Abstract
Toxoplasma gondii is a common life-threatening opportunistic infection. We used experimental murine T. gondii infection to optimize the PCR for diagnostic use, define its sensitivity, and characterize the time course and tissue distribution of experimental toxoplasmosis. PCR conditions were adjusted until the assay reliably detected quantities of DNA derived from less than a single parasite. Forty-two mice were inoculated intraperitoneally with T. gondii tachyzoites and sacrificed from 6 to 72 h later. Examination of tissues with PCR and histology revealed progression of infection from blood to lung, heart, liver, and brain, with PCR consistently detecting parasites earlier than microscopy and with no false-positive results. We then evaluated the diagnostic value of this PCR assay in human patients. We studied cerebrospinal fluid and serum samples from 12 patients with AIDS and confirmed toxoplasmic encephalitis (defined as positive mouse inoculation and/or all of the Centers for Disease Control clinical diagnostic criteria), 12 human immunodeficiency virus-infected patients with suspected cerebral toxoplasmosis who had neither CDC diagnostic criteria nor positive mouse inoculation, 26 human immunodeficiency virus-infected patients with other opportunistic infections and no signs of cerebral toxoplasmosis, and 18 immunocompetent patients with neurocysticercosis. Eleven of the 12 patients with confirmed toxoplasmosis had positive PCR results in either blood or cerebrospinal fluid samples (6 of 9 blood samples and 8 of 12 cerebrospinal fluid samples). All samples from control patients were negative. This study demonstrates the high sensitivity, specificity, and clinical utility of PCR in the diagnosis of toxoplasmic encephalitis in a resource-poor setting.
The emergence of human immunodeficiency virus (HIV) has increased the need for more sensitive and efficient diagnostic tests for opportunistic infections. Latent Toxoplasma gondii infections have a high risk of progressing to toxoplasmic encephalitis in immunocompromised patients. For example, 30 to 50% of HIV-positive Toxoplasma-infected individuals develop cerebral toxoplasmosis (6, 23, 26, 30). Undiagnosed primary toxoplasmosis in pregnant women can also cause severe congenital infection in the developing fetus (9, 10, 31). For these reasons, there has been a considerable effort to develop a more reliable and efficient technique for rapid diagnosis of active Toxoplasma infection (2, 19, 20, 23, 30).
Serological testing for anti-Toxoplasma immunoglobulin is one commonly used technique but has many limitations. In particular, the production of specific antibodies to T. gondii is often delayed or impaired in immunocompromised patients (6, 18). The seroprevalence of T. gondii antibody is approximately 50% in healthy HIV-positive patients; a significant rise in immunoglobulin G (IgG) levels occurs during active toxoplasmosis in only 30% of patients, and only 2% of patients demonstrate a change in IgM titers during cerebral infection (26). For these reasons, serology is not useful for diagnosis of cerebral abscess formation in immunocompromised patients. Other diagnostic methods, such as direct microscopic detection of T. gondii in blood and mouse infection, either have a low sensitivity or are slow and thus of little clinical use (15). Computed tomography and magnetic resonance imaging are important clinical methods of diagnosis but are often not available in Third World settings. A definitive diagnosis often requires brain biopsy, but this method is associated with significant morbidity and mortality and is insensitive, confirming only half of the cases of cerebral toxoplasmosis (11, 29, 30).
PCR has also been used to detect T. gondii infection (5, 8, 12, 13, 17, 24, 25, 29). Most studies have used the B1 gene repetitive sequence and reported sensitivities ranging between 13.3% and 65% when testing cerebrospinal fluid (3, 14, 25). Several studies have demonstrated the diagnostic value of PCR with blood samples, but sensitivities have varied widely, from 25% to 77% (11, 12, 14, 17).
Here, we used a murine toxoplasmosis infection model to optimize a PCR assay for the T. gondii B1 gene repetitive sequence. We then validated this test in tissues, blood, and secretions from mice as they developed toxoplasmosis, compared with control samples from healthy mice. In light of the results from these laboratory experiments, which demonstrated high sensitivity and specificity, we evaluated the clinical utility of this test for diagnosing active cerebral toxoplasmosis in cerebrospinal fluid and blood samples from Peruvian patients with and without suspected cerebral toxoplasmosis.
