Abstract
A large outbreak of aseptic meningitis occurred from April to November 2001 in Taiwan. Of the 1,130 enterovirus-infected patients, echovirus 30 (E30) infection was diagnosed in 188 (16.6%), with the patients having various clinical manifestations including aseptic meningitis (73.9%), young infant fever (6.9%), respiratory symptoms or herpangina (13.3%), or others (5.9%). The majority of the E30-infected patients were between 3 and 10 years old. Of the 264 E30 strains identified, 94.3, 71, and 67.4% were isolated from RD, MRC-5, and A549 cells, respectively. Primary isolation of E30 required mean times of 3.7 days for RD cells and 4.1 days for MRC-5 and A549 cells. Among all E30-positive patients, virus was most frequently isolated from throat swab specimens (85.2%) and, to a lesser extent, stool (76.4%) or cerebrospinal fluid (70.1%) specimens. The virus isolates were initially identified as echovirus 4 (E4) on the basis of immunofluorescence staining with anti-E4 and anti-E30 (Bastianni prototype) monoclonal antibodies. However, upon performance of the neutralization test, E30-specific reverse transcription-PCR, and sequencing of the VP1 gene, the results identified these isolates as E30, not E4, indicating that the reagent used to type E30, which is produced with the Bastianni strain as the immunogen, is inadequate for the identification of recent E30 isolates in Taiwan. Phylogenetic analyses of the VP1 genes of these isolates showed that their sequences differed from those of E30 isolates from the GenBank database by 9.1 to 25.2%, suggesting that this outbreak was caused by a new variant strain of E30 introduced into Taiwan in 2000 that resulted in the widespread aseptic meningitis epidemic in 2001.
Enteroviruses are the major etiologic agents of aseptic meningitis, which results in approximately 50,000 hospitalizations per year in the United States and Canada (14). A variety of clinical manifestations are associated with enteroviral infections, including respiratory illness (common colds), hand-foot-and-mouth disease, acute hemorrhagic conjunctivitis, myocarditis, neonatal sepsis-like disease, encephalitis, and acute flaccid paralysis (12). Sixty-six human enterovirus serotypes have been classified into coxsackieviruses A and B, echoviruses, polioviruses, and enteroviruses 68 to 71 (11, 17). Several epidemic outbreaks of enterovirus infection occurred in Taiwan between January 1994 and December 2000 (4, 21, 22, 23). An outbreak of aseptic meningitis occurred in Taiwan from April to November 2001. Many enteroviruses were isolated (from 1,130 patients) in our laboratory during this outbreak. The majority (17%) of the enteroviruses isolated were echovirus 30 (E30).
Diagnosis of enterovirus infections is generally based on viral isolation and identification by indirect immunofluorescence staining with commercially available monoclonal antibodies or serotyping by a neutralization test with Lim and Benyesh-Melnick pools (7). However, new antigenic variants or emerging serotypes cannot be consistently serotyped by the methods described above and are frequently found to be untypeable (2). Recently, molecular methods based on reverse transcription (RT)-PCR, DNA sequencing, and computerized analytical programs have been used for epidemiological investigations and classification of enteroviruses (2, 8, 15, 16, 17, 18).
E30 is a human enterovirus belonging to the family Picornaviridae. The picornavirus positive-stranded RNA genome possesses an unusually long, conserved 5′ untranslated region (UTR) of approximately 740 nucleotides. It has been demonstrated that this region plays a crucial role in the viral life cycle (13). In contrast to the highly conserved 5′ UTR, the open reading frame encoding capsid protein VP1 is more variable and confers distinct antigenic properties to the virus (1). Thus, this VP1-encoding region of the genome is considered the most suitable for use in sequence analysis for determination of the enterovirus genotype and genetic variation. In this study, analysis of E30 by RT-PCR and sequencing of the VP1 gene allowed us to understand the genetic diversity of E30 in an outbreak of aseptic meningitis. The results revealed that a variant genotype of E30 was responsible for the outbreak in Taiwan in 2001.
MATERIALS AND METHODS
Specimen collection and processing.
