Abstract
The sensitivity and specificity of the Cell-Dyn 4000 hematology analyzer in the diagnosis of imported malaria were studied with samples from patients in an academic hospital setting. The performance of the Cell-Dyn 4000 hematology analyzer was compared with that of conventional diagnostic methods for malaria. The Cell-Dyn 4000 hematology analyzer detected hemozoin-containing depolarizing monocytes in 29 of 58 patients with malaria and 2 of 55 patients without malaria. The presence or absence of depolarizing monocytes in patients with malaria was related to duration of symptoms before presentation for malaria analysis. A second parameter, pseudoreticulocytosis due to nuclear material of intraerythrocytic malaria parasites, was detected by the Cell-Dyn 4000 hematology analyzer almost exclusively in Plasmodium falciparum malaria patients with parasitemia levels of ≥0.5%. Attention to these abnormalities in medical centers without tropical disease expertise may decrease a delay in the diagnosis of malaria.
Microscopic investigation of stained thick and thin blood smears has been the reference standard for malaria detection and species identification for decades. Recently, a number of alternative diagnostic approaches have evolved, including detection of Plasmodium species DNA stained with acridine orange in a quantitative buffy coat analysis, PCR methods, and assays based on detection of circulating Plasmodium species-specific antigens (e.g., P. falciparum histidine-rich protein 2). Recent studies using automated hematology analyzers have demonstrated unexpected abnormalities in differential white blood cell plots and reticulocyte histograms from patients with malaria (1, 2, 4). Normal monocytes can be discriminated from monocytes that have ingested the malarial breakdown product hemozoin because of the ability of hemozoin to depolarize laser light used for routine differentiation of eosinophils (1, 4). Nuclear material of intraerythrocytic malaria parasites could be discriminated by fluorescent nucleic acid dye used in routine quantification of reticulocytes. The presence of infected erythrocytes leads to a distinct fluorescent spike in reticulocyte histograms, referred to as pseudoreticulocytosis (2). It has been suggested that this novel method is a useful addition to conventional microscopy (1). Here, we set out to prospectively study the sensitivity and specificity of the Cell-Dyn 4000 hematology analyzer (Abbott Diagnostics, Santa Clara, Calif.) in the diagnosis of imported malaria among patients in an academic hospital setting in The Netherlands. The Cell-Dyn 4000 hematology analyzer is a new-generation automated hematology analyzer that has found widespread use in routine laboratory hematology.
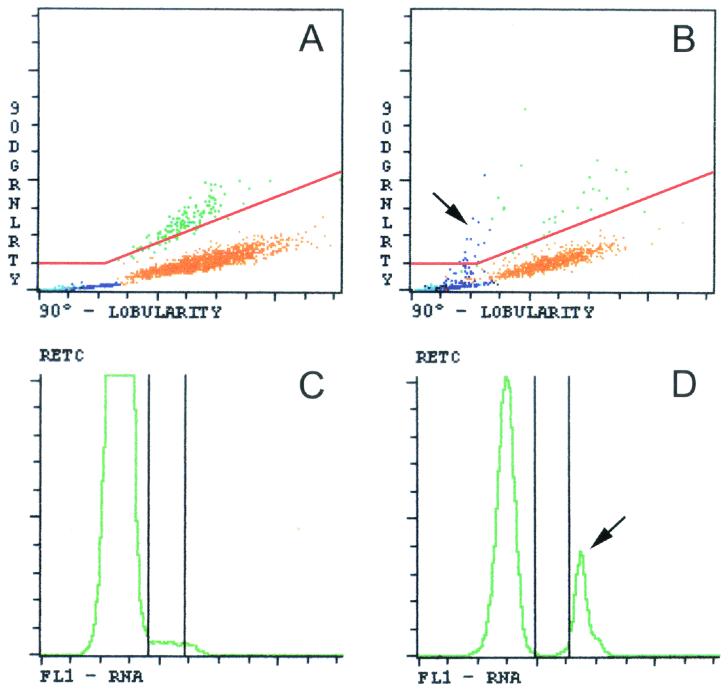
We prospectively studied 113 patients referred to the Section of Parasitology because of clinical suspicions of malaria by the Emergency Department or the Tropical Disease Center of the Academic Medical Center, Amsterdam, The Netherlands. Malaria was considered part of the differential diagnosis of pyrexia when a patient presented with a travel history to an area where malaria is endemic. Routine malaria analysis consisted of examination of stained thick and thin blood smears according to World Health Organization standard methods and quantitative buffy coat analysis. If malaria parasites were observed and a parasite density of ≥0.5% was expected, parasite density was expressed as the percentage of erythrocytes infected by determining in duplo the number of infected erythrocytes per 2,000 erythrocytes on a thin blood smear. In the case of an expected parasite density of <0.5%, parasite density was determined by counting in duplicate the number of parasites observed per 100 white blood cells on a thick blood smear. Species identification was based on morphological features. In addition, full blood count and reticulocyte analyses were performed at the Department of Clinical Chemistry with EDTA-anticoagulated blood on the Cell-Dyn 4000 hematology analyzer. Details of this technology have been described previously (2, 4). The presence of depolarizing monocytes was defined as the presence in a lobularity-granularity plot of any depolarizing purple event situated above a separation line constructed from a line making a 22.5° angle with the x axis and a horizontal line through granularity signal channel 25 (C. S. Scott, Abbott Diagnostics, Maidenhead, Berkshire, United Kingdom, personal communication) (Fig. 1A and B). Pseudoreticulocytosis was defined as a distinct spike with narrow fluorescence intensity in a reticulocyte histogram (Fig. 1C and D). Laboratory technicians at the Parasitology Section and the Department of Clinical Chemistry were blind to results obtained in the other laboratory.