MATERIALS AND METHODS
Parasite preparation.
T. gondii RH tachyzoites were grown in mouse ascites after intraperitoneal injection. After 3 days, mice were sacrificed, and the parasites were washed in phosphate-buffered saline and then counted in a Neubauer chamber. Tissues were chemically homogenized by incubating for 1 h at 37° Celsius with TESK buffer (50 mM Tris-HCl [pH 8.0], 50 mM EDTA, 1% sodium dodecyl sulfate, and 50 μg [500 μl] of protease K). The product of this reaction was then subjected to phenol-chloroform-isoamyl alcohol (25:24:1) DNA extraction (1, 13). Negative control samples were also prepared from samples of ascitic fluid taken from uninfected mice.
DNA preparation.
In order to compare two DNA extraction procedures, each experimental or clinical specimen was split into two equal portiions that were processed with the Qiagen QIAmp tissue kit exactly according to the manufacturer's instructions that utilize DNA affinity columns (Qiagen, Inc., Chatsworth, Calif.) and the Chelex 100 reagent (Sigma Chemical Co., St. Louis, Mo.), according to the published protocol (33). Samples were centrifuged at 4,000 × g for 10 min; the sediment was resuspended in 200 μl of 5% Chelex X-100 and then incubated at 56°C for 45 min, boiled for 10 min, and centrifuged. The resulting supernatant was stored at −20°C for PCR analysis. In order to evaluate the stability of the purified DNA, PCR analyses were performed monthly with the stored samples from both DNA extraction techniques for 1 year.
PCR.
PCR was performed for the T. gondii B1 gene, as previously described (3). Briefly, the primers 5′-GAT CAG AAA GGA ACT GCA TCC-3′ and 5′-TTA AAG CGT TCG TGG TCA AC-3′ were used to generate a 200-bp product. The samples were coamplified with primers for the human beta globin gene to serve as internal controls (5′-CAA CTT CAT CCA CGT TCA CC-3′ and 5′-GAA GAG CCA AGG ACA GGT AC-3′, product size 290 bp). DNA was amplified in 20-μl reaction volumes containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 200 μM each of the four deoxynucleotide triphosphates, 1 μM (each) of all oligonucleotide primers, 2.5 U of Taq DNA polymerase (Gibco-BRL Products, Rockville, Md.), and 5 μl of crude DNA with a PTC-150 thermal cycler (MJ Research, Inc., Watertown, Mass.). The PCR conditions were adjusted to give optimum sensitivity without the appearance of artifactual PCR products in negative controls. To minimize error, all samples were tested in two separate PCRs. Positive and negative controls, consisting of a sample containing DNA extract from tachyzoites and a sample without DNA, respectively, were included in every set of PCRs. The amplification products were electrophoresed on 1.5% agarose gels stained with ethidium bromide and visualized under UV light. A standard 200-bp DNA size marker was included in every gel.
PCR sensitivity.
The sensitivity of the PCR was tested with 5 × 10−2 pg, 0.1 pg, 1 pg, 10 pg, 1 ng, 10 ng, and 50 ng of purified parasite DNA and also with samples artificially contaminated with 1, 5, 10, 20, and 40 parasites as determined by the Neubauer method.
Experimental mouse model.
Forty-two 1.5-month-old Swiss mice were inoculated by the intraperitoneal route with 5 × 105 T. gondii RH tachyzoites and sacrificed in groups of five animals and one control at 6, 12, 18, 24, 36, 48, and 72 h to examine the time course of Toxoplasma dissemination. At sacrifice, samples of blood, ascitic fluid, brain, liver, heart, and lung tissue were taken from each mouse. Half of each sample was homogenized in TESK buffer and frozen at −80°C for DNA extraction. The other half was stored in phosphate-buffered saline-5% formalin for histological interpretation. The histopathological diagnosis of toxoplasmosis was defined by the visualization of T. gondii tachyzoites or, in one case, characteristic hepatic cysts.
Patients.