Specimens consisting of throat swab, stool or rectal swab, and vesicular swab specimens were collected in viral transport medium. Cerebrospinal fluid (CSF) specimens were directly collected in sterile tubes from inpatients or outpatients suspected of having enteroviral infection. Nonsterile specimens including throat swab, stool, and vesicular swab specimens (pretreated with penicillin [500 U/ml], gentamicin [500 μg/ml], and amphotericin B [Fungizone; 10 μg/ml]) were centrifuged at 3,000 × g for 20 min at 4°C before inoculation into cell cultures.
Cell lines, virus isolation, and virus identification.
RD, A549, Green monkey kidney (GMK), and MRC-5 cells were routinely used for enterovirus isolation. Cells were cultured in Eagle's minimum essential medium (Gibco, BRL, Grand Island, N.Y.) (supplemented with 10% fetal bovine serum, penicillin [100 U/ml], streptomycin [100 μg/ml], and amphotericin B [0.25 μg/ml]) and incubated at 35°C with 5% CO2. Each culture tube was inoculated with 0.2 ml of clinical specimens, and the specimens were examined for cytopathic effects for 14 days postinoculation. Enterovirus strains were typed antigenically by neutralization tests with Lim and Benyesh-Melnick pools (7) or immunofluorescence tests with available monoclonal antibodies including mouse anti-E30 (catalog no. 3315; Chemicon International Inc.) and mouse anti-echovirus 4 (anti-E4; catalog no. 3317; Chemicon International Inc.). Identification of E30 was confirmed by an E30-specific RT-PCR (10) or a neutralization test with polyclonal antibodies (anti-E30 serum, ATCC VR-1072; anti-E4 serum, ATCC VR-1041). E30 was pretreated with a low concentration of chloroform to disaggregate the virus before performance of the neutralization test (9).
Extraction of E30 RNA.
E30 was grown in RD cells, and the infected cells were scraped and pelleted by centrifugation when a 75% cytopathic effect was seen. The viral RNA was extracted with TRIZOL (Gibco, BRL) and chloroform, followed by precipitation with isopropanol. Purified RNA was resuspended in 100 μl of distilled water.
RT and PCR.
cDNA for panenterovirus RT-PCR was synthesized and amplified as described by Cornelissen et al. (5). Briefly, a 20-μl reaction mixture contained 75 mM KCl, 50 mM Tris-HCl (pH 8.3), 3 M MgCl2, 10 mM dithiothreitol, 0.2 mM each deoxynucleoside triphosphate, 50 pmol of primer 011 (antisense; 5′-GCICCIGAYTGITGICCRAA-3′; nucleotides [nt] 3408 to 3389), 200 U of moloney murine leukemia virus reverse transcriptase, and 5 μg of purified RNA. The PCR primers (primer 008 [sense; 5′-GCRTGCAATGAYTTCTCWGT-3′; nt 2411 to 2430] and primer 011[antisense]) amplified a cDNA sequence which encoded the enterovirus VP3, VP1, and 2A genes (14). The PCR mixture contained 50 mM KCl, 10 mM Tris-HCl (pH 8.9), 3.6 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 100 μg of bovine serum albumin per ml, and 40 pmol of primers. One unit of Taq DNA polymerase was added after denaturation of the cDNA at 95°C for 5 min. DNA amplification was performed for 40 cycles consisting of denaturation for 1 min at 95°C, primer annealing for 2 min at 45°C, and extension for 1 min at 72°C. The RT-PCR products were analyzed by electrophoresis on 1% agarose gels (20). The E30-specific RT-PCR was performed as described by Kilpatrick et al. (10) with primer 47A (5′-TKIACRTGICKIGTYTGCAT-3′; E30 VP1 nt 162 to 143) for cDNA synthesis and primer 120S (5′-GACCCIGARIRIGCIYTNAA-3′; E30 VP1 nt 4 to 25) and primer 47A for PCR.
Sequencing of RT-PCR products.
