Abstract
An evaluation of the AMPLICOR hepatitis C virus (HCV) monitor test, version 2.0 (Roche Diagnostics), was carried out to investigate whether this test overestimates the HCV RNA content of reference preparations. Satisfactory accuracy was observed when the World Health Organization HCV international standard was included in the assay and a modified formula was used to calculate the viral content.
The first World Health Organization (WHO) international standard for hepatitis C virus (HCV) RNA for nucleic acid amplification technology (NAT) (96/790) was established in 1997 based on the results of an international collaborative study, and its HCV RNA content was expressed in international units (IU) (1). Since then, calibration of working reagents has become possible and harmonization of data from individual laboratories has been feasible.
The Italian HCV RNA reference preparation ISS 0498 was recently calibrated, along with four more reference preparations, in an international collaborative study (2) in which 19 experienced laboratories participated, using mainly qualitative in-house or commercial NAT assays. Each participant was requested to test in parallel the ISS preparation and the WHO standard for HCV RNA by performing four independent assays on different days. In order to obtain an accurate value for the end point, i.e., the dilution at which 63% of samples tested are positive, a minimum of five half-log dilutions were assayed for both reference materials. Statistical evaluation was performed on the results from all laboratories, and a concentration of 103.23 IU/ml was assigned to the ISS 0498 preparation. To date, we have distributed 1,200 vials of this preparation to a number of Italian transfusion centers for NAT validation. Some of these centers also performed a quantitative evaluation of the HCV RNA content of ISS 0498 by using the AMPLICOR HCV monitor test, version 2.0 (Roche Diagnostics, Branchburg, N.J.), and observed a much higher titer than that established by the international collaborative study (personal communication). In the present study, we investigated this discrepancy by using a rather simple approach that requires a limited number of independent assays.
The design of the study consisted of performing four independent assays on different days by using the AMPLICOR HCV monitor test, version 2.0. In each assay, four undiluted samples of ISS 0498 and one sample of the WHO HCV international standard, diluted in a pool of 12 plasma units (negative for anti-human immunodeficiency virus and anti-HCV antibodies, HBsAg, and HCV RNA) to a final concentration of 103.00 IU/ml, were tested.
The AMPLICOR HCV monitor test, version 2.0, has a stated dynamic range of quantitation between 600 and 850,000 IU/ml. The assay includes a quantitation standard (QS), which is a noninfectious in vitro-transcribed RNA molecule to be added to each sample at the time of extraction. Its concentration is expressed in IU/milliliter. The assay was carried out in accordance with the manufacturer's instructions. Therefore, the two positive controls provided with the kit, with low and high HCV titers, were also tested. Briefly, after amplification and denaturation, the amplified products were serially diluted directly in separate microwells coated with HCV- and QS-specific probes. Measurement of the optical density (OD) at 450 nm yielded two series of values, one for the sample and the other for the QS. The concentration of HCV RNA in the sample was calculated by using two different approaches, namely, (i) the manufacturer's formula
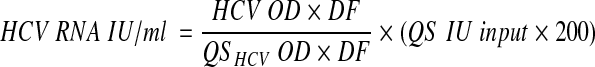 |
where OD is the lowest optical density value that falls between 0.15 and 2.0, DF is the dilution factor associated with the respective OD value, QS IU input is the lot-specific concentration assigned to the QS, and 200 is the dilution factor, and (ii) by generating a new formula in which the QS IU input is replaced by the international standard input (WHO IU input):
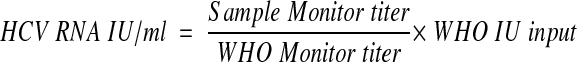 |
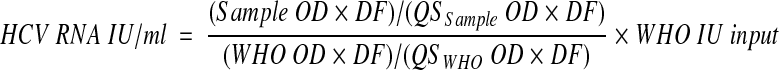 |
(3) |
The final formula is
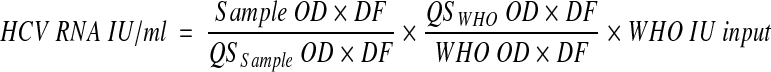 |
(4) |
where WHO IU input is the concentration of the diluted sample of the WHO standard subjected to extraction.
The results obtained by testing the 16 samples of ISS 0498 preparation and the four samples of WHO Standard by the AMPLICOR HCV monitor test, calculated according to the manufacturer's formula, are reported in Table 1. The mean value of the four samples of ISS preparation for each of the four independent assays ranged from 103.49 to 103.89 IU/ml, with an overall mean of 103.70 IU/ml. In the case of the WHO standard, the values, corrected by the predilution factor (1:100), ranged from 105.36 to 105.68 IU/ml, with a mean titer of 105.49 IU/ml. The interassay precision was comparable with the data provided by the manufacturer. With respect to the positive controls, the HCV titer fell within the range indicated on the data card provided with the kit.
TABLE 1.
