Abstract
Rotavirus RNA was detected in the cerebrospinal fluid (CSF) of a child with central nervous system disease symptoms associated with rotavirus gastroenteritis. The rotavirus isolates from the fecal and CSF samples were genotyped as G1P[8]. Sequence analysis of the VP7 and VP4 proteins derived from the fecal and CSF samples were remarkably similar to each other and to G1P[8] rotavirus strains commonly circulating in the community and associated with gastroenteritis.
CASE REPORT
A 22-month-old previously healthy male child was admitted to the hospital with a 2-day history of nonbloody diarrhea and vomiting. On the day of admission he developed fever and had a generalized tonic-clonic seizure lasting 2 min. On arrival at the hospital, he was apyrexial, mildly dehydrated, and drowsy. Physical examination was otherwise unremarkable with no neck stiffness.
A lumbar puncture was performed. The cerebrospinal fluid (CSF) contained 1,235 red cells/μl and eight white cells/μl (75% lymphocytes). A blood count showed a total white cell count of 9.4 × 109 cells/liter, with 2.4 × 109 lymphocytes/liter and 6.0 × 109 neutrophils/liter (normal for the patient's age). Intravenous penicillin, cefotaxime, and acyclovir were administered. Bacterial cultures of blood and CSF were negative, and antibiotic treatment was stopped after 72 h. Clinically the child improved over the first 24 h and subsequently made a full recovery.
Microbiologic analysis.
A stool sample was positive for rotavirus antigen by latex particle agglutination, and this result was confirmed by inhibition with specific antibodies. Both stool and CSF samples were tested for the presence of rotavirus RNA. Nucleic acid was extracted and reverse transcribed, and the cDNA was used in rotavirus VP7- and VP4-specific typing PCRs as described previously (7). Both the stool and CSF samples contained rotavirus of the genotype G1P[8]. The VP7 and VP4 first-round amplicons derived from the stool and CSF samples were purified and sequenced in both directions by using the same consensus primers as those used for the first-round amplification. Nucleic acid sequences were derived using an automated sequencer (CEQ2000; Beckman-Coulter). Sequence data were analyzed using the software programs Seqman and Megalign (DNA Star; Lasergene, Madison, Wis.).
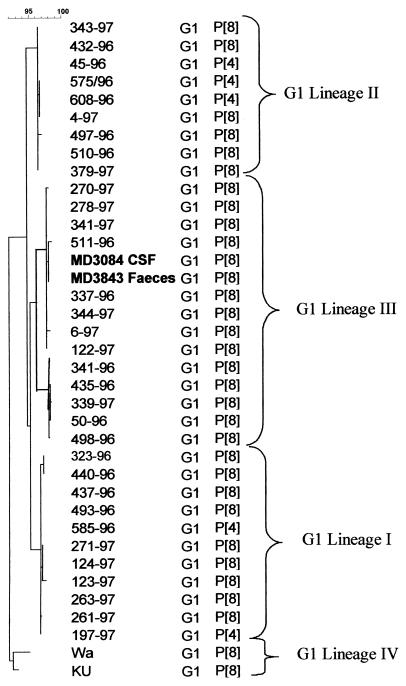
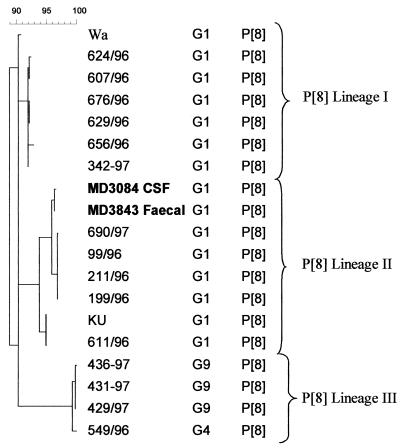
Partial cDNA sequences of the genes encoding the rotavirus VP7 (nucleotides [nt] 51 to 932) and VP4 (nt 11 to 887) obtained from the stool and CSF samples were highly homologous (>98.5 and 100% at the nucleotide and amino acid levels, respectively). The sequences of VP7 and VP4 clustered within the G1 genetic lineage III and the P[8] genetic lineage II, respectively (Fig. 1 and 2) (8, 9). Analysis of the deduced amino acid sequences revealed no amino acid substitutions between the VP7 amino acid sequences derived from the fecal and CSF samples and VP7 sequences of other rotavirus strains within their genetic lineage. The deduced amino acid sequence of VP4 revealed a single amino acid substitution at position 166 within the hypervariable region of VP8. An isoleucine residue at amino acid position 172 differentiated the sequences derived from feces and CSF from other rotavirus sequences within the genetic lineage P[8]-II, which had a conserved valine at this position.