The RT-PCR products were analyzed by a Sanger dideoxy cycle sequencing reaction protocol with primers 008, 011, 040 (sense; 5′-ATGTAYRTICCIMCIGGIGC-3′; nt 2951 to 2970), and 013 (antisense; 5′-GGIGCRTTICCYTCIGTCCA-3′; nt 3051 to 3032) according to the instructions of the manufacturer (PE Applied Biosystems) (14, 15). After completion of the cycle sequencing reactions, the products were analyzed in an automated DNA sequencer (model 373A; Applied Biosystems). Each RT-PCR product was sequenced in both directions to resolve possible ambiguous nucleotides.
Phylogenetic analysis of VP1 sequences.
Sixteen E30 isolates randomly sampled from patients with mild fever to aseptic meningitis were used for phylogenetic analysis (Table 1). Multiple-sequence alignments were performed with the PileUp program (version 10.1; University of Wisconsin Genetics Computer Group). Three different methods (the neighbor-joining, parsimony, and maximum-likelihood methods) of phylogenetic analysis from the PHYLIP program package (version 3.573c) were used to make a more reliable inference of phylogeny. The nucleotide distances were calculated by use of the DNADIST program from the PHYLIP program package and by use of the Kimura two-parameter model with a transition and transversion rate of 2.0. A phylogenetic tree was then constructed by using the neighbor-joining program of the PHYLIP package. The SEQBOOT program was used to bootstrap the data, in which 1,000 data sets were analyzed, and the CONSENSE program was used to compute a consensus tree. Statistical estimation of the significance of branch lengths was also determined by the maximum-likelihood method. Pairwise nucleotide and amino acid sequence comparisons were performed by use of the Distance program in DAMBE.
TABLE 1.
E30 isolates used for phylogenetic analysis
| Isolate | Sampling date (mo/yr) | Clinical presentation | Patient age (yr) | Specimen |
|---|---|---|---|---|
| N4111-TW-00 | 12/2000 | Aseptic meningitis | 0.2 | Stool |
| N0566-TW-01 | 03/2001 | Aseptic meningitis | 10.3 | Throat swab |
| N1006-TW-01 | 04/2001 | CNSa syndrome | 5.5 | Throat swab |
| N1251-TW-01 | 04/2001 | Fever | 6.6 | Stool |
| N1375-TW-01 | 05/2001 | Fever | 0.1 | Throat swab |
| N1591-TW-01 | 05/2001 | Meningitis | 10.9 | Throat swab |
| N1788-TW-01 | 05/2001 | Meningitis | 4.1 | Throat swab |
| N1938-TW-01 | 06/2001 | Fever | 8.3 | Throat swab |
| N2144-TW-01 | 06/2001 | Meningitis | 9.9 | CSF |
| N2252-TW-01 | 06/2001 | Aseptic meningitis | 8.7 | CSF |
| N2401-TW-01 | 06/2001 | Meningitis | 0.2 | CSF |
| N2730-TW-01 | 07/2001 | Meningitis | 0.1 | CSF |
| N2846-TW-01 | 07/2001 | Herpangina | 6.0 | CSF |
| N2884-TW-01 | 07/2001 | Meningitis | 3.9 | Throat swab |
| N2970-TW-01 | 07/2001 | Meningitis | 3.9 | Throat swab |
| N3108-TW-01 | 08/2001 | Meningitis | 5.3 | CSF |
CNS, central nervous system.
Nucleotide sequence accession numbers.
The E30 sequences reported in this study have been deposited in the GenBank database under accession nos. AY146069 to AY146084.
RESULTS
E30 outbreak description.