The study included a total of 68 patients. Twelve patients had HIV infection and confirmed cerebral toxoplasmosis, defined by our gold standard of a positive T. gondii culture in the diagnostic mouse inoculation test and/or all three Centers for Disease Control (CDC) criteria for AIDS-related cerebral toxoplasmosis. The CDC criteria for clinical diagnosis are (i) recent onset of a focal neurological abnormality consistent with intracranial disease or reduced consciousness; (ii) evidence from brain imaging of a lesion having a mass effect (on computed tomography or magnetic resonance imaging) or a lesion whose radiographic appearance is enhanced by injection of contrast medium, and (iii) serum antibody to T. gondii or successful response to treatment for toxoplasmosis (29).
Of the 12 confirmed toxoplasmosis patients, the mouse inoculation test was performed for 9, with 7 of these 9 mouse inoculations resulting in cultures positive for T. gondii. Ten of the 12 confirmed toxoplasmosis patients were positive by the clinical diagnostic criteria. Five of the 12 confirmed toxoplasmosis patients were positive by both clinical criteria and mouse inoculation. Twelve cerebrospinal fluid samples and nine blood samples were available from the confirmed toxoplasmosis patients. An additional 12 HIV-positive patients had suspected cerebral toxoplasmosis but did not meet the CDC diagnostic criteria and did not have a positive T. gondii mouse inoculation. Eleven cerebrospinal fluid samples and 11 blood samples were available from these 12 patients. Twenty-six HIV-positive control patients had an undiagnosed active opportunistic infection with neurological symptoms but no suspicion of cerebral toxoplasmosis. Six of these patients were later confirmed to have tuberculosis.
Cerebrospinal fluid samples were available from all 26 HIV-positive control patients, and blood samples were available from 23. Eighteen immunocompetent control patients (confirmed HIV negative by enzyme-linked immunosorbent assay and Western blot) had confirmed neurocysticercosis, all of whom provided both cerebrospinal fluid and blood samples. Neurocysticercosis patients were selected because cerebrospinal fluid sampling is part of the investigation of this condition, which is prevalent in Peru. This study was ethically approved by the institutional review boards of the Asociacion Benéfica Proyectos en Informatica, Salud, Medicina y Agricultura and the Universidad Cayetano Heredia. All study participants gave informed written consent and received appropriate treatment.
Serology.
Specific anti-T. gondii IgG antibodies were detected by Western blot with plasma diluted 1:100 and cerebrospinal fluid diluted 1:50, as previously described (18). Briefly, antigen was prepared from T. gondii tachyzoites derived from murine peritoneal infection, the secondary antibody was peroxidase-conjugated anti-human IgG (CDC), and diaminobenzidine was used as the chromogen. Samples were considered positive if either the 20-kDa or 32-kDa diagnostic band was present (7).
Diagnostic mouse inoculation.
Mouse inoculation was performed concurrently with PCR if there was sufficient blood and/or cerebrospinal fluid. Blood samples (5 ml) in tubes with EDTA were diluted 1:1 with Ficoll Histopaque (Sigma Diagnostic Inc., St. Louis, Mo.) and centrifuged at 2,000 × g for 20 min to isolate leukocytes. Mice were inoculated by the intraperitoneal route with 0.5 ml of leukocyte-rich fluid. Cerebrospinal fluid samples were injected directly in volumes of 0.5 to 1 ml. The inoculated mice were sacrificed after 2 months, intraperitoneal fluid was collected, and liver, lung, heart, and brain tissue were fixed in formalin and embedded in paraffin blocks. Then 5-μm sections from these blocks were stained with hematoxylin and eosin and examined by a pathologist. The mouse inoculation was considered positive if histology revealed tachyzoites, and these cases were considered laboratory-confirmed toxoplasmosis (8). On almost all occasions, uninfected control mice were sacrificed at the same time as the experimentally inoculated mice to ensure that there was no environmental source of T. gondii in this mouse colony.
CD4 counts.
A sandwich type immunoenzymatic test (Kit TRAX CD4; Diamedix Corporation, Miami, Fla.) was used according to the manufacturer's instructions to measure the number of CD4 lymphocytes per microliter of blood sample for all patients.
RESULTS
PCR sensitivity.