FIG. 1.
Phylogenetic tree constructed with VP7 (nt 51 to 932) cDNA sequences of G1 strains of genetic lineages I, II, and III (bootstrap values at the lineage branching points, >95%) isolated in the United Kingdom. Sequences of prototype strains Wa and Ku of G1 lineage IV were obtained from GenBank. Sequences derived from the CSF and paired fecal samples from the patient are represented in boldface type. The calibration bar at top indicates percent homology.
FIG. 2.
Phylogenetic tree constructed with VP4 cDNA (nt 11 to 887) sequences of P[8] strains of genetic lineages I, II, and III (bootstrap values at the lineage branching points, >95%) isolated in the United Kingdom. Sequences of prototype strains Wa and Ku were obtained from GenBank. Sequences derived from the CSF and paired fecal samples from the patient are represented in boldface type. The calibration bar at top indicates percent homology.
Discussion.
Rotaviruses are the most common cause of infantile diarrhea throughout the world and account for high mortality rates in the developing world as a result of severe dehydration (10). However, the tropism of rotavirus does not seem to be absolutely limited to enterocytes, and rotavirus-induced lesions, isolation of infectious particles, or the detection of rotavirus-specific antigens and/or nucleic acid at extraintestinal sites has been reported both in animals and humans, in immunocompetent as well as immunocompromised children (2). These sites include the lamina propria, mesenteric lymph nodes, lungs, liver, kidney, and bile ducts.
Since the first report of Salmi et al. in 1978 (19), central nervous system (CNS) involvement after rotavirus infection has been described by several authors. Different studies have reported various frequencies of CNS involvement in children with acute rotavirus gastroenteritis: 2% in Germany (20), 2.6% in Japan (1), 3.7% in the United States (12), 5.4% in Taiwan (12), and 5.7% in Hong Kong (26). A recent study analyzed the incidence of convulsions in children with acute gastroenteritis associated with either a bacterial infection or a rotavirus infection or in whom no organism was found (26). A statistically significant association was found between encephalopathy and rotavirus infection (P < 0.002) versus no organism found, and rotavirus infection also had a higher risk of encephalopathy in comparison to that for bacterial gastroenteritis (relative risk = 1.846) (26). Neurological complications include meningitis, encephalitis, convulsions, hemorrhagic shock and encephalopathy, central pontine myelinolysis, and Guillain-Barré syndrome (6, 12, 14; C. C. Smeets, W. Brussel, Q. H. Leyten, and F. Brus, Letter, Eur. J. Pediatr. 159:224, 2000). Infection in these patients was diagnosed by examining CSF by electron microscopy (EM) for morphologically characteristic rotavirus particles, testing for virus-specific antibodies, or detecting the rotavirus genome by reverse transcription (RT)-PCR (Table 1).
TABLE 1.
Rotavirus genotypes detected in CSF of patients with CNS symptoms
| Method used | Genotype(s) found | Reference |
|---|---|---|
| Detection of virus particles by EM | NKa | Wong et al. (25) |
| Detection of rotavirus- specific antibodies | NK NK | Ushijima et al. (21) Keidan et al. (11) |
| Detection of rotavirus RNA by RT-PCR | G1 P[NK] NK | Nishimura et al. (17) Keidan et al. (11) |
| G3 P[9] | Yoshida et al. (27) | |
| G1 P[NK] | Pang et al. (18) | |
| G2 P[NK] | Makino et al. (13) | |
| G1 P[NK], G3 P[NK] | Goldwater et al. (5) | |
| GNK P[4] | Lynch et al. (12) |
NK, not known.