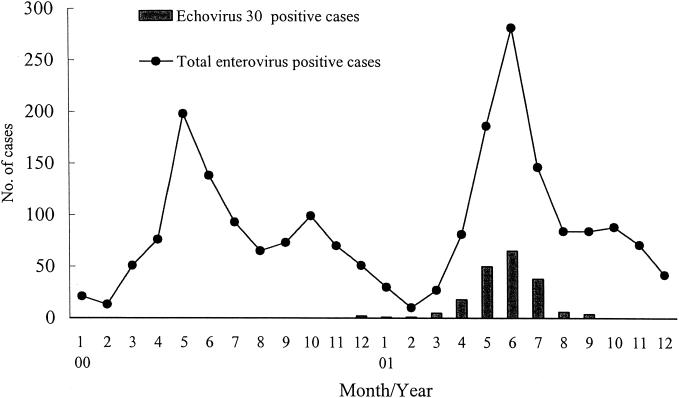
A total of 5,295 specimens were received for viral culture in the National Cheng Kung University Hospital (NCKUH) Virology Laboratory in 2001. Enteroviruses were isolated from 1,130 patients (1,301 specimens) who were suspected of being infected with enterovirus. Only two E30 infections were identified in the NCKUH Virology Laboratory between 1998 and 2000. However, E30 accounted for 16.6% of all enteroviruses isolated in 2001 (Table 2). The first recent isolation of E30, prior to the 2001 outbreak, was in December 2000. The peak period of E30 isolation in Taiwan was between May and June 2001 (Fig. 1). The clinical presentations of 188 E30-infected patients included aseptic meningitis (n = 139; 73.9%), respiratory symptoms or herpangina (n = 25; 13.3%), young infant fever (n = 13; 6.9%), and others (n = 11; 5.9%). The ages of the E30-infected patients ranged from 20 days to 36 years, but most were preschool-age and school-age children. The age distribution of the patients was as follows: <1 year old, 13.9% (26 patients); 1 to 3 years old, 10.7% (20 patients); 3 to 6 years old, 25.6% (48 patients); 6 to 10 years old, 38% (71 patients); 10 to 12 years old, 8.6% (16 patients); and >12 years old, 3.2% (6 patients). The ratio of males to females was 1.28 (104:84).
TABLE 2.
Enterovirus types isolated from 1998 to 2001
| Virus type | No. (%) of cases |
|||
|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | |
| E30 | 0 (0.0) | 0 (0.0) | 2 (0.2) | 188 (16.6) |
| Echovirus 6 | 4 (1.3) | 3 (0.5) | 0 (0) | 154 (13.6) |
| Enterovirus 71 | 132 (44.5) | 13 (2.0) | 195 (20.6) | 116 (10.3) |
| Coxsackievirus A16 | 54 (18.2) | 11 (1.7) | 172 (18.1) | 135 (11.9) |
| Coxsackievirus A10 | 0 (0.0) | 119 (18.4) | 2 (0.2) | 11 (1.0) |
| Coxsackievirus B1-6 | 31 (10.4) | 178 (27.6) | 85 (9.0) | 23 (2.0) |
| Other enteroviruses | 76 (25.6) | 322 (49.8) | 493 (51.9) | 503 (44.6) |
| Total | 297 (100) | 646 (100) | 949 (100) | 1,130 (100) |
FIG. 1.
Monthly distribution of all enterovirus-positive patients and E30-positive patients between 2000 and 2001.
Comparison of cells for detection of E30 by cell culture.
MRC-5, A549, GMK, and RD cells were used for the routine isolation of enterovirus in our laboratory. E30 could not grow in GMK cells. For primary isolation of E30, a mean of 4.1 days (range, 1 to 10 days) was required for MRC-5 cells, a mean of 4.1 days (range, 2 to 11 days) was required for A549 cells, and a mean of 3.7 days (range, 1 to 10 days) was required for RD cells (Table 3). The rates of isolation of E30 from MRC-5, A549, and RD cells were 71.0, 67.4, and 94.3%, respectively (Table 3). Among all E30-positive patients, virus was most frequently obtained from throat swab specimens (85.2%; 155 positive specimens of 182 total specimens tested from positive patients) but was isolated less often from stool specimens (76.4%; 13 of 17) and CSF (70.1%; 103 of 147). Among the patients with aseptic meningitis in 2001, the CSF of 14.3% (99 of 694) was positive for E30 by culture, the CSF of 10.5% (73 of 694) was positive for other enteroviruses, and the CSF of 75.2% (522 of 694) was culture negative.
TABLE 3.
Comparison of rate of growth and isolation of E30 among different cell lines on primary isolation
| Cell line | Growth rate (days)a | Isolation rate (%) |
|---|---|---|
| MRC-5 | 4.1 (1-10) | 71.0 (147/207)b |
| A549 | 4.1 (2-11) | 67.4 (118/175) |
| GMK | 0 | |
| RD | 3.7 (1-10) | 94.3 (249/264) |
Average to detection (range) time.
