, D. D. Howells, S. J. Picton, E E. Hanks, J. O. McGee, and J. J. O'Leary. 1997. HHV8 and Kaposi's sarcoma: a time cohort study. Mol. Pathol. 50:96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koelle, D. M., M. L. Huang, B. Chandran, J. Vieira, M. Piepkorn, and L. Corey. 1997. Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J. Infect. Dis. 176:94-102. [DOI] [PubMed] [Google Scholar]
- 16.Kwok, S., and R. Higuchi. 1989. Avoiding false positives with PCR. Nature 18:237-238. [DOI] [PubMed] [Google Scholar]
- 17.Lallemand, F., N. Desire, W. Rozenbaum, J. C. Nicolas, and V. Marechal. 2000. Quantitative analysis of human herpesvirus 8 viral load using a real-time PCR assay. J. Clin. Microbiol. 38:1404-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefrere, J. J., M. C. Meyohas, M. Mariotti, J. L. Meynard, M. Thauvin, and J. Frottier. 1996. Detection of human herpesvirus 8 DNA sequences before the appearance of Kaposi's sarcoma in human immunodeficiency virus (HIV)-positive subjects with a known date of HIV seroconversion. J. Infect. Dis. 174:283-287. [DOI] [PubMed] [Google Scholar]
- 19.Lennette, E. T., D. J. Blackbourn, and J. A. Levy. 1996. Antibodies to human herpesvirus type 8 in the general population and in Kaposi's sarcoma patients. Lancet 348:858-861. [DOI] [PubMed] [Google Scholar]
- 20.Locatelli, G., F. Santoro, F. Veglia, A. Gobbi, P. Lusso, and M. S. Malnati. 2000. Real-time quantitative PCR for human herpesvirus 6 DNA. J. Clin. Microbiol. 38:4042-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lock, M. J., P. D. Griffiths, and V. C. Emery. 1997. Development of a quantitative competitive polymerase chain reaction for human herpesvirus 8. J. Virol. Methods 64:19-26. [DOI] [PubMed] [Google Scholar]
- 22.Lusso, P. 1996. Human herpesvirus 6 (HHV-6). Antivir. Res. 31:1-21. [DOI] [PubMed] [Google Scholar]
- 23.Malnati, M. S., F. Broccolo, S. Nozza, L. Sarmati, S. Ghezzi, G. Locatelli, F. Delfanti, B. Capiluppi, A. Careddu, M. Andreoni, P. Monini, G. Poli, A. Lazzarin, P. Lusso, and G. Tambussi. 2002. Retrospective analysis of HHV-8 plasma viremia and cellular viral load in HIV-seropositive individuals receiving interleukin-2 in combination with antiretroviral therapy. Blood 100:1575-1578. [PubMed] [Google Scholar]
- 24.Mazzi, R., S. G. Parisi, L. Sarmati, I. Uccella, E. Nicastri, G. Carolo, F. Gatti, E. Concia, and M. Andreoni. 2001. Efficacy of cidofovir on human herpesvirus 8 viraemia and Kaposi's sarcoma progression in two patients with AIDS. AIDS 19:2061-2062. [DOI] [PubMed] [Google Scholar]
- 25.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 24:1167-1170. [DOI] [PubMed] [Google Scholar]
- 26.Moore, P. S., L. A. Kingsley, S. D. Holmberg, T. Spira, P. Gupta, D. R. Hoover, J. P. Parry, L. J. Conley, H. W. Jaffe, and Y. Chang. 1996. Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS 10:175-180. [DOI] [PubMed] [Google Scholar]
- 27.Nunez, M., A. Machuca, V. Soriano, D. Podzamczer, J. Gonzalez-Lahoz, et al. 2000. Clearance of human herpesvirus type 8 viraemia in HIV-1-positive patients with Kaposi's sarcoma treated with liposomal doxorubicin. AIDS 14:913-919. [DOI] [PubMed] [Google Scholar]
- 28.Oksenhendler, E., D. Cazals-Hatem, T. F. Schulz, V. Barateau, L. Grollet, J. Sheldon, J. P. Clauvel, F. Sigaux, and F. Agbalika. 1998. Transient angiolymphoid hyperplasia and Kaposi's sarcoma after primary infection with human herpesvirus 8 in a patient with human immunodeficiency virus infection. N. Engl. J. Med. 28:1585-1590. [DOI] [PubMed] [Google Scholar]
- 29.Oksenhendler, E., G. Carcelain, Y. Aoki, E. Boulanger, A. Maillard, J. P. Clauvel, and F. Agbalika. 2000. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood 15:2069-2073. [PubMed] [Google Scholar]
- 30.O'Leary, J. J., M. Kennedy, K. Luttich, V. Uhlmann, I. Silva, R. Russell, O. Sheils, M. Ring, M. Sweeney, C. Kenny, N. Bermingham, C. Martin, M. O'Donovan, D. Howells, S. Picton, and S. B. Lucas. 2000. Localisation of HHV-8 in AIDS related lymphadenopathy. Mol. Pathol. 53:43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen, S. J., Y. Chang, P. S. Moore, R. J. Biggar, and M. Melbye. 1998. Increasing Kaposi's sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi's sarcoma endemic region, Zambia in 1985. AIDS 12:1921-1925. [DOI] [PubMed] [Google Scholar]