FIG. 1.
Differential white blood cell plots and reticulocyte histograms generated by the Cell-Dyn 4000 hematology analyzer from the peripheral blood of patients with and without malaria. (A) Differential lobularity-granularity plot of the white blood cells from a patient without malaria. Monocytes are shown in purple, lymphocytes are shown in blue, neutrophils are shown in orange, eosinophils are shown in green, and basophils are shown in black. The differential lobularity-granularity plot depicts the results of a lobularity analysis (90° lobularity) versus those of a depolarization analysis (90° depolarized granularity [90DGRNLRTY]). (B) Lobularity-granularity plot of a patient with P. falciparum malaria. Note the presence of hemozoin-containing depolarizing monocytes as purple dots above the separation line (see arrow). (C and D) Reticulocyte histograms (RETC) of a patient without malaria (C) and of a patient with P. falciparum malaria (D). Note the presence of a distinct fluorescent spike representing nuclear material of intraerythrocytic malaria parasites (arrow). FL1, fluorescence intensity.
Of the 113 analyzed patients, 58 (51%) were found to have malaria by routine conventional methods. Forty-six patients suffered from P. falciparum malaria, of which 25 had parasitemia levels of ≥0.5% and 21 had parasitemia levels of <0.5%. Non-falciparum malaria was detected in 12 patients (5 P. vivax cases, 4 P. ovale cases, and 3 cases in which no differentiation could be made between P. vivax and P. ovale). Table 1 presents findings with the Cell-Dyn 4000 hematology analyzer for the 113 analyzed patients. Overall, either depolarizing monocytes or pseudoreticulocytosis was observed in 36 of 58 malaria patients (62%) and in 2 of 55 patients without malaria (4%). In the subgroup of P. falciparum malaria patients with parasitemia levels of ≥0.5%, either depolarizing monocytes or pseudoreticulocytosis was observed in 24 of 25 patients (96%). A statistically significant difference (unpaired t test, P = 0.03, SPSS software version 10.0) in mean durations of symptoms before presentation for malaria analysis was present among the 29 malaria patients in which depolarizing monocytes were detected (4.5 days; 95% confidence interval, 3.7 to 5.4) and the 29 malaria patients in which these cells were not detected (3.2 days; 95% confidence interval, 2.4 to 4.0). Mean levels of parasitemia as detected by routine malaria analysis did not differ between these two groups.
TABLE 1.
Findings with the Cell-Dyn 4000 hematology analyzer for 113 patients with or without malaria infectiona
| Plasmodium spp. and parasitemia level | Total no. of patients | No. (%) of patients with: |
||
|---|---|---|---|---|
| Depolarizing monocytes | Pseudoreticulocytosis | Either or both abnormalities | ||
| P. falciparum overall | 46 | 26 (57) | 23 (50) | 33 (72) |
| Parasitemia, ≥0.5% | 25 | 17 (68) | 22 (88) | 24 (96) |
| Parasitemia, <0.5% | 21 | 9 (43) | 1 (5) | 9 (43) |
| P. vivax or P. ovale | 12 | 3 (25) | 0 (0) | 3 (25) |
| Plasmodium spp. overall | 58 | 29 (50) | 23 (40) | 36 (62) |
| None | 55 | 2 (4)b | 0 (0) | 2 (4)b |
Findings with the Cell-Dyn 4000 hematology analyzer are for patients whose malaria or lack of malaria was determined by routine malaria analysis.