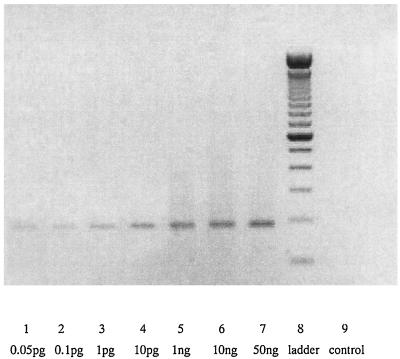
The T. gondii PCR was found to perform with optimum sensitivity and specificity with 2.5 mM MgCl2 and the following cycling parameters: 94°C for 3 min, then 38 cycles at 94°C for 40 s and 67oC for 1 min. The PCR was completed with 10 min at 72oC. Under these conditions, the PCR was sensitive enough to detect as a faint band a minimum of 5 × 10−14 g of DNA, which is equivalent to the DNA yield from 0.5 parasite. When DNA was extracted from a solution containing five parasites, a strong band was evident. All PCR results were identical in duplicate. A representative agarose gel of PCR products is shown in Fig. 1.
FIG. 1.
Agarose gel electrophoresis analysis of PCR amplification products (performed with various concentrations of DNA). The quantity of DNA is shown below each lane, from 0.05 pg in lane 1 to 50 ng in lane 7. Lane 8 contains a 100-bp DNA ladder, and lane 9 is the product from a negative control PCR.
DNA extraction.
If run immediately, PCRs performed on DNA obtained by both extraction techniques gave similar results. However, the DNA purified with Chelex 100 provided reproducible amplification only after up to 2 months of storage at −20°C. In contrast, DNA purified with the Qiagen kit provided reproducible amplifications after up to 1 year of storage at −20°C.
Chronological progression of toxoplasmosis.
The results from the mouse infection model PCR validation are shown in Table 1. After intraperitoneal infection, T. gondii DNA was detected by PCR first in intraperitoneal fluid 18 h postinfection, followed by progressive dissemination to blood, lung, heart, and liver by 36 h, and finally to the brain by 72 h postinfection. The study also showed that by 72 h, 100% of inoculated mice had positive PCR results in the intraperitoneal fluid. By histology, T. gondii was first detected 48 h postinfection in heart tissue and then subsequently in lung and liver. However, at 72 h postinfection, T. gondii was still not detected by histological examination of the brain. Histology was not performed on ascitic fluid and blood. All samples from the seven control mice were negative in both histology and duplicate PCR tests.
TABLE 1.
Chronological dissemination of T. gondii in experimental mouse infection demonstrated by PCR and histologya
| Technique | No. positive/no. analyzed at h postinfection: |
||||||
|---|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | 36 | 48 | 72 | |
| PCR | Negative | Negative | Ascites 2/5 | Ascites 4/5, blood 4/5 | Liver 2/5, ascites 4/5, heart 3/5, blood 4/5, lung 3/5 | Liver 2/5, ascites 4/5, heart 4/5, blood 5/5, lung 4/5 | Liver 2/4, ascites 3/3, heart 3/4, blood 3/3, lung 3/4, brain 3/4 |
| Histology | Negative | Negative | Negative | Negative | Negative | Heart 2/5 | Liver 1/4, heart 2/4, lung 2/4 |
Negative, PCR or histology gave negative results for the detection of T. gondii parasites. Liver, brain, heart, and lung were analyzed by both PCR and histology at each time, and ascites and blood samples were analyzed only by PCR. Tissues not listed in the table were negative. Although five mice were sacrificed at each time point, one mouse died before 72 h, and one sample each of blood and ascites were destroyed.
Clinical utility of T. gondii PCR.
PCR results were compared with the defined gold standard of positive results from mouse inoculation and/or clinical diagnosis (Table 2). All of the duplicate PCR assays were negative except for the 12 patients with diagnosed cerebral toxoplasmosis, 11 of whom had positive PCR results (92% sensitivity, 6 of 9 positive blood samples and 8 of 12 positive cerebrospinal fluid samples). These results give an identical sensitivity of 67% for PCR with either blood or cerebrospinal fluid. Of the seven positive mouse inoculations, one was negative by clinical diagnosis and by PCR of both cerebrospinal fluid and blood. All PCR results were negative except for samples from patients with confirmed toxoplasmosis, demonstrating that the PCR had 100% specificity in this study. The positive predictive value of the PCR was identical for cerebrospinal fluid and blood (100%), and the negative predictive value of the PCR with cerebrospinal fluid and/or blood was 97%.