Rotavirus has two outer capsid proteins, VP4 (which determines the P type) and VP7 (which determines the G type). These proteins elicit neutralizing antibodies and are involved in resistance to infection (4). G1 and P[8] types predominate worldwide (3). The G1P[8] rotaviruses isolated from the CNS and gut of this patient were remarkably similar to each other and also to rotaviruses circulating widely in the community and associated with gastroenteritis only. A previous report, in which sequence data were available for the VP7 genes of G1 rotaviruses associated with CNS symptoms, showed similar findings (22). The genotypes of rotaviruses detected in CSF are shown in Table 1. These results would suggest that transmission of rotavirus to extraintestinal sites may be host related rather than associated with changes in genes determining viral pathogenesis or cell tropism. However, by application of classical and molecular genetic techniques in the neonatal mouse model, hepatotropism has been found to segregate with the gene encoding NSP3, and it has been suggested that spread from the gut into the liver is likely to occur via the lymphatic pathway (16).
It is not known whether rotavirus replication is sustained in the CNS or whether the detection of rotavirus RNA may be the result of carriage in trafficking lymphocytes. However, it has been observed that rotaviruses can undergo limited in vitro replication in neuronal cells (23, 24). Although the majority of the patients with rotavirus infection with CNS involvement have made a complete recovery, a small number of patients have sustained severe sequelae or died (13, 21; C. Pager, D. Steele, P. Gwamanda, and M. Driessen, Letter, S. Afr. Med. J. 90:364-365, 2000).
The isolation of rotaviruses in the throat, serum, and CSF of patients with CNS involvement (22) and the appearance in some patients of an exanthemous rash (15) would suggest viremia following rotavirus infection in some cases. It has been suggested that genomes permeate the blood-brain barrier during convulsions or the virus infects the neuronal cells and releases the genome into the CSF (22). However, it is still unclear whether viremia always leads to CNS involvement or is a contributory factor. Further work is required in order to determine the frequency of rotavirus viremia and CNS disease.
Acknowledgments
Miren Iturriza-Gómara is supported by a grant from the MRC Research Training Fellowships program.
REFERENCES
- 1.Abe, T., M. Kobayashi, K. Araki, H. Kodama, Y. Fujita, T. Shinozaki, and H. Ushijima. 2000. Infantile convulsions with mild gastroenteritis. Brain Dev. 22:301-306. [DOI] [PubMed] [Google Scholar]
- 2.Conner, M., and R. Ramig. 1996. Viral enteric diseases, p. 713-743. In N. Nathanson et al. (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, Pa.
- 3.Desselberger, U., M. Iturriza-Gómara, and J. Gray. 2001. Rotavirus epidemiology and surveillance, p. 125-147. In D. Chadwick and J. A. Goode (ed.), Gastroenteritis viruses. Novartis symposium series, vol. 238. John Wiley & Sons, New York, N.Y. [DOI] [PubMed]
- 4.Estes, M. 2001. Rotaviruses and their replication, p. 1747-1785. In D. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 5.Goldwater, P. N., K. Rowland, M. Thesinger, K. Abbott, A. Grieve, E. A. Palombo, P. J. Masendycz, I. Wilkinson, and J. Bear. 2001. Rotavirus encephalopathy: pathogenesis reviewed. J. Paediatr. Child Health 37:206-209. [DOI] [PubMed] [Google Scholar]
- 6.Gregorio, L., C. L. Sutton, and D. A. Lee. 1997. Central pontine myelinolysis in a previously healthy 4-year-old child with acute rotavirus gastroenteritis. Pediatrics 99:738-743. [DOI] [PubMed] [Google Scholar]
- 7.Iturriza-Gómara, M., J. Green, D. W. Brown, U. Desselberger, and J. J. Gray. 1999. Comparison of specific and random priming in the reverse transcriptase polymerase chain reaction for genotyping group A rotaviruses. J. Virol. Methods 78:93-103. [DOI] [PubMed] [Google Scholar]