The values in parentheses are the number of positive specimens/total number of specimens from positive cases tested.
Identification of E30.
The virus isolates were initially identified as E4 on the basis of immunofluorescence staining with monoclonal antibodies from Chemicon Inc. The monoclonal antibodies used were anti-E4, which gave a positive result, and anti-E30, which gave a negative result. However, when the neutralization tests were performed with monovalent antisera, these isolates were neutralized by anti-E30 serum but not by anti-E4 serum. RT-PCR and sequencing of the VP1 gene were conducted to verify the virus type and to analyze the variations among the strains associated with this outbreak. Pairwise nucleotide sequence comparisons showed that these isolates were closer to E30 than to E4. In addition, the predicted RT-PCR products could be obtained from these isolates with E30-specific primers (data not shown) (10). The results confirmed that these echovirus isolates were E30 and not E4 (Table 4).
TABLE 4.
Identification of E30
| Isolate no. | Result bya |
Final identifi- cation | |||||
|---|---|---|---|---|---|---|---|
| IF |
NT |
E30 RT-PCR | Phylogenetic analysis | ||||
| E4 | E30 | E4 | E30 | ||||
| N0566-TW-01 | + | − | − | + | + | E30 | E30 |
| N1066-TW-01 | + | − | − | + | + | E30 | E30 |
| N1375-TW-01 | + | − | − | + | + | E30 | E30 |
| N1591-TW-01 | + | − | − | + | + | E30 | E30 |
| N2516-TW-01 | + | − | − | + | + | E30 | E30 |
If, immunofluorescence staining; NT, neutralization test.
Phylogenetic analysis of VP1 gene.
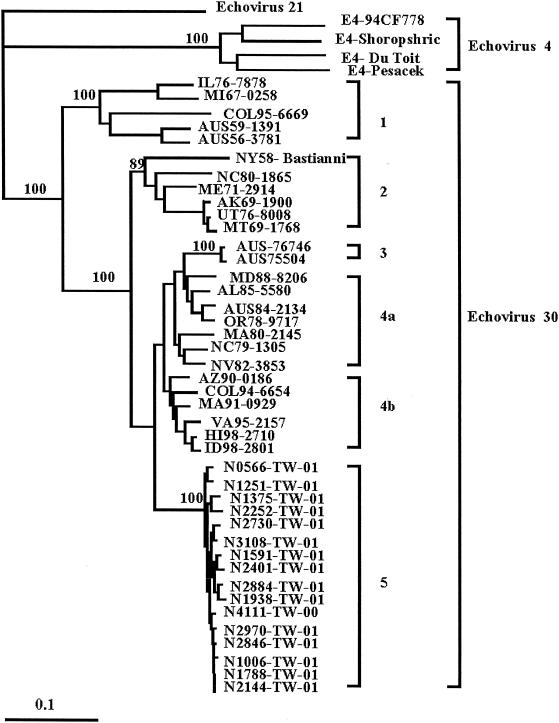
Previously, it has been shown that the VP1 gene contains the most variable region among enteroviruses. In the present study, we conducted molecular analyses with 16 newly isolated E30 strains by sequencing the VP1 genes of strains obtained from different clinical categories and various specimens. The E30 strains examined are listed in Table 1. A phylogenetic tree of these sequences was configured by the neighbor-joining method with echovirus 21 as the outgroup, and sequences from the GenBank database (from 26 E30 strains and 4 E4 strains) were included for comparison (14) (Fig. 2). To estimate the reliability of the phylogenetic tree, bootstrap analysis was performed by the neighbor-joining method. The tree constructed by the maximum-likelihood method is very similar to those produced by the neighbor-joining method and bootstrap analysis (data not shown). Genetic analysis of 16 strains of E30 obtained from the outbreak described here and the first isolate from 2000 showed that these isolates were closely related to each other (0 to 2.9% differences in the 876 bp sequenced), suggesting that the outbreak strain was introduced into Taiwan in December 2000, approximately 3 months prior to the start of the epidemic. In contrast, the sequences of these isolates were different from the E30 and E4 sequences retrieved from the GenBank database by 9.1 to 25.2% and 37.8 to 39.4%, respectively. The sequences of E30 segregated into five distinct branches: genotypes 1 to 4 consisted of E30 isolates from the GenBank database, and genotype 5 contained E30 isolates from the present epidemic. Pairwise comparison of the nucleotide sequences of the VP1 genes showed that the sequences of the strains within genotype 5 differed by up to 2.9% and that the sequences of the genotype 5 strains differed from those of genotype 1, 2, 3, 4a, and 4b isolates by 22.6 to 25.2, 14.5 to 17.6, 12.2 to 13.8, 10.7 to 13.0, and 9.1 to 11.5%, respectively (data not shown). The VP1 regions of these 2001 outbreak strains (genotype 5) had 95.6 to 100% amino acid sequence similarities, and their amino acid sequences differed from those of genotype 1, 2, 3, 4a, and 4b isolates by 8.2 to 11.2, 5.4 to 8.2, 3.4 to 5.4, 1.7 to 5.1, and 2.4 to 5.1%, respectively (data not shown).