TABLE 2.
Sensitivity of PCR techniques in detecting T. gondii in patients with confirmed toxoplasmic encephalitis versus mouse inoculation, clinical diagnosis, and the combination of both methods as a gold standard
| Sample testeda | PCR sensitivity (%) (no. positive/no. tested) |
||
|---|---|---|---|
| Mouse inoculation | CDC clinical diagnosis | Mouse inoculation plus clinical diagnosis | |
| CSF alone | 71 (5/7) | 70 (7/10) | 67 (8/12) |
| Blood alone | 50 (3/6) | 86 (6/7) | 67 (6/9) |
| CSF and blood | 86 (6/7) | 100 (10/10) | 92 (11/12) |
CSF, cerebrospinal fluid.
Diagnostic results of serology.
Patients were considered seropositive if Western blots of either cerebrospinal fluid or serum samples were positive for IgG. Sixty-eight patients were evaluated for T. gondii serology, and 62% (42 of 68) were positive. Of the 42 patients with positive Western blots, 8 had positive PCR results in cerebrospinal fluid and/or blood and the remaining 34 were negative by PCR. Of the 26 patients with negative Western blots, 22 also had negative PCR and the remaining 4 had positive PCR in blood and/or cerebrospinal fluid. Two patients with confirmed toxoplasmic encephalitis who were positive by PCR, mouse inoculation, and clinical criteria had negative Western blots for T. gondii. Comparison of Western blot (for antibodies) with PCR results (for active infection) revealed low concordance (Kappa = 0.03, no observed agreement; P = 0.35).
CD4 levels.
CD4 levels in HIV-positive patients with confirmed cerebral toxoplasmosis (84 ± 31.9 CD4 cells/μl of blood; range, 36 to 132) were significantly lower than in HIV-positive patients with other clinical diagnoses (180 ± 174 CD4 cells/μl of total blood; range, 24 to 635; P < 0.001 by Student's t test).
DISCUSSION
This research demonstrated that PCR is a relatively simple and rapid procedure that can be performed in a developing country with a reasonable laboratory infrastructure, such as Peru. The B1 primer has theoretical advantages because each tachyzoite contains approximately 35 DNA copies of this gene (7). The sensitivity of this primer has also been reported in nonimmunosuppressed cases with amniotic fluid, cerebrospinal fluid, aqueous humor, tissues, or blood for diagnosis of active disease (7, 12, 23, 25). The PCR sensitivity found in this study is significantly higher than that reported by previous studies. This discrepancy may in part reflect the fact that patients in Lima present with more advanced opportunistic infections, the high prevalence of toxoplasmosis in the Peruvian population selected for our study, or optimization of the laboratory techniques that we used (7, 16).
One of the advantages of the PCR assay over diagnostic mouse inoculation, biopsy, and other assays is the rapidity of PCR. DNA extraction took approximately 2 h, the thermocycling reaction itself took 1.5 h, and visualization of PCR products with ethidium bromide-stained gels typically took 1 h. All of these procedures can easily be performed in one working day, and recently developed technology for the real-time identification of PCR products could be used to further reduce the time taken for this assay. However, this would currently increase costs considerably and make this test less suitable for use in the developing countries where most HIV-positive people live.
In this and other studies, the majority of patients were seropositive for IgG against T. gondii (6, 10, 18, 28). However, two patients with confirmed cerebral toxoplasmosis (by clinical diagnosis, mouse inoculation, and PCR) had negative serology by Western blot. This is comparable to a previous study that also found that 5 to 10% of a study population of HIV-positive patients with confirmed toxoplasmosis may have negative serology to T. gondii (32). This may be due to immunoparesis in advanced AIDS or the result of an acute primary T. gondii infection rather than reactivation (6,,18,,27).
Previous serological studies have indicated that toxoplasmosis manifests as encephalitis when a latent T. gondii infection undergoes reactivation due to a low CD4 count (19, 22, 28, 33), and cerebral toxoplasmosis develops in approximately 30% of AIDS patients with prior evidence of positive T. gondii serology (6, 23, 33). In our study, the average CD4 count of patients who were HIV positive and had confirmed cerebral toxoplasmosis was significantly lower than that of the HIV-positive patients without cerebral toxoplasmosis and the HIV-negative patients. Although this is consistent with cerebral toxoplasmosis generally developing in patients with advanced immunosuppression, it is possible that the toxoplasmosis itself could have caused suppression of the CD4 count.