- 8.Iturriza-Gómara, M., J. Green, D. W. Brown, U. Desselberger, and J. J. Gray. 2000. Diversity within the VP4 gene of rotavirus P[8] strains: implications for reverse transcription-PCR genotyping. J. Clin. Microbiol. 38:898-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iturriza-Gómara, M., B. Isherwood, U. Desselberger, and J. Gray. 2001. Reassortment in vivo: driving force for diversity of human rotavirus strains isolated in the United Kingdom between 1995 and 1999. J. Virol. 75:3696-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapikian, A., Y. Hoshino, and R. Chanock. 2001. Rotaviruses, p. 1787-1834. In D. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 11.Keidan, I., I. Shif, G. Keren, and J. H. Passwell. 1992. Rotavirus encephalopathy: evidence of central nervous system involvement during rotavirus infection. Pediatr. Infect. Dis. J. 11:773-775. [PubMed] [Google Scholar]
- 12.Lynch, M., B. Lee, P. Azimi, J. Gentsch, C. Glaser, S. Gilliam, H. G. Chang, R. Ward, and R. I. Glass. 2001. Rotavirus and central nervous system symptoms: cause or contaminant? Clin. Infect. Dis. 33:932-938. [DOI] [PubMed] [Google Scholar]
- 13.Makino, M., Y. Tanabe, K. Shinozaki, S. Matsuno, and T. Furuya. 1996. Haemorrhagic shock and encephalopathy associated with rotavirus infection. Acta Paediatr. 85:632-634. [DOI] [PubMed] [Google Scholar]
- 14.Makino, Y., L. N. Mutanda, P. M. Tukei, and E. O. Lichenga. 1983. Enzyme-linked immunosorbent assay (ELISA) for screening rotavirus in faeces. East Afr. Med. J. 60:386-392. [PubMed] [Google Scholar]
- 15.McCormack, J. G. 1982. Clinical features of rotavirus gastroenteritis. J. Infect. 4:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossel, E., and R. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 73:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura, S., H. Ushijima, H. Shiraishi, C. Kanazawa, T. Abe, K. Kaneko, and Y. Fukuyama. 1993. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev. 15:457-459. [DOI] [PubMed] [Google Scholar]
- 18.Pang, X. L., J. Joensuu, and T. Vesikari. 1996. Detection of rotavirus RNA in cerebrospinal fluid in a case of rotavirus gastroenteritis with febrile seizures. Pediatr. Infect. Dis. J. 15:543-545. [DOI] [PubMed] [Google Scholar]
- 19.Salmi, T. T., P. Arstila, and A. Koivikko. 1978. Central nervous system involvement in patients with rotavirus gastroenteritis. Scand. J. Infect. Dis. 10:29-31. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher, R. F., and J. Forster. 1999. The CNS symptoms of rotavirus infections under the age of two. Klin. Paediatr. 211:61-64. [DOI] [PubMed] [Google Scholar]
- 21.Ushijima, H., K. Bosu, T. Abe, and T. Shinozaki. 1986. Suspected rotavirus encephalitis. Arch. Dis. Child. 61:692-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ushijima, H., K. Q. Xin, S. Nishimura, S. Morikawa, and T. Abe. 1994. Detection and sequencing of rotavirus VP7 gene from human materials (stools, sera, cerebrospinal fluids, and throat swabs) by reverse transcription and PCR. J. Clin. Microbiol. 32:2893-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weclewicz, K., K. Kristensson, H. B. Greenberg, and L. Svensson. 1993. The endoplasmic reticulum-associated VP7 of rotavirus is targeted to axons and dendrites in polarized neurons. J. Neurocytol. 22:616-626. [DOI] [PubMed] [Google Scholar]
- 24.Weclewicz, K., L. Svensson, M. Billger, K. Holmberg, M. Wallin, and K. Kristensson. 1993. Microtubule-associated protein 2 appears in axons of cultured dorsal root ganglia and spinal cord neurons after rotavirus infection. J. Neurosci. Res. 36:173-182. [DOI] [PubMed] [Google Scholar]
- 25.Wong, C. J., Z. Price, and D. A. Bruckner. 1984. Aseptic meningitis in an infant with rotavirus gastroenteritis. Pediatr. Infect. Dis. 3:244-246. [DOI] [PubMed] [Google Scholar]
- 26.Wong, V. 2001. Acute gastroenteritis-related encephalopathy. J. Child Neurol. 16:906-910. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida, A., T. Kawamitu, R. Tanaka, M. Okumura, S. Yamakura, Y. Takasaki, H. Hiramatsu, T. Momoi, M. Iizuka, and O. Nakagomi. 1995. Rotavirus encephalitis: detection of the virus genomic RNA in the cerebrospinal fluid of a child. Pediatr. Infect. Dis. J. 14:914-916. [PubMed] [Google Scholar]