FIG. 2.
Phylogenetic analysis of VP1 gene of echovirus. The dendrogram of the 16 E30 outbreak strains and 30 reference strains from GenBank is based on the sequence of 876 nucleotides (nt 2460 to 3335) of the VP1 gene and was obtained by the neighbor-joining method with the DNADIST distance measure program (version 3.573c; PHYLIP). The percent bootstrap frequency of each branch in the tree is indicated. Enterovirus 21 was included as an outgroup.
DISCUSSION
Enteroviruses are common human pathogens and are associated with a broad spectrum of clinical presentations including asymptomatic infections, respiratory symptoms, aseptic meningitis, and severe diseases in newborns and immunocompromised hosts (16). A large outbreak of enterovirus 71 infection occurred in Taiwan in 1998. The outbreak resulted in a hand-foot-and-mouth disease epidemic that caused sudden death among young children (20). In contrast, E30 causes either mild symptoms or aseptic meningitis. Outbreaks of aseptic meningitis associated with E30 are often reported (14, 19). A recent report by the World Health Organization indicated that from 1997 to 1999, of the 1,672 nonpoliomyelitis-associated enteroviruses isolated in the United States, E30 accounted for the highest percentage (27.5%) (3). Very few E30 isolates were detected in the NCKUH Virology Laboratory between 1995 and 2000. However, in the spring and summer of 2001 there was an unusually high rate of isolation of E30 (188 of 1,130 [16.6%] enterovirus-infected patients) at the NCKUH Virology Laboratory. Among these 188 patients, 139 patients (73.9%) were hospitalized with central nervous system syndromes indicating that the virus was neurovirulent.
The large number of E30 isolates obtained in this study allowed analysis of laboratory findings including growth rates in different cell lines and rates of isolation from various specimens. In the present study E30 was most successfully isolated from throat swab specimens but was less successfully isolated from stool and CSF specimens. The reason for the better recovery of virus from throat specimens is probably because throat specimens were usually taken during the early onset of symptoms. In addition, higher detection rates were achieved with throat swab specimens than with stool and CSF specimens, suggesting that throat swab specimens contained higher titers of E30 and allowed the recovery of viruses at higher rates. A pitfall of virus identification solely on the basis of immunofluorescence staining with commercial monoclonal antibodies was noted in this study. The mouse anti-E30 monoclonal antibody (against the Bastianni strain, which was isolated in 1958) could not recognize the recent E30 outbreak strains in Taiwan, suggesting that antigenic drift has occurred. Several reports have indicated that antigenic drift has occurred in enteroviruses (11, 14, 19). Pairwise comparisons of nucleotide sequences showed that the sequences of these isolates differed from the E30 and E4 sequences in the GenBank database by 9.1 to 25.2 and 37.8 to 39.4%, respectively. This indicates that these isolates are E30 strains, since the nucleotide sequences of strains of the homologous serotype were at least 75% identical (2, 15). Thus, it is important to use a neutralization test in conjunction with genetic analysis for evaluation of enteroviruses associated with outbreaks or epidemiological studies.