This is the first report of PCR validated against evolving experimental infection. This murine model demonstrated the time course and dissemination pattern of toxoplasmosis and the high sensitivity of the assay, which consistently detected parasite DNA before the development of histopathological changes. Previous studies have shown that PCR has high sensitivity (<10 organisms on PCR) in experimental acute murine toxoplasmosis (32, 34). Interestingly, the dissemination pattern of toxoplasmosis also demonstrated that cerebral infection occurred later than in other tissues, possibly marking the development of murine immunity and resultant encystment in the immunologically privileged brain tissues. We also found that mice infected by the intraperitoneal route had positive PCR results from ascitic fluid as little as 18 h after infection. This suggests that the speed of diagnosis in the mouse inoculation model may be increased if PCR of murine ascitic fluid is added to the diagnostic protocol when used in the diagnosis of toxoplasmosis, although direct PCR analysis of patient cerebrospinal fluid and blood may make the mouse inoculation assay redundant.
Southern blot hybridization was not used to confirm the identity of the PCR products. However, the reliability of these PCR primers has been validated in previous research (3, 13, 31), and their identity in the current study was confirmed by the size of the PCR product in comparison with molecular weight markers. Furthermore, our use of positive and negative control samples from parasite cultures, experimental mouse tissues, diagnostic mouse tissues, and patient samples provided further evidence of the sensitivity and specificity of the PCR assay.
The B1-specific gene probe used for the detection of T. gondii does not cross-react with other microorganisms found in immunocompromised patients such as Sarcocystis spp., Neospora spp., Plasmodium spp., Aspergillus spp., Candida spp., and Cryptococcus spp. Studies have reported that the B1 gene is able to detect approximately a single tachyzoite in ocular fluids (2, 3) and also that B1 primers were not compromised when large amounts of human lymphocyte DNA were present. This level of sensitivity is similar to the PCR assay of this study, which detected DNA equivalent to half of one tachyzoite.
This study demonstrates that PCR is a diagnostic technique with relatively high sensitivity when used with either cerebrospinal fluid or blood samples. Analyzing both cerebrospinal fluid and plasma from each patient increased the clinical reliability of this assay. Collection of cerebrospinal fluid as well as blood is therefore advantageous when there is no suspicion of raised intracranial pressure and lumbar puncture is therefore safe. Future recommendations include the use of both cerebrospinal fluid and blood samples from the same patient in order to increase the sensitivity of PCR detection and the use of PCR conditions such as those reported here that provide high sensitivity.
In developing countries, diagnostic imaging by computed tomography scanning or magnetic resonance imaging is often not available, whereas PCR can be performed at much lower cost. These results demonstrate that this simple and inexpensive assay has sufficient sensitivity and specificity to usefully augment clinical diagnosis of cerebral toxoplasmosis in immunocompromised patients.
Acknowledgments
We thank César Cárcamo, Hugo Garcia, Domitila Campomanes, and Inés Salas for their contributions to this investigation. We thank J. B. Phu and D. Sara for technical support.
This work was partially supported by the Hipolito Unanue Foundation (Perú). Carlton Evans is funded by a Wellcome Trust Career Development Fellowship in Clinical Tropical Medicine.