- 32.Prins, J. M., C. J. Sol, N. Renwick, J. Goudsmit, J. Veenstra, and P. Reiss. 1999. Favourable effect of chemotherapy on clinical symptoms and human herpesvirus-8 DNA load in a patient with Kaposi's sarcoma presenting with fever and anemia. Eur. J. Clin. Microbiol. Infect. Dis. 18:499-502. [DOI] [PubMed] [Google Scholar]
- 33.Renwick, N., T. Halaby, G. J. Weverling, N. H. Dukers, G. R. Simpson, R. A. Coutinho, J. M. Lange, T. F. Schulz, and J. Goudsmit. 1998. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi's sarcoma. AIDS 24:2481-2488. [DOI] [PubMed] [Google Scholar]
- 34.Rettig, M. B., H. J. Ma, R. A. Vescio, M. Pold, G. Schiller, D. Belson, A. Savage, C. Nishikubo, C. Wu, J. Fraser, J. W. Said, and J. R. Berenson. 1997. Kaposi's sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science 276:1851-1854. [DOI] [PubMed] [Google Scholar]
- 35.Schatz, O., P. Monini, R. Bugarini, F. Neipel, T. F. Schulz, M. Andreoni, P. Erb, M. Eggers, J. Haas, S. Butto, M. Lukwiya, J. R. Bogner, S. Yaguboglu, J. Sheldon, L. Sarmati, F. D. Goebel, R. Hintermaier, G. Enders, N. Regamey, M. Wernli, M. Sturzl, G. Rezza, and R. Ensoli. 2001. Kaposi's sarcoma-associated herpesvirus serology in Europe and Uganda: multicentric study with multiple and novel assays. J. Med. Virol. 65:123-132. [PubMed] [Google Scholar]
- 36.Secchiero, P., D. R. Carrigan, Y. Asano, L. Benedetti, R. W. Crowley, A. L. Komaroff, R. C. Gallo, and P. Lusso. 1995. Detection of human herpesvirus 6 in plasma of children with primary infection and immunosuppressed patients by polymerase chain reaction. J. Infect. Dis. 171:273-280. [DOI] [PubMed] [Google Scholar]
- 37.Secchiero, P., D. Zella, R. W. Crowley, R. C. Gallo, and P. Lusso. 1995. Quantitative PCR for human herpesviruses 6 and 7. J. Clin. Microbiol. 33:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson, G. R., T. F. Schulz, D. Whitby, P. M. Cook, C. Boshoff, L. Rainbow, M. R. Howard, S. J. Gao, R. A. Bohenzky, P. Simmonds, C. Lee, A. de Ruiter, A. Hatzakis, R. S. Tedder, I. V. Weller, R. A. Weiss, and P. S. Moore. 1996. Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet 26:1133-1138. [DOI] [PubMed] [Google Scholar]
- 39.Sitas, F., H. Carrara, V. Beral, R. Newton, G. Reeves, D. Bull, U. Jentsch, R. Pacella-Norman, D. Bourboulia, D. Whitby, C. Boshoff, and R. Weiss. 1999. Antibodies against human herpesvirus 8 in black South African patients with cancer. N. Engl. J. Med. 17:1863-1871. [DOI] [PubMed] [Google Scholar]
- 40.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 41.Spira, T. J., L. Lam, S. X. Dollard, Y. X. Meng, C. P. Pau, J. B. Black, D. Burns, B. Cooper, M. Hamid, J. Huong, K. Kite-Powell, and P. E. Pellett. 2000. Comparison of serologic assays and PCR for diagnosis of human herpesvirus 8 infection. J. Clin. Microbiol. 38:2174-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamey, F. R., M. M. Patel, B. P. Holloway, and P. E. Pellett. 2001. Quantitative, fluorogenic probe PCR assay for detection of human herpesvirus 8 DNA in clinical specimens. J. Clin. Microbiol. 39:3537-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedeschi, R., M. Enbom, E. Bidoli, A. Linde, P. De Paoli, and J. Dillner. 2001. Viral load of human herpesvirus 8 in peripheral blood of human immunodeficiency virus-infected patients with Kaposi's sarcoma. J. Clin. Microbiol. 39:4269-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 12:117-122. [DOI] [PubMed] [Google Scholar]
- 45.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. E. Suggett, D. M. Aldam, A. S. Denton, et al. 1995. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 23:799-802. [DOI] [PubMed] [Google Scholar]
- 46.Whitby, D., M. Luppi, P. Barozzi, C. Boshoff, R. A. Weiss, and G. Torelli. 1998. Human herpesvirus 8 seroprevalence in blood donors and lymphoma patients from different regions of Italy. J. Natl. Cancer Inst. 90:395-397. [DOI] [PubMed] [Google Scholar]
- 47.White, I. E., and T. B. Campbell. 2000. Quantitation of cell-free and cell-associated Kaposi's sarcoma-associated herpesvirus DNA by real-time PCR. J. Clin. Microbiol. 38:1992-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]