A recent report (6) indicated that there was an outbreak of E4 in Israel and the Palestinian Authority from June 1997 to beyond February 1998. That outbreak was also associated with aseptic meningitis. Genetic analysis of the 5′ UTR of the E4 genome showed that a new variant genotype of E4 was responsible for the epidemic (6). Molecular analysis of E30 strains isolated from patients involved in an outbreak of aseptic meningitis in Taiwan was undertaken in this study to understand the genetic variations. The phylogenetic analysis showed that the E30 strains from the 2000 and 2001 outbreaks were very similar and belonged to one genotype, with >97% identity in nucleotide sequences and >95% identity in amino acid sequences. In contrast, a recent study (21) showed that two genotypes were cocirculating in Taiwan during the 1998 enterovirus 71 epidemic. In addition, the sequences of the enterovirus 71 isolates from the 1998 and 2000 outbreaks were closely related to the sequences reported by others (21) and placed in the GenBank database. However, the sequences of the E30 strains isolated in the 2001 outbreak in Taiwan were at least 9% different from the sequences of the E30 strains in the GenBank database, indicating that a new variant genotype was circulating in Taiwan. The present investigation of an E30-associated aseptic meningitis outbreak in Taiwan showed that the outbreak had the following epidemiological features: (i) the incidence of the E30 outbreak peaked in southern Taiwan between May and June 2001; (ii) the prevalent genotype was type 5, whose sequence differed from those of genotype 1 to 4 strains in GenBank by 9.1 to 25.2%; and (iii) the outbreak strain was introduced into Taiwan approximately 3 months prior to the epidemic.
Acknowledgments
This study was supported by National Health Research Institutes grant NHRI-CN-CR8801S and by Department of Health grant DOH90-DC-1072.
We thank the members of the Virology Laboratory for efforts in isolating and typing the viruses analyzed in this study. We thank M. Steven Oberste (Centers for Disease Control and Prevention, Atlanta, Ga.) for suggestions in the identification of E30.
REFERENCES
- 1.Brown, B. A., and M. A. Pallansch. 1995. Complete nucleotide sequence of enterovirus 71 is distinct from poliovirus. Virus Res. 39:195-205. [DOI] [PubMed] [Google Scholar]
- 2.Caro, V., S. Guillot, F. Delpeyroux, and R. Craini. 2001. Molecular strategy for ′serotyping' of human enteroviruses. J. Gen. Virol. 82:79-91. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Enterovirus surveillance—United States, 1997-1999. Morb. Mortal. Wkly. Rep. 49:913-916 [PubMed] [Google Scholar]
- 4.Chang, L. Y., Y. C. Huang, and T. Y. Lin. 1998. Fulminant neurogenic pulmonary oedema with hand, foot, and mouth disease. Lancet 352:367-368. [DOI] [PubMed] [Google Scholar]
- 5.Cornelissen, M. T., H. L. Smits, M. A. Briet, J. G. van den Tweel, A. P. Struyk, J. van der Noordaa, and J. ter Schegget. 1990. Uniformity of the splicing pattern of the E6/E7 transcripts in human papillomavirus type 16-transformed human fibroblasts, human cervical premalignant lesions and carcinomas. J. Gen. Virol. 71:1243-1246. [DOI] [PubMed] [Google Scholar]
- 6.Handsher, R., L. M. Shulman, B. Abramovitz, I. Siberstein, M. Neuman, M. Tepperberg-Oikawa, E. Frisher, and T. Mendelson. 1999. A new variant of echovirus 4 associated with a large outbreak of aseptic meningitis. J. Clin. Virol. 13:29-36. [DOI] [PubMed] [Google Scholar]
- 7.Hsiung, G. D. 1994. Picornaviridae, p. 119-140. In G. D. Hsiung, C. K. Y. Fong, and M. L. Landry (ed.), Hsiung's diagnostic virology, 4th ed. Yale University Press, New Haven, Conn.