REFERENCES
- 1.Blanco, J. C., S. Angel, E. Maero, V. Pszenny, P. Serpente, and J. C. Garberi. 1992. Cloning of repetitive DNA sequences from T.gondii and their usefulness for parasite detection. Am. J. Trop. Med. Hyg. 46:350-357. [DOI] [PubMed] [Google Scholar]
- 2.Bretagne, S., J. M. Costa, M. Vidaud, J. Tran Van Nhieu, and J. F. Feith. 1993. Detection of T. gondii by competitive DNA amplification of bronchoalveolar lavage sample. J. Infect. Dis. 168:1585-1588. [DOI] [PubMed] [Google Scholar]
- 3.Burg, J. L., C. M. Grover, P. Pouletty, and J. C. Boothroyd. 1989. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27:1782-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantella, R., A. Colichon, L. Lopez, C. Wu, A. Goldfarb, E. Cuadra, C. Latorre, R. Kanashiro, M. Delgado, and Z. Piscoya. 1974. Geographic prevalence of Toxoplasma gondii antibodies in Peru studied by indirect fluorescent antibody technique. Trop. Geogr. Med. 26:204-209. [PubMed] [Google Scholar]
- 5.Chardes, T., I. Bourguin, M. Mevelec, J. Dubremetz, and D. Bout. 1990. Antibody responses to Toxoplasma gondii in sera, intestinal secretions, and milk from mice and characterization of target antigens. Infect. Immun. 20:1240-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danneman, B. R., D. M. Israelski, G. S. Leoung, T. McGraw, J. Mills, and J. S. Remington. 1991. Toxoplasma serology, parasitemia and antigenemia in patients at risk for toxoplasmic encephalitis. AIDS 5:1363-1365. [DOI] [PubMed] [Google Scholar]
- 7.Da Silva, A. V., and H. Langoni. 2001. The detection of Toxoplasma gondii by comparing cytology, histopathology, bioassay in mice, and the polymerase chain reaction (PCR). Vet. Parasitol. 97:191-198. [DOI] [PubMed] [Google Scholar]
- 8.Derouin, F., M. C. Mazeron, and Y. Garin. 1987. Comparative study of tissue culture and mouse inoculation methods for demonstration of Toxoplasma gondii. J. Clin. Microbiol. 25:1597-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desmonts, G., F. Forester, P. Thulliez, F. Daffos, M. Capella-Pavlovsky, and M. Hartier. 1985. Prenatal diagnosis of congenital toxoplasmosis. Lancet i:500-504. [DOI] [PubMed]
- 10.Doehring, E., and I. Reiter-Owona. 1995. Toxoplasma gondii antibodies in pregnant women and their newborns in Dar es Salaam, Tanzania. Am. J. Trop. Med. Hyg. 52:546-548. [DOI] [PubMed] [Google Scholar]
- 11.Dupon, M., J. Cazenave, J. L. Pellegrin, J. M. Ragnaud, A. Cheyrou, I. Fischer, B. Leng, and J. Lacut. 1995. Detection of Toxoplasma gondii by PCR and tissue culture in cerebrospinal fluid and blood of HIV-seropositive patients. J. Clin. Microbiol. 33:2421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupouy-Camet, J., S. Lavareda de Souza, C. Maslo, A. Paugam, A. Saimot, R. Benarous, C. Tourte-Schaefer, and F. Derouin. 1993. Detection of Toxoplasma gondii in venous blood from AIDS patients by polymerase chain reaction. J. Clin. Microbiol. 31:1866-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filice, G., J. A. Hutt, D. Charles, M. Blackstead, and S. W. Sorenson. 1993. Diagnosis of Toxoplasma parasitemia in patients with AIDS by gene detection after amplification by polymerase chain reaction. J. Clin. Microbiol. 31:2327-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzen, C., M. Altfeld, P. Hegener, P. Hartmann, G. Arendt, H. Jablonowoski, J. Rockstroh, V. Diehl, B. Salzberger, and G. Fatkenheuer. 1997. Limited value of PCR for detection of Toxoplasma gondii in blood from human immunodeficiency virus-infected patients. J. Clin. Microbiol. 35:2639-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenkel, J. K. 1985. Toxoplasmosis. Pediatr. Clin. N. Am. 32:917-932. [DOI] [PubMed] [Google Scholar]
- 16.Grigg, M. E., and J. C. Boothroyd. 2001. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J. Clin. Microbiol. 39:398-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guy, E. C., and D. H. Joynson. 1995. Potential of the polymerase chain reaction in the diagnosis of active toxoplasma infection by detection of parasite in blood. J. Infect. Dis. 172:319-322. [DOI] [PubMed] [Google Scholar]