HCV RNA titers (log10 IU/ml) of the ISS preparation and WHO standard calculated by using the manufacturer's formula
| Day | Titer (log10 IU/ml) |
|||||
|---|---|---|---|---|---|---|
| ISS 0498 Sample |
WHO standarda | |||||
| A | B | C | D | Mean | ||
| 1 | 3.62 | 3.81 | 3.76 | 3.42 | 3.65 | 5.45 |
| 2 | 3.63 | 3.90 | 3.68 | 3.82 | 3.76 | 5.45 |
| 3 | 3.77 | 3.86 | 4.15 | 3.78 | 3.89 | 5.68 |
| 4 | 3.49 | 3.31 | 3.54 | 3.62 | 3.49 | 5.36 |
| Overall mean | 3.70 | 5.49 | ||||
Figures corrected by the predilution factor (1:100).
These results proved that the commercial assay actually overestimates the viral content of both the international standard and the ISS 0498 preparation with respect to the values previously assigned to them in international collaborative studies, namely, 105.00 IU/ml and 103.23 IU/ml, respectively (1, 2). It is worth noting, based on the data obtained in the present study, that the differences between the inputs and measured concentrations for the two reference materials were comparable, as they were 0.49 log in the case of the WHO standard and 0.47 log in the case of the ISS 0498 preparation. We feel that this overestimation can be attributed to an erroneous assignment of the number of IU/milliliter to the HCV RNA concentration of the QS. This assumption is supported by the observation that using the newly generated formula, where the QS IU input is replaced by the WHO IU input, we obtained for the ISS 0498 preparation an HCV RNA content of 103.21 IU/ml, which reflects the value originally assigned (Table 2). The package insert of the AMPLICOR HCV monitor test, version 2.0, states that the QS was standardized against the WHO standard and that the concentration was expressed in IU/milliliter. As the insert does not describe how this standardization was performed, one hypothesis is that a conversion of copies/milliliter into IU/milliliter was done by using an inappropriate conversion factor. This could have generated the mistake.
TABLE 2.
HCV RNA titers (log10 IU/ml) of the ISS preparation calculated by using our newly generated formula
| Day | Titer (log10 IU/ml) |
||||
|---|---|---|---|---|---|
| ISS 0498 Sample |
Mean | ||||
| A | B | C | D | ||
| 1 | 3.17 | 3.36 | 3.32 | 2.97 | 3.21 |
| 2 | 3.18 | 3.44 | 3.22 | 3.37 | 3.31 |
| 3 | 3.09 | 3.18 | 3.47 | 3.10 | 3.21 |
| 4 | 3.12 | 2.95 | 3.17 | 3.26 | 3.12 |
| Overall mean | 3.21 | ||||
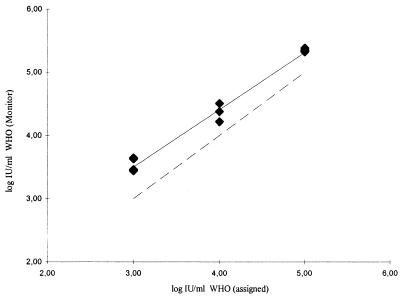
As, according to the Monitor test instructions, the QS has to be added to the lysis reagent in order to process the samples, the value of the QS IU input remains the same in all assays, no matter what the viral content of the sample is. This means that a similar overestimation can be expected throughout the entire linear range of the Monitor test. Indeed, this is what we observed when, to confirm this hypothesis, we tested the international standard at three different concentrations, 103.00, 104.00, and 105.00 IU/ml, in four independent assays (Fig. 1). The mean values were 3.55, 4.33, and 5.35 log IU/ml, respectively.
FIG. 1.
Linearity in the overestimation of the HCV RNA content of the WHO standard by the Roche AMPLICOR HCV monitor test, version 2.0. The solid line represents the linear regression of the values obtained with the Monitor test, while the dotted line represents 100% concordance.
In conclusion, we confirmed that the Roche AMPLICOR HCV monitor test, version 2.0, overestimates the HCV RNA content of reference materials. It should be noted, however, that any attempt to use this or any other quantitative assay to calibrate a reference material not within the framework of a collaborative study would be misleading, as it would not take into consideration the many factors that influence the outcome of a collaborative study.
Acknowledgments
This work was supported by grant “Progetto 1% ricerca corrente” no. 9L2/C from the Italian Ministry of Health.
REFERENCES
- 1.Saldanha, J., N. Lelie, A. Heath, and the WHO collaborative study group. 1999. Establishment of the first international standard for nucleic acid amplification technology (NAT) assays for HCV RNA. Vox Sang. 76:149-158. [DOI] [PubMed] [Google Scholar]
- 2.Saldanha, J., A. Heath, N. Lelie, G. Pisani, M. Nubling, M. Yu, and the collaborative study group. 2000. Calibration of HCV working reagents for NAT assays against the HCV international standard. Vox Sang. 78:217-224. [DOI] [PubMed] [Google Scholar]