- 8.Hyypia, T., T. Hovi, N. J. Knowless, and G. Stanway. 1997. Classification of enteroviruses based on molecular and biological properties. J. Gen. Virol. 78:1-11. [DOI] [PubMed] [Google Scholar]
- 9.Kapsenberg, J. G., A. Ras, and J. Korte. 1980. Improvement of enterovirus neutralization by treatment with sodium deoxycholate or chloroform. Intervirology 12:329-334. [DOI] [PubMed] [Google Scholar]
- 10.Kilpatrick, D. R., J. Quay, M. A. Pallansch, and M. S. Oberste. 2001. Type-specific detection of echovirus 30 isolates using degenerate reverse transcriptase PCR primers. J. Clin. Microbiol. 39:1299-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkel, U., and E. Schreier. 2000. Genetic variability within the VP1 coding region of echovirus type 30 isolates. Arch. Virol. 145:1455-1464. [DOI] [PubMed] [Google Scholar]
- 12.Morens, D. M., and M. A. Pallansch. 1995. Epidemiology, p. 3-23. In H. A. Rotbart (ed.), Human enterovirus infections. American Society for Microbiology, Washington, D.C.
- 13.Muir, P., U. Kammerer, K. Korn, M. N. Mulders, T. Poyry, B. Weissbrich, R. Kandolf, G. M. Cleatro, and A. M. van Loon. 1998. Molecular typing of enteroviruses: current status and future requirements. Clin. Microbiol. Rev. 11:202-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberste, M. S., K. Maher, M. L. Kennett, J. J. Campbell, M. S. Carpenter, D. Schnurr, and M. A. Pallansch. 1999. Molecular epidemiology and genetic diversity of echovirus type 30 (E30): genotypes correlate with temporal dynamics of E30 isolation. J. Clin. Microbiol. 37:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberste, M. S., K. Maher, D. R. Kilpatrick, M. R. Flemister, B. A. Brown, and M. A. Pallansch. 1999. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 37:1288-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberste, M. S., K. Maher, D. R. Kilpatrick, and M. A. Pallansch. 1999. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73:1941-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberste, M. S., K. Maher, M. R. Flemister, G. Marchetti, D. R. Kilpatrick, and M. A. Pallansch. 2000. Comparison of classic and molecular approaches for the identification of untypeable enterovirus. J. Clin. Microbiol. 38:1170-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacios, G., I. Casas, A. Tenorio, and C. Freire. 2002. Molecular identification of enterovirus by analyzing a partial VP1 genomic region with different methods. J. Clin. Microbiol. 40:182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savolainen, C., T. Hovi, and M. N. Mulders. 2001. Molecular epidemiology of echovirus 30 in Europe: succession of dominant sublineages within a single major genotype. Arch. Virol. 146:521-537. [DOI] [PubMed] [Google Scholar]
- 20.Wang, J. R., H. P. Tsai, P. F. Chen, Y. J. Lai, J. J. Yan, D. Kiang, K. H. Lin, C. C. Liu, and I. J. Su. 2000. An outbreak of enterovirus 71 infection in Taiwan, 1998. II. Laboratory diagnosis and genetic analysis. J. Clin. Virol. 17:91-99. [DOI] [PubMed] [Google Scholar]
- 21.Wang, J. R., Y. C. Tuan, H. P. Tsai, J. J. Yan, C. C. Liu, and I. J. Su. 2002. Change of major genotype of enterovirus 71 in outbreaks of hand-foot-and-mouth disease in Taiwan between 1998 and 2000. J. Clin. Microbiol. 40:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, S. M., C. C. Liu, Y. J. Chen, Y. C. Chang, and C. C. Huang. 1996. Alice in Wonderland syndrome caused by coxsackievirus B1. Pediatr. Infect. Dis. J. 15:470-471. [DOI] [PubMed] [Google Scholar]
- 23.Wang, S. M., C. C. Liu, Y. J. Yang, H. B. Yang, C. H. Lin, and J. R. Wang. 1998. Fatal coxsackievirus B infection in early infancy characterized by fulminant hepatitis. J. Infect. 37:270-273. [DOI] [PubMed] [Google Scholar]