- 18.Hafid, J., R. Tran Manh Sung, H. Raberin, Z. Y. Akono, B. Pozzetto, and M. Jana. 1995. Detection of circulating antigens of Toxoplasma gondii in human infection. Am. J. Trop. Med. Hyg. 52:336-339. [DOI] [PubMed] [Google Scholar]
- 19.Holliman, R. E. 1994. Recent developments in the diagnosis of toxoplasmosis. Serodiagn. Immunother. Infect. Dis. 6:05-16. [Google Scholar]
- 20.Huskinson, J., P. Thulliez, and J. S. Remington. 1990. Toxoplasma antigens recognized by human immunoglobulin A antibodies. J. Clin. Microbiol. 28:2632-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, J. D., P. D. Butcher, D. Savva, R. E. Holliman. 1993. Application of the polymerase chain reaction to the diagnosis of human toxoplasmosis. J. Infect. 26:147-158. [DOI] [PubMed] [Google Scholar]
- 22.Julander, I., C. Martín, M. Lappalainen, E. Guy, B. Isberg, and B. Evenger. 2001. Polymerase chain reaction for diagnosis of cerebral toxoplasmosis in cerebrospinal fluid in HIV-positive patients. Scand J. Infect. Dis. 33:538-541. [DOI] [PubMed] [Google Scholar]
- 23.Khalifa, K. S., A. Roth, R. Roth, K. Araseth, and K. Janetschke. 1994. Value of PCR for evaluating occurrence of parasitemia in immunocompromised patients with cerebral and extracerebral toxoplasmosis. J. Clin. Microbiol. 32:2813-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebech, M., A. Lebech, S. Nelsing, J. Vuust, L. Mathiesen, and E. Petersen. 1992. Detection of T. gondii DNA by PCR in cerebral fluid from AIDS patients with cerebral toxoplasmosis. J. Infect. Dis. 165:982-983. [DOI] [PubMed] [Google Scholar]
- 25.Lin, M., T. Chen, T. Kuo, C. Tseng, and C. Tseng. 2000. Real-time PCR for quantitative detection of Toxoplasma gondii. J. Clin. Microbiol. 38:4121-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luft, B. J., and J. S. Remington. 1998. Toxoplasma encephalitis. J. Infect. Dis. 157:1-6. [DOI] [PubMed] [Google Scholar]
- 27.Parmley, S. F., F. D. Goebel, and J. S. Remington. 1992. Detection of Toxoplasma gondii in cerebrospinal fluid from AIDS patients by polymerase chain reaction. J. Clin. Microbiol. 30:3000-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partenen, P., H. J. Turunen, R. T. Parasivuo, and P. O. Leinileki. 1984. Immunoblot analysis of Toxoplasma gondii antigens by human immunoglobulin G, M, and A antibodies at different stages of infection. J. Clin. Microbiol. 20:133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts, T. C., and G. A. Storch. 1997. Multiplex PCR for diagnosis of AIDS-related central nervous system lymphoma and toxoplasmosis. J. Clin. Microbiol. 35:268-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoondermark, E., J. Galama, C. Kraaijeueld, J. Van Druten, J. Mencuissen, and W. Melchers. 1992. Value of polymerase chain reaction for the detection of Toxoplasma gondii in cerebrospinal fluid from patients with AIDS. Clin. Infect. Dis. 16:661-666. [DOI] [PubMed] [Google Scholar]
- 31.Schoondermark, E., W. Melchers, J. Galama, W. Camps, T. Eskes, and J. Meuwissen. 1993. Congenital toxoplasmosis: an experimental study in rhesus monkeys for transmission and prenatal diagnosis. Exp. Parasitol. 77:200-221. [DOI] [PubMed] [Google Scholar]
- 32.Skiest, D. J., W. Erdman, W. E. Chang, O. K. Oz, A. Ware, and J. Fleckenstein. 2000. SPECT thallium-201 combined with Toxoplasma serology for the presumptive diagnosis of focal central nervous system mass lesions in patients with AIDS. J Infect. 40:274-281. [DOI] [PubMed] [Google Scholar]
- 33.Walsh, P. Sean, D. A. Metzger, and R. Higuchi. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10:506-513. [PubMed] [Google Scholar]
- 34.Weiss, L. M., S. Udem, M. Salgo, H. Tanoqitz, and M. Wittner. 1991. Sensitive and specific detection of toxoplasma DNA in an experimental murine model: use of Toxoplasma gondii-specific cDNA and the polymerase chain reaction. J. Infect. Dis. 163:180-186. [DOI] [PubMed] [Google Scholar]