One of the two patients without malaria in whom depolarizing monocytes were observed had been treated for malaria the previous month.
Our data indicate that the Cell-Dyn 4000 hematology analyzer is capable of detecting specific abnormalities in the blood of patients with imported malaria. Its overall sensitivity in an academic hospital setting with technicians with expertise in tropical medicine and diagnostic parasitology is, however, limited compared to the sensitivities of conventional diagnostic methods.
Pseudoreticulocytosis was observed almost exclusively in P. falciparum malaria patients with parasitemia levels of ≥0.5%. One P. falciparum malaria patient with a parasitemia level of 0.4% presented with pseudoreticulocytosis, while three P. falciparum malaria patients with parasitemia levels of 0.5, 0.5, and 0.6% did not exhibit this phenomenon. This indicates that a parasitemia level around 0.5% is a cutoff point for pseudoreticulocytosis to appear. Pseudoreticulocytosis did not occur in non-falciparum malaria patients. Parasitemia levels in non-falciparum malaria patients are low and might, in general, be below the detection level of the Cell-Dyn 4000 technique. In addition, it is known that P. vivax preferentially infects reticulocytes. P. vivax-infected reticulocytes will be detected by the Cell-Dyn 4000 hematology analyzer as true reticulocytes. In theory, therefore, they might not contribute to the pseudoreticulocytosis phenomenon.
In their initial report on the detection of depolarizing monocytes in areas where malaria is endemic, Mendelow et al. reported sensitivities for black and white patients in South Africa of 90 and 42%, respectively, using the Cell-Dyn 3500 hematology analyzer. The reason for this racial difference was not found, but speculations were made regarding certain genetic factors, previous exposure, or different responses to infection (4). We show that in imported malaria, the presence or absence of depolarizing monocytes detectable by the Cell-Dyn 4000 hematology analyzer depends on the duration of symptoms before presentation for malaria analysis. Thus, the difference in sensitivities for black and white malaria patients in the South African study may reflect socioeconomic or cultural differences in access to health services and, therefore, in duration of symptoms before presentation for malaria analysis.
Recently, a Canadian study reported on the diagnosis and management of imported malaria in medical centers whose technicians lack expertise in tropical medicine versus the diagnosis and management in medical centers with a tropical disease unit. There were significant delays in the recognition, laboratory diagnosis, and initiation of treatment of malaria when patients presented to medical centers where technicians lacked expertise in tropical medicine (3). Although the Cell-Dyn 4000 hematology analyzer has a low sensitivity for the detection of malaria, the specificity is high. Therefore, it is feasible that the Cell-Dyn 4000 hematology analyzer may contribute to the diagnosis of imported malaria in medical centers whose technicians lack expertise in tropical medicine. As it is likely that general screening tests like a full blood count are always undertaken for patients who present with pyrexia, it can be expected that attention to these abnormalities can decrease a delay in the diagnosis of severe P. falciparum malaria if such a diagnosis was not initially considered. However, our data also indicate that one should not rely on the Cell-Dyn 4000 hematology analyzer as the sole diagnostic test for malaria. A good patient history and repeated routine malaria analysis are essential, especially for patients suspected of being early in the course of a P. falciparum infection.
REFERENCES
- 1.Hänscheid, T., J. Melo-Cristino, and B. G. Pinto. 2001. Automated detection of malaria pigment in white blood cells for the diagnosis of malaria in Portugal. Am. J. Trop. Med. Hyg. 64:290-292. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman, J. J. M. L., and J. M. A. Pennings. 1999. Pseudo-reticulocytosis as a result of malaria parasites. Clin. Lab. Haematol. 21:257-260. [DOI] [PubMed] [Google Scholar]
- 3.Kain, K. C., M. A. Harrington, S. Tennyson, and J. S. Keystone. 1998. Imported malaria: prospective analysis of problems in diagnosis and management. Clin. Infect. Dis. 27: 142-149. [DOI] [PubMed] [Google Scholar]
- 4.Mendelow, B. V., C. Lyons, P. Nhlangothi, M. Tana, M. Munster, E. Wypkema, L. Liebowitz, L. Marshall, S. Scott, and T. L. Coetzer. 1999. Automated malaria detection by depolarization of laser light. Br. J. Haematol. 104:499-503. [DOI] [PubMed] [Google Scholar]